Key Points
In older cHL patients, survival was longer with conventional chemotherapy regimens.
Geriatric fitness markers (eg, ADLs, medical comorbidities) correlated strongly with patient outcomes, despite performance status of 0 to 2.
Abstract
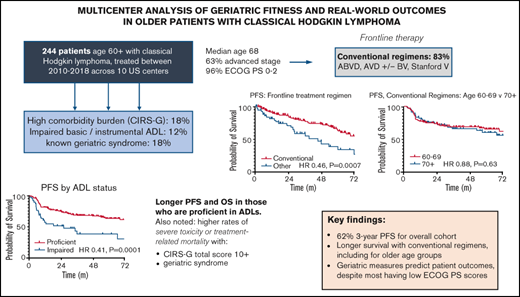
We performed a multicenter retrospective analysis across 10 US academic medical centers to evaluate treatment patterns and outcomes in patients age ≥60 years with classic Hodgkin lymphoma (cHL) from 2010-2018. Among 244 eligible patients, median age was 68, 63% had advanced stage (III/IV), 96% had Eastern Cooperative Oncology Group performance status (PS) 0-2, and 12% had documented loss of ≥1 activity of daily living (ADL). Medical comorbidities were assessed by the Cumulative Illness Rating Scale–Geriatric (CIRS-G), where n = 44 (18%) had total scores ≥10. Using multivariable Cox models, only ADL loss predicted shorter progression-free (PFS; hazard ratio [HR] 2.13, P = .007) and overall survival (OS; HR 2.52, P = .02). Most patients (n = 203, 83%) received conventional chemotherapy regimens, including doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; 56%), AVD (14%), and AVD with brentuximab vedotin (BV; 9%). Compared to alternative therapies, conventional regimens significantly improved PFS (HR 0.46, P = .0007) and OS (HR 0.31, P = .0003). Survival was similar following conventional chemotherapy in those ages 60-69 vs ≥70: PFS HR 0.88, P = .63; OS HR 0.73, P = .55. Early treatment discontinuation due to toxicity was more common with CIRS-G ≥10 (28% vs 12%, P = .016) or documented geriatric syndrome (28% vs 13%, P = .02). A competing risk analysis demonstrated improved disease-related survival with conventional therapy (HR 0.29, P = .02) and higher mortality from causes other than disease or treatment with high CIRS-G or geriatric syndromes. This study suggests conventional chemotherapy regimens remain a standard of care in fit older patients with cHL, and highlights the importance of geriatric assessments in defining fitness for cHL therapy going forward.
Introduction
Patients age >60 years represent 20% to 30% of new classical Hodgkin lymphoma (cHL) diagnoses annually but remain vastly underrepresented in clinical trials, comprising only 5% to 13% of participants in modern phase 3 studies.1-5 These individuals more commonly present with advanced-stage disease, mixed cellularity subtype, and varying burdens of age-related medical comorbidities, all of which may affect fitness for and outcomes with conventional cHL regimens such as doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and Stanford V.2,6,7 Older patients have historically experienced inferior efficacy and greater toxicity with these regimens compared with younger individuals with cHL.8 However, it remains unclear how these experiences compare in the context of novel combinations without a conventional chemotherapy backbone,9-11 particularly in those who otherwise seem fit enough to receive intensive therapy.
Geriatric fitness measures have been increasingly studied across oncologic settings and may allow for more objective insight into a patient’s candidacy for a proposed treatment intensity.12 Although geriatric assessment (GA) has been studied extensively in the management of older patients with solid tumors, data are more limited in hematologic malignancies, and it is unknown how widely GA is used in the United States to inform decisions on lymphoma-directed therapy in routine practice.13 Medical comorbidity burden, a key component of geriatric fitness testing, has been shown in multiple cHL studies to affect the tolerability and efficacy of a given regimen.14,15 Functional impairment of basic and/or instrumental activities of daily living (ADLs) has likewise been shown to have a significant impact on treatment tolerability and disease-related outcomes, in geriatric oncology more broadly and cHL in particular.15-17
Given the wide range of current treatment options available to older cHL patients as well as the impact that a more nuanced assessment of geriatric fitness may have on treatment consideration and outcomes, we performed a large retrospective analysis to assess real-world outcomes in this population.
