Key Points
Allogeneic HSCT is an effective therapeutic modality for the HR subset of infants with ALL.
Reduction of pretransplant MRD and selection of appropriate conditioning and donor is crucial for successful transplantation.
Abstract
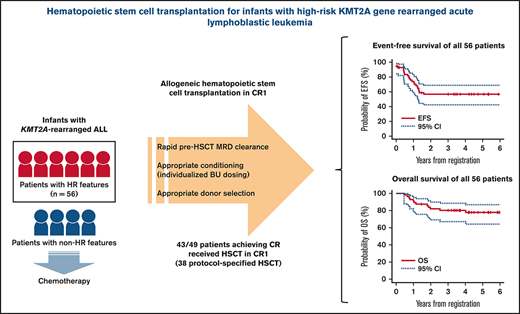
The role of allogeneic hematopoietic stem cell transplantation (HSCT) for infants with acute lymphoblastic leukemia (ALL) and KMT2A gene rearrangement (KMT2A-r) is controversial in terms of both its efficacy and potential for acute and late toxicities. In Japanese Pediatric Leukemia/Lymphoma Study Group trial MLL-10, by introducing intensive chemotherapy, indication of HSCT was restricted to patients with high-risk (HR) features only (KMT2A-r and either age <180 days or presence of central nervous system leukemia). Of the 56 HR patients, 49 achieved complete remission. Forty-three patients received HSCT in first remission including 38 patients receiving protocol-specified HSCT with conditioning consisting of individualized targeted doses of busulfan, etoposide, and cyclophosphamide. Three-year event-free survival (EFS) of 56.8% (95% confidence interval [CI], 42.4% to 68.8%) and overall survival of 80.2% (95% CI, 67.1% to 88.5%) were accomplished. Univariable analysis showed that Interfant-HR criteria and flow cytometric minimal residual disease (MRD; ≥0.01%), both at the end of induction and at the end of consolidation (EOC), were significantly associated with poorer EFS. In the multivariable analysis, positive MRD at EOC was solely associated with poor EFS (P < .001). Rapid pretransplant MRD clearance and tailored HSCT strategy in the MLL-10 trial resulted in a favorable outcome for infants with HR KMT2A-r ALL. However, considering the high rate of potentially life-threatening toxicities and the risk of late effects, its indication should be further restricted or even eliminated in the future by introducing more effective therapeutic modalities with minimal toxicities. This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as #UMIN000004801.
Introduction
Acute lymphoblastic leukemia (ALL) in infants, particularly those with the KMT2A (MLL) gene rearrangement (KMT2A-r) that accounts for ∼80% of cases, has been a challenging disease with reported event-free survival (EFS) rates of <50%.1–3 Attempts to improve the outcome with allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR1) have been carried out by the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG); non–total-body irradiation (TBI) myeloablative conditioning with targeted-dose busulfan (BU) was used to avoid acute and late conditioning-related toxicities and HSCT in the early phase of therapy was attempted to prevent early relapses, which are commonly observed in infants with KMT2A-r ALL.4–6 Nearly 90% of the patients who achieved remission could receive HSCT in CR1 with these approaches, but relatively high rates of post-HSCT relapse remained an issue.6 In addition, the role of HSCT itself for infants with KMT2A-r ALL is controversial,7–9 and at least its benefit seems to be restricted to a high-risk (HR) subset, such as those presenting at younger age (<6 months old) either with a high leukocyte count (white blood cells [WBCs], ≥300 × 109/L) or with poor initial prednisolone response (PPR).10
In the JPLSG MLL-10 trial, introduction of a risk stratification according to KMT2A status, age at diagnosis, and presence of central nervous system (CNS) leukemia and intensive chemotherapy for KMT2A-r patients yielded an excellent 3-year event-free survival (EFS) of 66.2% for 75 infants with KMT2A-r ALL, although the HSCT option was limited to the 56 HR cases.11 In this report, we focused on the infants with HR features stratified to receive HSCT in CR1 in order to clarify both the positive and negative role of this treatment modality by analyzing a homogeneously treated cohort.
Methods
Patients
Details of the JPLSG MLL-10 trial are described elsewhere.11 Briefly , patients included infants younger than 1 year of age with ALL; those with B-cell/myeloid mixed phenotype acute leukemia were eligible, but those with T-cell ALL, T-cell/myeloid mixed phenotype acute leukemia, mature B-cell ALL, or Philadelphia chromosome–positive ALL were excluded. The infants were stratified into HR, intermediate-risk (IR), or low-risk (LR) groups as follows: HR = KMT2A-r and either age <180 days at diagnosis or presence of CNS leukemia defined as CNS-3 (WBC ≥ 5/μL in cerebrospinal fluid with blasts); IR = non-HR KMT2A-r; and LR = germline KMT2A. The factors that defined the risk groups were selected according to results from previous studies (MLL96 and MLL98).12 Patients with KMT2A-r ALL (IR and HR) were treated with modified chemotherapy per Children’s Oncology Group (COG) AALL0631,13 and HR cases were allocated to receive HSCT in CR1. Minimal residual disease (MRD) was prospectively evaluated at certain time points (TPs) with 3 methodologies, but was not used to guide therapy (supplemental Figure 1). The following procedures were performed: flow cytometric (FCM)-MRD targeting leukemia-specific immunophenotypes; immunoglobulin/T-cell receptor (TCR) polymerase chain reaction (PCR)-MRD targeting genomic rearrangements of leukemia-specific immunoglobulin and/or the TCR gene; and KMT2A fusion PCR-MRD targeting certain KMT2A fusion transcripts (KMT2A-AFF1, KMT2A-AFDN, KMT2A-MLLT3, and KMT2A-MLLT1). Written informed consent was obtained from the guardians of the patients according to the Declaration of Helsinki, and institutional review board approval was obtained for all aspects of the study from the 120 institutions participating in the trial.
