TO THE EDITOR:
Primary mediastinal B-cell lymphoma (PMBL) is recognized as a unique clinicopathologic entity. It has a predilection for female adolescents and young adults, and is characterized by distinct morphologic, immunophenotypic, and molecular features. PMBL is rare, and this has resulted in a paucity of prospective data and lack of randomized studies, leading to controversy regarding optimal frontline therapy. In this issue of Blood Advances, Camus and colleagues describe real-world patient outcomes following frontline immunochemotherapy across 25 centers in France and Belgium.
Three hundred and thirteen treatment-naïve patients with PMBL were retrospectively evaluated in the study. A total of 180 received rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP), 76 received rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone every 14 days (R-CHOP-14), and 57 received R-CHOP every 21 days (R-CHOP-21), respectively. Outcomes were excellent with high end-of-treatment (EOT) complete metabolic response rates between 76% and 86%, and mediastinal radiation was administered in just 5.4%. Progression-free survival at 3 years favored R-ACVBP and R-CHOP-14 (89.4%, 89.4%), with statistically lower rates for R-CHOP-21 (74.7%). Overall survival at 3 years also supported the dose-intensive options (R-ACVBP, 92.4%; R-CHOP-14, 100%; R-CHOP-21, 87.5%). The results presented here are of interest and importance, especially considering the high number of patients included, and the authors raise many provocative questions regarding optimal management of the disease. Despite this, the study is limited to answer the key question of whether or not increased dose-intensity approaches are superior to R-CHOP-21. Following R-ACVBP and R-CHOP-14 therapy, a low proportion of patients needed radiation. However, a significant number of these patients underwent consolidation transplantation (25.6% and 31.6%, respectively), which may have affected outcomes. Additionally, transplantation is associated with high added toxicity and is not a standard approach in upfront management of PMBL. Further data that confounds interpretation of results were the higher proportion of older patients (>60 years), who are frequently not considered candidates for dose-intensive approaches, in the R-CHOP-21 cohort.
Early retrospective studies in PMBL demonstrated that more aggressive chemotherapy regimens were associated with higher response rates in comparison with CHOP.1 This has not proven to be the case in diffuse large B-cell lymphoma, where R-CHOP remains the optimal approach following comparisons with higher intensity, more toxic therapies. Higher sensitivity of PMBL to dose intensity may potentially be explained by its much younger age distribution as well as its close biological resemblance to classic Hodgkin lymphoma, a disease that benefits from increased therapeutic intensity.2 Although R-CHOP has been commonly used in studies of PMBL, its use has relied quite significantly on consolidation mediastinal radiation, which has significant long-term toxicities, such as secondary malignancies including breast cancer.3 Though there has been some recent progress with positron emission tomography (PET)-adapted approaches, it remains unclear if radiation can be removed from treatment algorithms based on EOT-PET findings, so the ongoing International Extranodal Lymphoma Study Group-37 study (NCT01599559) was designed to answer this important question.4,5 Regarding other experiences with more dose-intense regimens, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab was investigated prospectively as an approach to obviate the need for mediastinal radiation; in an initial prospective study of 51 patients and later follow-up report of 93 patients, >90% of patients were event-free with long follow-up without the need for radiation.6,7 Similar results were observed in a retrospective series of 156 pediatric and adult patients with PMBL treated with the regimen.8 In the absence of definitive randomized comparison studies, strategies that achieve high cure rates without reliance on mediastinal radiation such as dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab or R-ACVBP should be favored, we believe, for PMBL.
Another interesting finding in the Camus study is the analysis of EOT-PET correlation with outcome. One of the challenges in treating patients with PMBL is that large mediastinal masses are typical at diagnosis and usually persist to some degree even at the end of successful therapy. This can significantly confound EOT response assessment and was particularly problematic before the advent of PET.9 Despite improvement compared with traditional cross-sectional imaging, PET continues to have limitations, and EOT-PET positivity is not always associated with inferior outcomes. In this study, the authors report cases that were Deauville score 4 (considered positive) at EOT with superb outcomes, notably a 3-year overall survival rate of 90%. Similar results for interim or EOT-PET has been demonstrated by others,10,11 raising the question of whether the predictive value of Deauville score 4 is dependent on regimen used. Further prospective studies regarding this issue are ongoing, and alternative mechanisms to enhance or even replace PET imaging, such as circulating tumor DNA are currently being evaluated (NCT04824950).
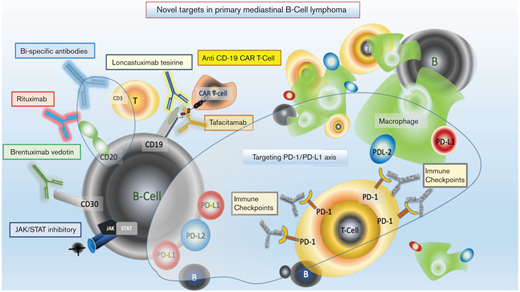
What about approaches beyond conventional immunochemotherapy for PMBL? Recent biological insights into the disease, as well as the development of novel agents for common B-cell markers, provide a number of potential therapeutic options (see Figure 1).12-14 Translocation and/or amplifications of chromosome 9p24.1 leads to the common overexpression of PD-L1 and PD-L2 in PMBL, a shared finding with classic Hodgkin lymphoma. The biologic similarity suggests both tumors have mechanisms to overcome immune surveillance, a theory strengthened by clinical responsiveness to immune checkpoint inhibitors. Pembrolizumab is now US Food and Drug Administration approved in relapsed/refractory PMBL, based on data published by Armand and colleagues.15 The phase 1B/2 use of pembrolizumab in PMBL reported an overall response rate of 48% in the phase 1B (33% complete response rate) and 45% (13% complete response rate) in the phase 2 trial. Neither study had achieved a median duration of response at time of publication. Nivolumab, another immune checkpoint inhibitor, is now being studied in combination with immunochemotherapy for frontline use in PMBL pediatric and adult patients (NCT04759586). Several functional genomic studies in PMBL have identified that JAK-STAT signaling is an important mechanism of oncogenic activation; inhibitors of this pathway are in development.12,16 Anti-CD19 chimeric antigen receptor T-cell therapy has recently been approved for relapsed and refractory aggressive B-cell lymphoma and is highly effective in PMBL.17-19 Additional novel agents targeting CD19 and CD20/CD3 (bispecific T-cell engager therapy) require further study, but may be attractive future options for this disease.20-22 In conclusion, until there is a randomized study in PMBL to inform on optimal therapy, we support dose-intensive approaches with low reliance on radiation. Considering the armamentarium of novel approaches in development for PMBL, we expect that incorporation of the most effective of these in the up-front setting will lead to new highly curative strategies with reduced treatment-related toxicity.
Contribution: M.R.C. and K.D. wrote the paper.
Conflict-of-interest disclosure: M.R.C. has no competing financial interests. K.D. has participated as an advisor for Abbvie, Genmab, Morphosys, Incyte, Genentech, ADC Therapeutics, Astra Zeneca, Kyte, Bayer, Beigene, and Kymera.
Correspondence: Kieron Dunleavy, Division of Hematology and Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Hospital, Washington, DC 20057; e-mail: Kmd322@georgetown.edu.