Methods
Eligibility
After institutional review board approval was obtained at each site, we collected detailed clinical and pathologic data from patients with cHL across 10 US academic medical centers. Eligible patients were age ≥60 years at the time of cHL diagnosis and started on lymphoma-directed therapy between January 2010 and December 2018. A total of 264 patients were identified, of whom 244 met full eligibility for inclusion. Those with a preceding hematologic malignancy (n = 4) or inadequate clinicopathologic or outcome data (n = 16) were excluded; no cases of nodular lymphocyte-predominant HL were submitted. The study was conducted in accordance with the Declaration of Helsinki.
Variables and end points
Medical comorbidities were quantified by the Cumulative Illness Rating Scale–Geriatric (CIRS-G), a validated assessment tool specifically designed to capture comorbidities in older individuals18 ; cHL was not included in CIRS-G scoring. Data regarding ADLs and presence of a geriatric syndrome (dementia, delirium, depression, osteoporosis, incontinence, falls, failure to thrive, and/or neglect/abuse) were abstracted from patient medical records. Treatment response and progression were determined by local investigators and clinical documentation. Survival estimates were calculated for progression-free (PFS; time from cHL diagnosis to disease progression, death, or last follow-up) and overall survival (OS; time from diagnosis to death or last follow-up).
In assessing frontline therapy, we compared recipients of conventional cHL regimens (defined here as ABVD; doxorubicin, vinblastine, and dacarbazine [AVD] with or without brentuximab vedotin [BV]; or Stanford V) with those who received alternative regimens (ie, all other therapies). Although anthracyclines are considered necessary for curative-intent therapy in cHL,19 we did not consider cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) a conventional cHL regimen for these analyses, given the substantial differences in dose density, which has been shown in cHL to affect response rates and survival, between CHOP and A(B)VD.20 On the basis of this decision, a separate 3-arm comparison was also performed to assess PFS and OS after CHOP relative to conventional and non-CHOP alternative therapies. Growth factor use and chemotherapy dose adjustments were not collected.
Statistical methods
Data were deidentified before submission for analysis, including the censoring of age as ≥90 years for relevant cases. Categorical data were analyzed using the χ2 test, the Fisher exact test, and logistic regression modeling. Continuous variable data were compared using the 2-sample Student t test. Kaplan-Meier estimates and the log-rank test were used for time-to-event analyses. Findings significant on univariate analysis were subsequently assessed in multivariable logistic regression models. Associations of baseline patient characteristics with survival were further evaluated using a multivariable Cox proportional hazards model, using backward elimination to help mitigate potential collinearity between factors and using a variable retention threshold of 0.05. Analyses were performed in SAS (version 9.4; SAS institute, Cary NC), and figures were created using GraphPad Prism (version 8; GraphPad Software, Inc., San Diego, CA).
Multivariate competing-risk regression based on the Fine-Gray method21 was conducted using the cmprsk package in R (R Core Team, Vienna, Austria) to compare the cumulative incidence of disease-related deaths and treatment-related deaths between groups, accounting for death resulting from other causes as a competing event. Associations for each analysis were considered significant at a 2-sided P value of <.05.
Trial eligibility, treatment standards, and disease-related outcomes generally differ in patients with stage I cHL and those with stage II to IV disease.22 As such, outcomes for these individuals (n = 22) were analyzed separately; unless otherwise stated, reported analyses include those with stage II to IV disease (n = 222).
Results
Patient characteristics
Among the 244 eligible patients, median age at diagnosis was 68 years (range, 60-90+), with 32% (n = 78) between ages 70 and 79 years and 10% (n = 24) age ≥80 years; 57% were male. At diagnosis, 63% had advanced-stage disease, 46% had B symptoms, and 96% had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2. Sixty-nine percent had nodular sclerosis, 19% had mixed cellularity, and 12% had other subtypes; Epstein-Barr virus was detectable by immunohistochemistry and/or peripheral blood in 39% of evaluable cases (n = 98). These characteristics were similar when restricted to patients with stage II to IV disease.