Donor selection
HLA typing of at least HLA-A, HLA-B, and HLA-DR loci was performed when the patients met the HR criteria. Either of the following could be selected: HLA 5 of 6 to 6 of 6 serologically matched familial donor (MFD) or HLA 4 of 6 to 6 of 6 matched unrelated cord blood donor (UCBD) with total nucleated cells of 2 × 107/kg of recipient’s body weight or higher. In the absence of appropriate MFD or UCBD, selection of matched unrelated donor (MUD) from the Japan Marrow Donor Program was allowed. Either bone marrow (BM) transplantation (BMT) or peripheral blood stem cell (PBSC) transplantation (PBSCT) could be chosen from either MFD or MUD according to the institutional preference.
Conditioning regimen and individual BU dose adjustment
The protocol-specified conditioning regimen was myeloablative conditioning consisting of targeted doses of IV BU (4 times daily over 2 hours on 4 consecutive days from day −8 to −5) in combination with etoposide (ETP; 60 mg/kg IV, once daily over 12 hours on day −4) and cyclophosphamide (CY; 60 mg/kg IV, once daily over 2 hours on days −3 and −2). Use of clonazepam was recommended for preventing BU-related convulsions, but phenytoin or sodium valproate were also permitted.
Initial BU dose was determined based on a pharmacokinetics (PK) test (test PK) performed 1 week prior to the start of conditioning to target within 600 to 900 ng/mL of the steady-state concentration (Css); the plasma BU concentration was measured at 0, 3, 6, and 8 hours of test BU (0.6 mg/kg, 2-hour IV). Further BU dose adjustments were conducted in each patient according to the PK results at the initial BU administration (initial-dose PK) based on the plasma BU sampling data at 0, 3, and 6 hours. The plasma BU concentration was measured by high-performance liquid chromatography centrally at Shinshu University Hospital. PK parameters were calculated using a 1-compartment PK model at Chiba Institute of Science. To evaluate characteristics of PK in infants, distribution of test PK data were displayed by correlation diagrams. To evaluate the significance of dose adjustment by initial-dose PK, actual total area under the curve (tAUC) was retrospectively calculated and compared with the estimated tAUC (16 times the initial-dose area under the curve [AUC]). The proper range of tAUC was set as 57.5 to 86.3 mg/L × hour, which is equivalent to a Css of 600 to 900 ng/mL.
GVHD prophylaxis
Prophylaxis for graft-versus-host-disease (GVHD) consisted of either tacrolimus (24-hour continuous IV from day −1, and maintaining a blood concentration level of 5-10 ng/mL) or cyclosporine (24-hour continuous IV from day −1, and maintaining a blood concentration level of 250-400 ng/mL; or 2-hour to 4-hour IV twice daily from day −1, and maintaining a blood concentration trough level of 150-250 ng/mL) in combination with short-term methotrexate. The methotrexate doses consisted of 15 mg/m2 (on day +1) and 10 mg/m2 (on days +3, +6, and +11) for MFD or MUD, whereas it was 7.5 mg/m2 (on days +1, +3, and +6) for UCBD. If there were no clinical signs of GVHD, recommendations were made for both tacrolimus and cyclosporine to start tapering from day +50.
Statistical analysis
EFS was defined as time from registration to first event (non–complete remission [CR] [defined as event at day 0] evaluated at the end of early consolidation [EOC; TP2], relapse, lineage switch to acute myeloid leukemia [AML], secondary malignancy, or any cause death). Overall survival (OS) was defined as time from registration to death from any cause. Disease-free survival (DFS) in the HSCT subanalysis was defined as time from the date of HSCT to first event (relapse, lineage switch to AML, secondary malignancy, or any cause death). The probability of EFS, DFS, and OS was estimated using the Kaplan-Meier method and standard error (SE) with the Greenwood formula and was then compared using the log-rank test. The 95% confidence intervals (95% CIs) were also computed. Known prognostic variables were examined in the univariable analyses. Cox proportional hazards regression was used to identify the risk factors associated with the EFS rate, and the variables that were significant at P < .1 were included in the model. Variables significantly associated with EFS were then identified by a significance level of .05. All P values were 2-sided, and no statistical adjustment was made for multiple testing. All data analyses were performed with STATA Release 14 statistical software (StataCorp, College Station, TX).
Results
Main analyses for all 56 HR cases
Patient characteristics.
The main analyses were performed for all 56 patients categorized as HR in the MLL-10 trial with the intention to perform HSCT in CR1 (Figure 1). The characteristics of the 56 patients are summarized in Table 1. There was a slight female predominance (57.1%) and one-half of the patients had t(4;11)/KMT2A-AFF1. Reflecting their HR features, 64.3% of the patients had WBC > 100 × 109/L, 46.5% were either CNS-2 (WBC < 5/μL in cerebrospinal fluid with blasts) or CNS-3, and 44.6% were PPR. According to the Interfant-06 risk groups, 58.9% were categorized as Interfant-HR (KMT2A-r and age <6 months either with WBC ≥ 300 × 109/L or PPR) and 41.1% as Interfant-medium risk (MR; other KMT2A-r).
CONSORT flow diagram of the 56 MLL-10 HR patients. AMoL , acute monocytic leukemia; T-ALL, T-cell ALL.
CONSORT flow diagram of the 56 MLL-10 HR patients. AMoL , acute monocytic leukemia; T-ALL, T-cell ALL.
Three-year EFS according to characteristics of the 56 HR patients
| . | n (%) . | 3-y EFS (SE), % . | P . |
|---|---|---|---|
| Sex | |||
| Male | 24 (42.9) | 67.5 (10.2) | .255 |
| Female | 32 (57.1) | 50.0 (8.8) | |
| Age at diagnosis, d | |||
| 0-89 | 21 (37.5) | 62.0 (11.4) | .719 |
| 90-179 | 35 (62.5) | 54.1 (8.5) | |
| WBCs at diagnosis, ×109/L | |||
| <100 | 20 (35.7) | 73.7 (10.1) | .170 |
| 100 to <300 | 14 (25.0) | 54.2 (13.8) | |
| ≥300 | 22 (39.3) | 43.1 (10.8) | |
| CNS status at diagnosis* | |||
| CNS-1 | 29 (51.8) | 58.6 (9.2) | .815 |
| CNS-2 | 16 (28.6) | 46.9 (12.9) | |
| CNS-3 | 10 (17.9) | 63.5 (16.9) | |
| Unknown | 1 (1.8) | — | |
| KMT2A fusion | |||
| t(4;11)/KMT2A-AFF1 | 28 (50.0) | 53.6 (9.4) | .595 |
| t(9;11)/KMT2A-MLLT3 | 3 (5.4) | 100 | |
| t(11;19)/KMT2A-MLLT1 | 14 (25.0) | 57.1 (13.2) | |
| Other KMT2A-r | 11 (19.6) | 50.0 (17.7) | |
| Interfant-06 risk group† | |||
| Interfant-MR | 23 (41.1) | 72.9 (9.5) | .051 |
| Interfant-HR | 33 (58.9) | 45.4 (9.0) | |
| Day 8 prednisolone response | |||
| PGR | 31 (55.4) | 60.1 (8.9) | .615 |
| PPR | 25 (44.6) | 52.5 (10.4) | |
| BM status at TP2‡ | |||
| M1 | 47 (90.4) | 59.5 (7.2) | .205 |
| M2 | 4 (7.7) | 25.0 (21.7) | |
| M3 | 0 (0.0) | — | |
| Not evaluated | 1 (1.9) | — | |
| FCM-MRD at TP2‡ | |||
| <0.01% | 29 (56.9) | 65.5 (8.8) | .036 |
| ≥0.01% | 19 (37.3) | 36.8 (11.1) | |
| Not evaluated | 3 (5.9) | — | |
| FCM-MRD at TP3‡ | |||
| <0.01% | 33 (64.7) | 69.6 (8.0) | <.001 |
| ≥0.01% | 8 (15.9) | 0 | |
| Not evaluated | 10 (19.6) | — |
| . | n (%) . | 3-y EFS (SE), % . | P . |
|---|---|---|---|
| Sex | |||
| Male | 24 (42.9) | 67.5 (10.2) | .255 |
| Female | 32 (57.1) | 50.0 (8.8) | |
| Age at diagnosis, d | |||
| 0-89 | 21 (37.5) | 62.0 (11.4) | .719 |
| 90-179 | 35 (62.5) | 54.1 (8.5) | |
| WBCs at diagnosis, ×109/L | |||
| <100 | 20 (35.7) | 73.7 (10.1) | .170 |
| 100 to <300 | 14 (25.0) | 54.2 (13.8) | |
| ≥300 | 22 (39.3) | 43.1 (10.8) | |
| CNS status at diagnosis* | |||
| CNS-1 | 29 (51.8) | 58.6 (9.2) | .815 |
| CNS-2 | 16 (28.6) | 46.9 (12.9) | |
| CNS-3 | 10 (17.9) | 63.5 (16.9) | |
| Unknown | 1 (1.8) | — | |
| KMT2A fusion | |||
| t(4;11)/KMT2A-AFF1 | 28 (50.0) | 53.6 (9.4) | .595 |
| t(9;11)/KMT2A-MLLT3 | 3 (5.4) | 100 | |
| t(11;19)/KMT2A-MLLT1 | 14 (25.0) | 57.1 (13.2) | |
| Other KMT2A-r | 11 (19.6) | 50.0 (17.7) | |
| Interfant-06 risk group† | |||
| Interfant-MR | 23 (41.1) | 72.9 (9.5) | .051 |
| Interfant-HR | 33 (58.9) | 45.4 (9.0) | |
| Day 8 prednisolone response | |||
| PGR | 31 (55.4) | 60.1 (8.9) | .615 |
| PPR | 25 (44.6) | 52.5 (10.4) | |
| BM status at TP2‡ | |||
| M1 | 47 (90.4) | 59.5 (7.2) | .205 |
| M2 | 4 (7.7) | 25.0 (21.7) | |
| M3 | 0 (0.0) | — | |
| Not evaluated | 1 (1.9) | — | |
| FCM-MRD at TP2‡ | |||
| <0.01% | 29 (56.9) | 65.5 (8.8) | .036 |
| ≥0.01% | 19 (37.3) | 36.8 (11.1) | |
| Not evaluated | 3 (5.9) | — | |
| FCM-MRD at TP3‡ | |||
| <0.01% | 33 (64.7) | 69.6 (8.0) | <.001 |
| ≥0.01% | 8 (15.9) | 0 | |
| Not evaluated | 10 (19.6) | — |
MR, medium risk; PGR, prednisolone good responder; TP2, time point 2 (at the end of induction); TP3, time point 3 (at the end of early consolidation).