Geriatric fitness
Before initiation of therapy, 31 patients (12%) had documented loss of at least 1 basic or instrumental ADL, 40 (16%) had a documented geriatric syndrome, and 16 (6.3%) had both ADL impairment and a geriatric syndrome. In terms of medical comorbidities, median CIRS-G score was 5 (range, 0-22). One hundred eleven (45%) had at least 1 comorbidity classified as severe (ie, individual CIRS-G category score of 3-4), and 44 (18%) had a total score of ≥10, indicating increased overall burden. Geriatric fitness markers varied by age; patients age >70 years were more likely to have impaired ADLs (odds ratio [OR], 1.78; P = .09), CIRS total score of ≥10 (OR, 2.27; P = .001), and/or a geriatric syndrome (OR, 2.82; P = .0002). Further analysis of ECOG PS was limited by a small number of patients with PS of 3 to 4 (n = 7), and notably, only 1 patient underwent formal GA before treatment.
Frontline therapy
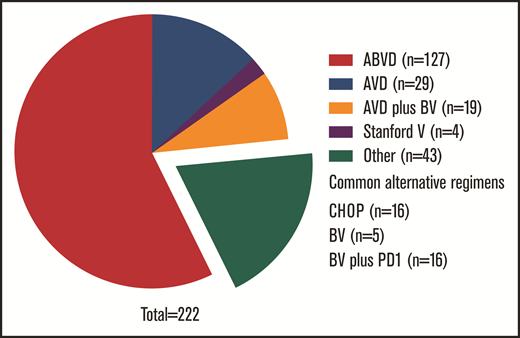
Key clinical features are listed in Table 1, overall and by frontline therapy. Common regimens (Figure 1) included ABVD (n = 136; 56%), AVD (n = 31; 13%), and CHOP (n = 17; 7%). Six patients (2.4%) received BV monotherapy; 19 (8%) received BV with AVD, either concurrently (n = 10) or sequentially (n = 9). Of 9 patients who did not receive systemic therapy, all received radiotherapy alone for stage I (n = 7) or II disease (n = 2). Fifty-eight received radiotherapy with systemic treatment; they predominantly had early-stage cHL (n = 41; 71%). In total, 12.6% (n = 31) received BV as part of frontline therapy, including 5 who also received upfront programmed death-1 inhibition. No patients received bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP; conventional or escalated) at any point during therapy.
Patient demographics and clinical characteristics at diagnosis by frontline therapy
| . | Total (N = 244) . | Conventional* (n = 190) . | Other (n = 54) . | . | |||
|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | P† . |
| Male sex | 113 | 46.3 | 85 | 44.7 | 28 | 51.9 | .36 |
| Age, y | .004 | ||||||
| Median | 68 | 67 | 73 | ||||
| Range | 60 to ≥90 | 60-88 | 60 to ≥90 | ||||
| Age category, y | |||||||
| 60-69 | 140 | 57.4 | 123 | 64.7 | 18 | 33.3 | Ref |
| 70-79 | 79 | 32.4 | 55 | 28.9 | 24 | 44.4 | .7 |
| ≥80 | 24 | 9.8 | 12 | 6.3 | 12 | 22.2 | .002 |
| Stage | |||||||
| Limited (I-II) | 89 | 36.5 | 70 | 36.8 | 19 | 35.2 | Ref |
| Advanced (III-IV) | 155 | 63.5 | 120 | 63.2 | 35 | 64.8 | .77 |
| B symptoms present | 112 | 45.9 | 95 | 50.0 | 17 | 31.5 | .01 |
| ECOG PS | |||||||
| 0-2 | 214 | 87.7 | 166 | 87.4 | 48 | 88.9 | Ref |
| 3-4 | 8 | 3.3 | 6 | 3.2 | 2 | 3.7 | .03 |
| Unknown/missing data | 22 | 9.0 | 18 | 9.4 | 4 | 7.4 | .65 |
| Impaired ADLs/instrumental ADLs at baseline | |||||||
| No | 206 | 84.4 | 163 | 85.9 | 43 | 79.6 | Ref |
| Yes | 30 | 12.3 | 19 | 10.0 | 11 | 20.4 | .054 |
| Unknown/missing data | 8 | 3.3 | 8 | 4.2 | 0 | 0 | — |
| Geriatric syndrome at baseline | |||||||
| No | 195 | 79.9 | 156 | 82.1 | 39 | 72.2 | Ref |
| Yes | 43 | 17.6 | 28 | 14.7 | 15 | 27.8 | .03 |
| Unknown/missing data | 6 | 2.5 | 6 | 3.2 | 0 | 0 | — |
| CIRS-G total score | |||||||
| <10 | 196 | 80.3 | 153 | 80.5 | 43 | 79.6 | Ref |
| ≥10 | 48 | 19.7 | 37 | 19.5 | 11 | 20.4 | .02 |
| Unknown/missing data | 0 | 0 | 0 | 0 | 0 | 0 | — |
| . | Total (N = 244) . | Conventional* (n = 190) . | Other (n = 54) . | . | |||
|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | P† . |
| Male sex | 113 | 46.3 | 85 | 44.7 | 28 | 51.9 | .36 |
| Age, y | .004 | ||||||
| Median | 68 | 67 | 73 | ||||
| Range | 60 to ≥90 | 60-88 | 60 to ≥90 | ||||
| Age category, y | |||||||
| 60-69 | 140 | 57.4 | 123 | 64.7 | 18 | 33.3 | Ref |
| 70-79 | 79 | 32.4 | 55 | 28.9 | 24 | 44.4 | .7 |
| ≥80 | 24 | 9.8 | 12 | 6.3 | 12 | 22.2 | .002 |
| Stage | |||||||
| Limited (I-II) | 89 | 36.5 | 70 | 36.8 | 19 | 35.2 | Ref |
| Advanced (III-IV) | 155 | 63.5 | 120 | 63.2 | 35 | 64.8 | .77 |
| B symptoms present | 112 | 45.9 | 95 | 50.0 | 17 | 31.5 | .01 |
| ECOG PS | |||||||
| 0-2 | 214 | 87.7 | 166 | 87.4 | 48 | 88.9 | Ref |
| 3-4 | 8 | 3.3 | 6 | 3.2 | 2 | 3.7 | .03 |
| Unknown/missing data | 22 | 9.0 | 18 | 9.4 | 4 | 7.4 | .65 |
| Impaired ADLs/instrumental ADLs at baseline | |||||||
| No | 206 | 84.4 | 163 | 85.9 | 43 | 79.6 | Ref |
| Yes | 30 | 12.3 | 19 | 10.0 | 11 | 20.4 | .054 |
| Unknown/missing data | 8 | 3.3 | 8 | 4.2 | 0 | 0 | — |
| Geriatric syndrome at baseline | |||||||
| No | 195 | 79.9 | 156 | 82.1 | 39 | 72.2 | Ref |
| Yes | 43 | 17.6 | 28 | 14.7 | 15 | 27.8 | .03 |
| Unknown/missing data | 6 | 2.5 | 6 | 3.2 | 0 | 0 | — |
| CIRS-G total score | |||||||
| <10 | 196 | 80.3 | 153 | 80.5 | 43 | 79.6 | Ref |
| ≥10 | 48 | 19.7 | 37 | 19.5 | 11 | 20.4 | .02 |
| Unknown/missing data | 0 | 0 | 0 | 0 | 0 | 0 | — |
Conventional cHL regimens included AVD, ABVD, BV plus AVD (concurrent or sequential), and Stanford V.
All P values are 2 sided.
Frontline treatment regimens for stage II to IV disease. Common alternative regimens enumerated in figure; regimens used for less than 5 patients are not listed.
Frontline treatment regimens for stage II to IV disease. Common alternative regimens enumerated in figure; regimens used for less than 5 patients are not listed.