CNS-3, WBC ≥ 5/μL in cerebrospinal fluid (CSF) with blasts; CNS-2, WBC < 5/μL in CSF with blasts; CNS-1, absence of blasts in CSF regardless of the number of WBCs.
Interfant-06 HR, KMT2A-r, and age <6 mo either with WBC ≥ 300 ×109/L or PPR; MR, other KMT2A-r.
Fifty-one patients were evaluated (4 patients withdrew from the study before CR evaluation and the MRD target was not set up for 1 patient).
Outcomes.
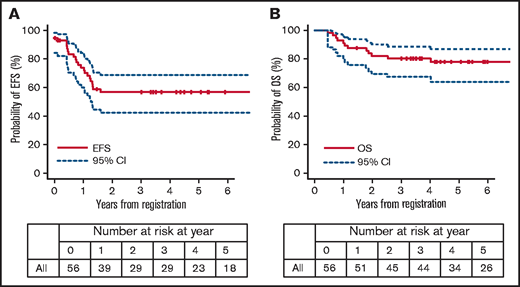
The 3-year EFS and OS rates of all 56 patients were 56.8% (95% CI, 42.4% to 68.8%) and 80.2% (95% CI, 67.1% to 88.5%), respectively (Figures 1 and 2). During and after the remission-induction courses (induction B and early consolidation B), 3 patients failed to achieve CR, 1 patient experienced a lineage switch to AML, and 3 patients withdrew from the study because of adverse events; thus, 49 patients (87.5%) were confirmed to have achieved CR1 at EOC (TP2). Thirty-eight patients (67.9%) received HSCT as specified in the protocol. There were 5 extra patients who received HSCT in CR1 outside of the study (protocol-unspecified HSCT): 1 withdrew from the study by clinical decision after developing cerebellitis during postinduction therapy (later received HSCT in CR1) and 4 withdrew by guardian’s choice (2 underwent HSCT with reduced-intensity conditioning and 2 received postinduction chemotherapy using Erwinia asparaginase because of asparaginase hypersensitivity; neither therapy was allowed within the study). Therefore, a total of 43 of all 56 HR patients (76.8%) were able to receive HSCT in CR1. Six patients did not undergo HSCT although they achieved CR1, mainly because of relapse before HSCT; there were 3 on-study relapses and 2 relapses after study withdrawal (1 due to an adverse event and 1 after referral to a nontrial participating center). There is 1 patient who discontinued the study because of norovirus enterocolitis, and is alive in CR1 with chemotherapy only.
The 3-year EFS rates according to the different variables are presented in Table 1. Among all, FCM-MRD ≥ 0.01% at both end of induction (EOI; TP2) and at EOC (TP3) were significantly associated with a poor prognosis. In the multivariable analysis, positive FCM-MRD at EOC (TP3) was solely associated with poor EFS (P < .001; Table 2). There were no differences in background characteristics between the patients with or without MRD availability at TP3 (supplemental Table 1). The number of patients with positive MRD at each TP is shown in supplemental Figure 1.
Multivariable analysis of prognostic factors for EFS in infants with 56 HR KMT2A-r ALL
| Variables* . | N . | Hazard ratio . | 95% CI, % . | P . |
|---|---|---|---|---|
| Interfant-06 risk group | ||||
| Interfant-MR | 23 | 1 | — | |
| Interfant-HR | 33 | 1.918 | 0.666-5.527 | .228 |
| FCM-MRD at TP2 | ||||
| Negative | 29 | 1 | — | |
| Positive | 19 | 0.903 | 0.321-2.540 | .847 |
| FCM-MRD at TP3 | ||||
| Negative | 33 | 1 | — | |
| Positive | 8 | 31.220 | 7.38-132.1 | <.001 |
| Variables* . | N . | Hazard ratio . | 95% CI, % . | P . |
|---|---|---|---|---|
| Interfant-06 risk group | ||||
| Interfant-MR | 23 | 1 | — | |
| Interfant-HR | 33 | 1.918 | 0.666-5.527 | .228 |
| FCM-MRD at TP2 | ||||
| Negative | 29 | 1 | — | |
| Positive | 19 | 0.903 | 0.321-2.540 | .847 |
| FCM-MRD at TP3 | ||||
| Negative | 33 | 1 | — | |
| Positive | 8 | 31.220 | 7.38-132.1 | <.001 |
TP2, time point 2 (at the end of induction); TP3, time point 3 (at the end of early consolidation).
Variables with P < .1 in univariable analysis were selected.
As of 30 March 2019, a total of 31 patients are in CR1, 10 patients are in CR2, 2 patients are in CR3, 1 patient is alive with disease (third relapse), and 12 patients have died (1 death from primary refractory leukemia, 6 relapse deaths, 2 early deaths, 1 death in CR1, 1 death in CR2, and 1 death in CR3) with a median follow-up of 5.7 years (range, 1.5-7.8 years) for living patients. Among the patients who are alive in CR2, 6 survived after the second HSCT. The clinical course of all 56 cases is illustrated in supplemental Figure 2 as swimmer plots.
Subanalyses on HSCT outcomes for the 43 patients who received transplants
HSCT outcomes.
Subanalyses on HSCT outcomes were performed for the 43 patients who actually received HSCT in CR1, including the 38 patients who underwent protocol-specified HSCT. Thirty-six patients received HSCT from UCBD, 6 from MFD (5 BMT and 1 PBSCT), and 1 from the haploidentical mother (PBSCT) (Table 3). Forty-one patients received conditioning consisting of BU, ETP, and CY, and 2 received reduced-intensity conditioning, mainly consisting of fludarabine and melphalan. The median number of days from trial registration to HSCT was 159 (range, 135-196 days). A graft failure occurred in 1 patient who received a UCBD transplant; this patient received rescue HSCT and successfully engrafted, but eventually died of HSCT-related pulmonary disease 1 month after the first HSCT. Acute GVHD of any grade developed in 22 of the 42 evaluated patients, and chronic GVHD developed in 5 of the 40 evaluated patients. Thirteen patients relapsed after HSCT, 1 patient died in CR, and 29 patients are in continuous CR. Neither donor source, acute GVHD grade, occurrence of chronic GVHD, nor HLA compatibility correlated with DFS (supplemental Table 2).
Results of the 43 HR patients who underwent HSCT in CR1
| . | Protocol-specified HSCT . | Protocol-unspecified HSCT, n = 5 . | P* . | |
|---|---|---|---|---|
| UCBD, n = 32 . | MFD, n = 6 . | |||
| FCM-MRD at TP3 | ||||
| Negative | 22 | 6 | 3 | |
| Positive | 1 | 0 | 0 | |
| Not evaluated | 9 | 0 | 2 | |
| Days from registration to HSCT | ||||
| Median (range) | 156 (135-194) | 185 (138-196) | 163 (150-190) | .27 |
| Donor | ||||
| UCBD | 32 | — | 4 | |
| MFD | — | 6 (5 BM and 1 PBSC) | 0 | |
| Haploidentical mother | — | — | 1 | |
| Conditioning regimen | ||||
| BU + ETP + CY | 32 | 6 | 3 | |
| FLU + MEL + other | — | — | 2 | |
| GVHD prophylaxis | ||||
| TAC + sMTX | 22 | 4 | 5 | |
| CsA + sMTX | 10 | 1 | 0 | |
| MTX only | 0 | 1 | 0 | |
| Infused cell dose, ×107/kg | ||||
| Median (range) | 12.3 (0.113-115) | 41.5 (19.3-69.0) | — | .0077 |
| Neutrophil engraftment† | ||||
| n | 31 | 6 | 5 | |
| Median (range), d | 17 (12-39) | 15.5 (14-17) | — | .0024 |
| Platelet engraftment‡ | ||||
| n | 31 | 6 | 5 | |
| Median (range), d | 43 (23-85) | 32.5 (28-51) | — | .12 |
| Acute GVHD | ||||
| Any | 17 | 3 | 2 | |
| Grade I | 6 | 2 | 1 | |
| Grade II | 6 | 1 | 1 | |
| Grade III | 2 | 0 | 0 | |
| Grade IV | 3 | 0 | 0 | |
| Not evaluated | 1 | 0 | 0 | |
| Chronic GVHD | ||||
| Any | 5 | 0 | 0 | |
| Limited | 5 | 0 | 0 | |
| Extensive | 0 | 0 | 0 | |
| Not evaluated | 3 | 0 | 0 | |
| Relapse | ||||
| Any | 10 | 2 | 1 | |
| BM relapse | 5 | 0 | 1 | |
| Combined BM/EM relapse | 4 | 1 | 0 | |
| Isolated EM relapse | 1 | 1 | 0 | |
| Nonrelapse death | 1 | 0 | 0 | |
| Continuous CR (%) | 22 (68.8) | 3 (50) | 4 (80) | |
| . | Protocol-specified HSCT . | Protocol-unspecified HSCT, n = 5 . | P* . | |
|---|---|---|---|---|
| UCBD, n = 32 . | MFD, n = 6 . | |||
| FCM-MRD at TP3 | ||||
| Negative | 22 | 6 | 3 | |
| Positive | 1 | 0 | 0 | |
| Not evaluated | 9 | 0 | 2 | |
| Days from registration to HSCT | ||||
| Median (range) | 156 (135-194) | 185 (138-196) | 163 (150-190) | .27 |
| Donor | ||||
| UCBD | 32 | — | 4 | |
| MFD | — | 6 (5 BM and 1 PBSC) | 0 | |
| Haploidentical mother | — | — | 1 | |
| Conditioning regimen | ||||
| BU + ETP + CY | 32 | 6 | 3 | |
| FLU + MEL + other | — | — | 2 | |
| GVHD prophylaxis | ||||
| TAC + sMTX | 22 | 4 | 5 | |
| CsA + sMTX | 10 | 1 | 0 | |
| MTX only | 0 | 1 | 0 | |
| Infused cell dose, ×107/kg | ||||
| Median (range) | 12.3 (0.113-115) | 41.5 (19.3-69.0) | — | .0077 |
| Neutrophil engraftment† | ||||
| n | 31 | 6 | 5 | |
| Median (range), d | 17 (12-39) | 15.5 (14-17) | — | .0024 |
| Platelet engraftment‡ | ||||
| n | 31 | 6 | 5 | |
| Median (range), d | 43 (23-85) | 32.5 (28-51) | — | .12 |
| Acute GVHD | ||||
| Any | 17 | 3 | 2 | |
| Grade I | 6 | 2 | 1 | |
| Grade II | 6 | 1 | 1 | |
| Grade III | 2 | 0 | 0 | |
| Grade IV | 3 | 0 | 0 | |
| Not evaluated | 1 | 0 | 0 | |
| Chronic GVHD | ||||
| Any | 5 | 0 | 0 | |
| Limited | 5 | 0 | 0 | |
| Extensive | 0 | 0 | 0 | |
| Not evaluated | 3 | 0 | 0 | |
| Relapse | ||||
| Any | 10 | 2 | 1 | |
| BM relapse | 5 | 0 | 1 | |
| Combined BM/EM relapse | 4 | 1 | 0 | |
| Isolated EM relapse | 1 | 1 | 0 | |
| Nonrelapse death | 1 | 0 | 0 | |
| Continuous CR (%) | 22 (68.8) | 3 (50) | 4 (80) | |
CsA, cyclosporine; EM, extramedullary; FLU, fludarabine; MEL, melphalan; MTX, methotrexate; sMTX, short-term methotrexate; TAC, tacrolimus; TP3, time point 3 (at the end of early consolidation).