Outcomes and prognostication
With a median follow-up time of 3.1 years, median PFS and OS for the total cohort have not yet been reached. Overall, 3-year PFS was 61.8%, and 3-year OS was 83.7%.
Geriatric fitness
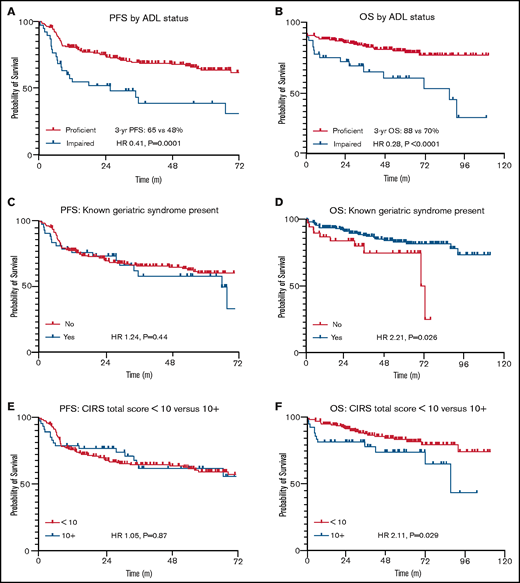
Figure 2 highlights survival by different measures of geriatric fitness. Patients with at least 1 documented impaired ADL had significantly inferior PFS (hazard ratio [HR], 2.46; P = .0001) and OS (HR, 3.58; P < .0001) compared with those who were functionally proficient. Other geriatric measures did not seem predictive of PFS but showed modest OS impact. These included presence of a geriatric syndrome (PFS: HR, 1.24; P = .44; OS: HR, 2.21; P = .03) and a total CIRS-G score of ≥10 (PFS: HR, 1.05; P = .87; OS: HR, 2.11; P = .03). Patient characteristics listed in Table 2 were then included in backward-elimination multivariable Cox proportional hazards models for PFS and OS, respectively, where only ADL impairment continued to correlate significantly with either end point (PFS: multivariable HR, 2.13; P = .007; OS: multivariable HR, 2.52; P = .02). Alternative CIRS-G total score thresholds were not predictive of PFS, nor were previously reported scoring systems in elderly patients with lymphoma focusing on individual CIRS-G categories6,23 (data supplement).
PFS and OS by geriatric fitness measures in stage II to IV disease. Time is listed in months for all figures. (A) PFS by ADL status. (B) OS by ADL status. (C) PFS by documented geriatric syndrome. (D) OS by documented geriatric syndrome. (E) PFS by CIRS-G total score. (F) OS by CIRS-G total score.
PFS and OS by geriatric fitness measures in stage II to IV disease. Time is listed in months for all figures. (A) PFS by ADL status. (B) OS by ADL status. (C) PFS by documented geriatric syndrome. (D) OS by documented geriatric syndrome. (E) PFS by CIRS-G total score. (F) OS by CIRS-G total score.