Comparison between UCBD and MFD among the 38 patients who received protocol-specified HSCT.
Neutrophil engraftment, the first day of the 3 consecutive days achieving an absolute neutrophil count ≥0.5 × 109/L.
Platelet engraftment, the first day of achieving a platelet count ≥20 × 109/L without transfusions.
BU PK and HSCT-related toxicities.
PK data were available in 37 of the 38 patients who received protocol-specified HSCT. The distribution of test PK parameters demonstrated the typical PK patterns observed in infants (Figure 3).14 Although the volume of distribution (Vd) and clearance (CL) values were relatively uniform, the distribution of Css was scattered. The coefficient of variation (CV) of BU clearance in this study was 27%.
Characteristics of BU PK in 37 infants with available test PK. Volume of distribution (A) (Vd; [liters per kilogram]), clearance (B) (CL; [liters per hour per kilogram]), average steady-state concentration (C) (Css; [nanograms per mililiter]).
Characteristics of BU PK in 37 infants with available test PK. Volume of distribution (A) (Vd; [liters per kilogram]), clearance (B) (CL; [liters per hour per kilogram]), average steady-state concentration (C) (Css; [nanograms per mililiter]).
Initial-dose PK data were available in 32 patients; therefore, paired data of PK and HSCT outcome were analyzed in these patients. The average initial BU dose determined by the test PK in this study cohort was 0.70 mg/kg (range, 0.39-1.54 mg/kg), which was less than the officially labeled dosage (1.0 mg/kg for patients with body weight <9 kg and 1.2 mg/kg for those 9-16 kg in Japan and in Europe; 1.1 mg/kg for those 12 kg or less in the United States). Based on the PK data for this cohort, it is estimated that only 8 of the 37 cases (21.6%) would have achieved the target Css if administered the officially labeled doses. We confirmed that 23 of the 32 patients (71.9%) achieved 600 to 900 ng/mL Css from the initial-dose PK data as a result of dose adjustment after the test PK results. We retrospectively calculated and confirmed that 87.5% of the patients had achieved a proper range of tAUC by further readjustment based on the initial-dose PK.
Among the 32 patients, 7 (21.9%) developed sinusoidal obstruction syndrome (SOS) and 9 patients (28.1%) developed pulmonary complications (acute respiratory distress syndrome, n = 1; cryptogenic organizing pneumonia, n = 1; interstitial pneumonia, n = 3; pulmonary hemorrhage, n = 1; pulmonary hypertension, n = 3). Both types of complications are generally associated with higher Css and/or higher tAUC of BU administration; however, it is notable that 6 of the 7 cases with SOS and 8 of the 9 cases with pulmonary complications were within the proper Css range (Table 4 Css). Similarly, all 7 cases with SOS and all 9 cases with pulmonary complications were within the proper tAUC range (Table 4 AUC). Cytomegalovirus antigenemia was observed in 2 patients (1 with MFD and another with UCBD) and cytomegalovirus infection was observed in 1 patient (with UCBD). Other grade 3 or greater adverse events evaluated by Common Terminology Criteria for Adverse Events (version 3) related to the HSCT conditioning are shown in supplemental Table 2.
Correlation between BU PK (initial-dose PK) results and HCT-related complications: Css and tAUC
| . | Css . | AUC . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | Total, n = 38 . | n = 5 . | n = 11 . | n = 12 . | n = 4 . | Total, n = 38 . | n = 1 . | n = 10 . | n = 19 . | n = 2 . |
| Css, ng/mL; AUC, mg/L × h . | . | <600 . | 600-749.9 . | 750-900 . | >900 . | . | <57.5 . | 57.5-71.9 . | 72-86.3 . | >86.3 . |
| SOS, n (%) | 8 (21) | 0 (0) | 4 (36) | 2 (17) | 1 (25) | 8 (21) | 0 (0) | 3 (30) | 4 (21) | 0 (0) |
| Pulmonary complications, n (%) | 9 (24) | 0 (0) | 3 (27) | 5 (42) | 1 (25) | 9 (24) | 0 (0) | 3 (30) | 6 (32) | 0 (0) |
| PA hypertension | 3 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 2 | 0 |
| Interstitial pneumonia | 3 | 0 | 1 | 2 | 0 | 3 | 0 | 1 | 2 | 0 |
| COP | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| ARDS | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Pulmonary hemorrhage | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| . | Css . | AUC . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | Total, n = 38 . | n = 5 . | n = 11 . | n = 12 . | n = 4 . | Total, n = 38 . | n = 1 . | n = 10 . | n = 19 . | n = 2 . |
| Css, ng/mL; AUC, mg/L × h . | . | <600 . | 600-749.9 . | 750-900 . | >900 . | . | <57.5 . | 57.5-71.9 . | 72-86.3 . | >86.3 . |
| SOS, n (%) | 8 (21) | 0 (0) | 4 (36) | 2 (17) | 1 (25) | 8 (21) | 0 (0) | 3 (30) | 4 (21) | 0 (0) |
| Pulmonary complications, n (%) | 9 (24) | 0 (0) | 3 (27) | 5 (42) | 1 (25) | 9 (24) | 0 (0) | 3 (30) | 6 (32) | 0 (0) |
| PA hypertension | 3 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 2 | 0 |
| Interstitial pneumonia | 3 | 0 | 1 | 2 | 0 | 3 | 0 | 1 | 2 | 0 |
| COP | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| ARDS | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Pulmonary hemorrhage | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
ARDS, acute respiratory distress syndrome; COP, cryptogenic organizing pneumonia; PA, pulmonary artery.
Discussion
The role of HSCT for infants with ALL is an open question and its indication varies among the major study groups. None received HSCT in the recent COG studies following their legacy data that there was no difference in 5-year EFS between patients who did and did not receive transplants in the CCG1953 and POG9407 studies.9,13,15 In contrast, a subset of the patients categorized as HR (KMT2A-r and age <6 months either with WBC ≥ 300 × 109/L or PPR) or MR (KMT2A-r other than HR) with MRD ≥ 10−4 at the start of reinduction received HSCT in CR1 in the Interfant-06 study following the data from the previous Interfant-99 study.3,10 In Japan, all infants with KMT2A-r ALL had been allocated to HSCT in the past 3 consecutive studies (MLL96, MLL98, and MLL03).4–6 However, the modest improvement in outcomes observed and consideration of potential risk of developing late effects prompted us to restrict the indication only to the patients with HR features (defined as KMT2A-r and either age <180 days or CNS-3) in the current MLL-10 study.6,12 Fortunately, the study resulted in a 66.2% EFS rate in 3 years for infants with KMT2A-r ALL, which is the highest among all reports to date.11 The 3-year EFS rate of the 56 HR patients was 56.8%, and 43 patients (76.8%) were able to undergo HSCT in CR1.
As already mentioned, indication of HSCT in CR1 is still broader in MLL-10 compared with the other 2 major study groups. In Interfant-06, 164 patients in the Interfant-HR group (25.2% of all registered patients) were supposed to undergo HSCT, whereas 56 patients in the MLL-10-HR group (62.2% of all registered patients) were supposed to undergo HSCT.3 When adjusting to the Interfant-06 risk group definition, the 6-year EFS rate of the Interfant-HR patients (n = 164) in Interfant-06 was 20.9%, whereas the 3-year EFS rate of the same counterpart in MLL-10 (n = 33) was 45.2%. However, only 76 of 164 patients (46.3%) could actually receive HSCT in CR1 in the Interfant-06 study, mainly because of an early event in many patients; 4-year DFS of those who received transplants in CR1 was 44.0%, which does not seem different from the outcome of the MLL-10 counterpart. Considering that 76.8% of the MLL-10 HR patients (23 of 33 [69.7%] of the Interfant-HR patients) in MLL-10 actually received HSCT, it is likely that the lower rate of pre-HSCT events is 1 of the factors responsible for the favorable outcomes observed in the MLL-10 study.
The low rate of early events before HSCT observed in MLL-10 could be attributed to the high percentage of the patients with EOC MRD clearance, which was a significant prognostic factor for favorable EFS (Table 1). Twenty-nine of the 48 evaluated HR patients (60.4%) and 33 of the 41 evaluated HR patients (80.5%) had negative MRD (FCM-MRD < 0.01%) at EOI and at EOC, respectively (Table 1, supplemental Figure 1). In contrast, only 19.4% of the evaluated patients (includes non-HR KMT2A-r patients) were MRD negative at EOI in Interfant-06, although the MRD methodology was different.16 We speculate that the lower leukemia burden before HSCT contributed to the low number of early events and to the favorable outcome in the MLL-10 cohort; whether the pre-HSCT chemotherapy itself (with more stringent age-based dose reduction criteria), or the registered patients possibly having been more sensitive to chemotherapy, is the reason is debatable.6,14
Subanalyses on patients who actually received transplants are potentially biased because they do not include the patients who could not reach HSCT, and some of the data were only available for highly selected cases with protocol-specified HSCT. However, it is likely that the low number of HSCT-related deaths in MLL-10 (1 death in CR among the 43 patients who received HSCT in CR1) contributed to the favorable outcome as well. This is consistent with the results observed in the previous MLL03 trial in Japan (2 deaths in CR among the 44 patients who received HSCT in CR1); therefore, the conditioning regimen consisting of targeted BU, ETP, and CY was feasible and well tolerated.6 A total of 14.4% of patients who received HSCT died of HSCT-related toxicity in Interfant-06.3 In fact, death in CR was 26% in the Interfant-06 when the conditioning regimen of BU, CY, and melphalan was used, which improved to 5% after amending to the regimen using BU plus treosulfan, fludarabine, and thiotepa. These data suggest that the selection of conditioning regimen is crucial in managing HSCT-related toxicities in infants with ALL. TBI is usually avoided as a conditioning regimen for infants considering the risk of severe late toxicities; therefore, the BU-containing regimen is often selected.12,17 However, our results showed that the officially labeled BU dosing was inappropriate for the majority (nearly 80%) of the infants. Because excess dosing would lead to severe toxicities, such as SOS and pulmonary complications, and underdosing would lead to the risk of graft failure or low antileukemia effect, determining the BU dose according to the individual PK results is extremely important. As a result of appropriate BU dosing in the majority of the HSCT recipients in MLL-10, there was only 1 death in CR. However, one cannot ignore the fact that 6 of 7 cases with SOS and 8 of 9 cases with pulmonary complications were within the targeted Css range, although there was no fatal case. In that sense, introducing a safer regimen, such as treosulfan instead of BU, would be important.18 Besides the selection of conditioning, availability of an appropriate donor might have contributed to the low number of nonrelapse deaths in MLL-10. Notably, 36 of 43 of the patients who received HSCT (84%) received transplants from UCBDs. The patients registered in MLL-10 were all Japanese, and Japan is a nation with a relatively homogeneous population and less HLA disparity than typically found in other countries.19 Although a statistically significant difference could not be found due to the small number analyzed, a trend of lower DFS associated with higher HLA allele incompatibilities was observed (supplemental Table 2); therefore, selection of a well-matched donor also seems to be crucial for successful HSCT.