Analysis of survival by baseline patient characteristics
| . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| Patient characteristic . | HR . | 95% CI . | P* . | HR . | 95% CI . | P* . |
| Male sex | 1.19 | 0.78-1.80 | .42 | 1.44 | 0.76-2.71 | .26 |
| Age 60-69 vs ≥70 y | 0.81 | 0.53-1.23 | .31 | 0.59 | 0.29-1.06 | .07 |
| Advanced stage | 1.75 | 1.14-2.70 | .02 | 1.75 | 0.92-3.33 | .12 |
| Impaired ADLs/instrumental ADLs | 2.11 | 1.03-4.30 | .006 | 3.58 | 1.42-8.92 | <.0001 |
| Geriatric syndrome | 1.24 | 0.70-2.18 | .44 | 2.21 | 0.88-5.54 | .03 |
| CIRS-G total <10 vs ≥10 | 1.05 | 0.62-1.77 | .87 | 2.11 | 0.92-4.85 | .03 |
| Multivariable Cox proportional hazards model (backward elimination) | ||||||
| Impaired ADLs/instrumental ADLs | 2.13 | 1.23-3.69 | .007 | 2.52 | 1.13-5.58 | .02 |
| . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| Patient characteristic . | HR . | 95% CI . | P* . | HR . | 95% CI . | P* . |
| Male sex | 1.19 | 0.78-1.80 | .42 | 1.44 | 0.76-2.71 | .26 |
| Age 60-69 vs ≥70 y | 0.81 | 0.53-1.23 | .31 | 0.59 | 0.29-1.06 | .07 |
| Advanced stage | 1.75 | 1.14-2.70 | .02 | 1.75 | 0.92-3.33 | .12 |
| Impaired ADLs/instrumental ADLs | 2.11 | 1.03-4.30 | .006 | 3.58 | 1.42-8.92 | <.0001 |
| Geriatric syndrome | 1.24 | 0.70-2.18 | .44 | 2.21 | 0.88-5.54 | .03 |
| CIRS-G total <10 vs ≥10 | 1.05 | 0.62-1.77 | .87 | 2.11 | 0.92-4.85 | .03 |
| Multivariable Cox proportional hazards model (backward elimination) | ||||||
| Impaired ADLs/instrumental ADLs | 2.13 | 1.23-3.69 | .007 | 2.52 | 1.13-5.58 | .02 |
All P values are 2 sided.
Conventional vs alternative frontline therapy
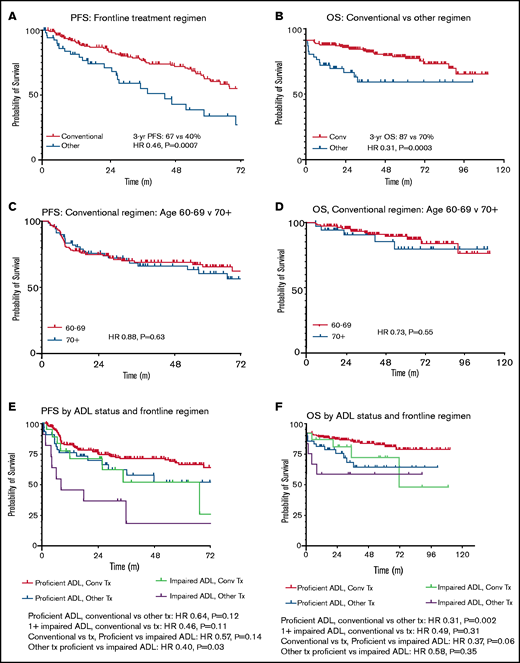
Recipients of conventional cHL regimens showed superior PFS (HR, 0.46; P = .0007) and OS (HR, 0.31; P = .0003) compared with those treated with alternative regimens (Figure 3). Patient age group (age 60-69 vs ≥70 years) did not affect survival outcomes after conventional regimens (PFS: HR, 0.88; P = .63; OS: HR, 0.73; P = .55). Assessed across all frontline therapies, there was no significant age-related PFS difference (HR, 0.81; P = .35), although there was a trend toward longer OS in patients age <70 years (HR, 0.53; P = .052). Given there were relatively few patients age ≥80 years, further subdivision by age was not performed. When adjusting for ADL status, conventional regimens continued to show improved PFS (adjusted HR, 0.59; 95% confidence interval [CI], 0.36-0.94; P = .03) and OS (adjusted HR, 0.34; 95% CI, 0.17-0.69; P = .003) vs alternative therapies.
PFS and OS by frontline treatment regimen in stage II to IV disease. Time is listed in months for all figures. (A) PFS by frontline treatment regimen. (B) OS by frontline treatment regimen. (C) PFS in conventional (conv) regimen recipients age <70 vs ≥70 years. (D) OS in conv regimen recipients age <70 vs ≥70 years. (E) PFS by ADL status and frontline regimen. (F) OS by ADL status and frontline regimen. Tx, treatment.
PFS and OS by frontline treatment regimen in stage II to IV disease. Time is listed in months for all figures. (A) PFS by frontline treatment regimen. (B) OS by frontline treatment regimen. (C) PFS in conventional (conv) regimen recipients age <70 vs ≥70 years. (D) OS in conv regimen recipients age <70 vs ≥70 years. (E) PFS by ADL status and frontline regimen. (F) OS by ADL status and frontline regimen. Tx, treatment.
To assess the relative efficacy of CHOP compared with other therapies, a separate 3-arm comparison was performed for PFS and OS (data supplement). CHOP demonstrated inferior PFS compared with conventional regimens (HR, 0.28; P = .02), with no significant difference in OS (HR, 0.86; P = .84). In contrast, non-CHOP alternative therapies were similar to CHOP in terms of PFS (HR, 1.46; P = .46), and there was a nonsignificant trend toward improved OS with CHOP vs non-CHOP alternative regimens (HR, 0.33; P = .13). Stratified analysis by ADL status was not performed because of the low sample size.
Stage I disease
Twenty-two patients (9% of total cohort) had stage I disease at diagnosis; median age was 71 years (range, 60-88), with 13 patients (59%) age >70 years. A majority (n = 21; 95%) received radiotherapy, and 14 (64%) received systemic therapy (range, 2-4 cycles); the latter was most commonly ABVD (n = 9) and also included AVD (n = 2) and CHOP (n = 1), all with radiotherapy. Two patients received BV, 1 with radiotherapy and 1 with bendamustine. Overall response for those with stage I disease was 95% (n = 21), with 91% (n = 20) achieving a complete response (CR). Two-year PFS and OS were 82% and 95%, respectively; relapses within the first 2 years of completing therapy (n = 4; 18%) were evenly divided between patients who received ABVD with radiotherapy and those who received radiotherapy alone. Long-term outcomes in this cohort remain unknown because of the excellent early outcomes and limited follow-up thus far.
Toxicity
Low-grade toxicities are common if not expected with cHL therapy; such events are not reported here unless otherwise associated with clinical outcomes. Severe toxicity leading to early treatment discontinuation (treatment-limiting toxicity [TLT]) was noted in 15% (n = 36) of patients. Eight patients (3.2%) experienced treatment-related mortality (TRM), including 3 as a result of bleomycin-related lung injury. TLTs occurred more commonly in those with CIRS-G of ≥10 (28% vs 12%; P = .016), those with a geriatric syndrome (28% vs 13%; P = .02), and those age ≥70 years (25% vs 8%; P = .001). ADL impairment also trended toward higher incidence of TLT but did not meet statistical significance (27% vs 13%; P = .096). Multivariable analysis of TLTs by age (≥70 vs <70 years), presence of a geriatric syndrome, and high CIRS-G total score found that only age remained significant (multivariable HR, 3.1; P = .004), with a nonsignificant trend for CIRS-G of ≥10 (multivariable HR, 2.2; P = .059). Most TLTs were infectious in nature (n = 19; 52.8%), with or without associated neutropenia; other hematologic toxicities (eg, severe anemia) were cited in only 1 other treatment discontinuation. Cardiac toxicities were rare (n = 11) but tended to be treatment limiting (n = 8; 73%).
TLT was associated with shorter treatment duration by a median of 2 cycles (6 vs 4 cycles; P < .0001). This in turn resulted in a lower CR rates (50% vs 77%; P < .0001) as well as inferior PFS (HR, 3.19; 95% CI, 2.00-5.09; P < .0001) and OS (HR, 3.80; 95% CI, 1.94-7.45; P = .0001). When evaluating TLT along with ADL status and achievement of a CR in a multivariable model, only response to frontline therapy remained significant for PFS (adjusted HR, 0.17; P < .0001) or OS (adjusted HR, 0.30; P = .003).
Bleomycin lung injury
ABVD recipients received a median of 7 bleomycin doses (range, 1-14), and 26 (18.4%) had documented pulmonary toxicity. Although uncommon, incidence of bleomycin lung injury was higher among men (25.4% vs 11.4%; P = .03) and was not significantly associated with any other individual factor, including age, stage, smoking history, pulmonary CIRS-G score, number of bleomycin doses, or number of chemotherapy cycles (data not shown).