Novel therapeutic approaches are emerging in the field of leukemia. Among them, agents such as epigenetic modifiers, DOT1L inhibitors, bromodomain inhibitors, BCL-2 inhibitors, and menin inhibitors have shown certain effects in preclinical or early clinical studies.20–24 In addition, immunotherapies such as anti-CD19-bispecific antibody blinatumomab and autologous and allogeneic chimeric antigen receptor T-cell therapy have shown tremendous effect against relapsed or refractory B-cell ALL.25–27 Considering the limitation of the therapeutic effect of HSCT and its potential for developing severe late effects especially in infants, the aforementioned therapeutic modalities should substitute for HSCT in the future.
In conclusion, the risk-stratified HSCT strategy of MLL-10 was successful. However, improvement in HSCT methodologies to reduce both acute and late toxicities is required. Moreover, considering the high rate of potentially life-threatening toxicities and the risk of late effects, further restriction or even elimination of HSCT for infants with ALL is preferable by introducing emerging novel therapeutics.
Acknowledgments
The authors thank all of the JPLSG and Japan Children’s Cancer Group (JCCG) investigators involved in the MLL-10 trial, and Yoshie Okano, Noriko Sato, and Kaori Nagai from the JPLSG Data Center for managing the trial data. The authors also thank the medical editor from the Division of Education for Clinical Research of the National Center for Child Health and Development for assistance with editing this manuscript.
This work was supported by a grant for clinical cancer research from the Ministry of Health, Labour, and Welfare of Japan (H23-GanRinsho-Ippan-014 [K.H.]), grants for Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED; 15ck0106071h0002 [A.M.] and 18ck0106436h0001 [T.M.]), a grant from the National Center for Child Health and Development (30-1 [D.T.]), and a research grant from the Children’s Cancer Association of Japan.
Authorship
Contribution: T.T., T.M., Y.A., S.I., S.S., J.O., K.K., and D.T. (principal investigator) conceived and designed the study; T.T., T.W., and D.T. reviewed the data analysis and interpretation and served as the main authors of the manuscript; A.M.S. and T.W. conducted the statistical analysis; T.D. performed immunodiagnostics and FCM-MRD analyses; T.H. and T.Y. performed immunoglobulin/TCR PCR-MRD analysis; S.O. and M.H. performed the BU PK study; A.I., Y.T., and N.H. recruited patients; D.T., T.M., A.M., K.H., and E.I. contributed to the financial and administrative support of the study; and all authors contributed to the conduct of the trial, were involved in the review of the results, and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests .
Correspondence: Daisuke Tomizawa, Division of Leukemia and Lymphoma, Children’s Cancer Center, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan; e-mail: tomizawa-d@ncchd.go.jp.
References
Author notes
The Japan Children’s Cancer Group (JCCG) is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by the JCCG steering committee based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
The full-text version of this article contains a data supplement.


![Characteristics of BU PK in 37 infants with available test PK. Volume of distribution (A) (Vd; [liters per kilogram]), clearance (B) (CL; [liters per hour per kilogram]), average steady-state concentration (C) (Css; [nanograms per mililiter]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/19/10.1182_bloodadvances.2020004157/7/m_advancesadv2020004157f3.png?Expires=1770312726&Signature=LfLIyBxsFUAVdgBeTEoWEzwX9pE2HxYq3pO-RQD59qxSSIOWvcSWa9kZ~ooULD9nOAXfVEOwSLMwyJfcdSxcO5kjNzEuRx8omnR77M16scuQjnVYVbj29lKQ7I~wdv2uvMPWC4Q9DWcvKcpUD7jEccj~sWAyqePT-INLR-zyMyHs3HvS60SaBXPOkE9y6WLa5QSeA9JnvQ1wVJfZYgBHULYvAvkq66tQqmX2DEA7ABMnzlkWs98jIE7S~pKkVDpTy-5~5qpND3pAI~JKcuVYShR996~Eo8DxU~2Bhqs9Z6QoGgbTcOB8fbJKub0W6QMPaNcoedwfh7SjTReal1g8vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)