TRM
Eight patient deaths (3.3% of total cohort) were attributed to complications of HL therapy. Median age of patients who experienced TRM was 72.5 years (range, 61-83). TRM was most common after receipt of ABVD (n = 7); 3 deaths after ABVD were attributed to bleomycin lung injury. One patient had a pretreatment ECOG PS of 3, 3 (37.5%) had CIRS-G of ≥10 (each with ECOG PS of 0-2), and 2 (25%) had impaired instrumental ADLs and/or a known geriatric syndrome before frontline therapy. Three patients experienced TRM after presenting initially with ECOG PS of 0 to 2, CIRS-G of <10, and intact ADLs.
A competing-risk analysis was performed to compare deaths resulting from disease-related, treatment-related, and other causes, which showed significant improvement in disease-related survival after conventional cHL regimens (HR, 0.29; P = .02). Although there was a suggestion that patients with high CIRS-G or a geriatric syndrome were at increased risk for death resulting from alternative causes, this was not significant on multivariate analysis (data supplement).
Discussion
To our knowledge, these data represent 1 of the largest detailed analyses of older cHL patients in the modern era. Most patients in this study derived significant benefit from conventional cHL regimens, and many remained disease free at the time of last follow-up. We believe a significant portion of these individuals may be cured of their lymphoma, because their responses have been observed beyond 5 years. Importantly, a number of these individuals with durable responses are age >70 years, a group of patients who, if treated according to their chronologic age, may not have been offered potentially curative therapy. Given the substantial survival benefit seen with conventional therapies, undertreatment based solely on age thus presents a major risk to older cHL patients, and such decisions should require justification based on physiologic measures of fitness rather than age alone.
This in many ways echoes modern experiences in treating older patients with diffuse large B-cell lymphoma (DLBCL), where anthracycline-based therapy is a clear standard of care, even in patients of advanced age.24,25 Because of low numbers, analysis was limited for those age >80 years in this cohort, and further study is warranted. Notably, there is no mini-CHOP equivalent when prescribing an AVD-based regimen, and as mentioned, there are significant differences in dose density between AVD and CHOP (eg, chemotherapy every 3 vs every 2 weeks, respectively), which can affect respective therapeutic outcomes in cHL.20,24 Common dose modifications in older cHL patients include avoiding chemotherapy escalation (no patient in this cohort received standard or escalated BEACOPP) as well as limiting bleomycin exposure to a maximum of 4 doses.22 The latter was clearly not observed in this cohort, where the median bleomycin course was 7 doses, and 86 (63%) of 136 ABVD recipients received ≥5 doses. Of note, bleomycin lung injury as an entity is not well defined; we considered this diagnosis as any reported pulmonary toxicity in a bleomycin recipient, although numerous definitions have been employed, with rates varying from 9.3% to 91% across studies.8,26,27 We interpret the relatively low rates of bleomycin lung injury in this context to be in part a consequence of this lack of consensus on a definition as well as a byproduct of retrospective collection, although administration of up to 4 bleomycin doses, which the current National Comprehensive Cancer Network guidelines22 list as appropriate for select older patients, seemed to be well tolerated in this study.
Although older patients showed significant benefit overall with more aggressive therapy, this is not to say that age and age-related factors do not affect treatment considerations. ADL impairment was seen more commonly with advancing age, and the impact of other geriatric measures on treatment efficacy by itself seems more nuanced and challenging to quantify. Toxicities showed a consistent relation to presence of a geriatric syndrome and high burden of medical comorbidities, especially for toxicities that limited further planned therapy. Risk of such TLT showed a clear increase with advancing age, and the truncation of treatment length even by 1 cycle led to inferior responses and, by extension, to shorter PFS and OS. Of note, few patients (15%) received novel agents with frontline therapy, and it remains unclear whether 6 cycles will remain the optimal treatment duration when such agents are included in the upfront setting.
Only 1 patient in this large sampling of US academic cancer centers underwent formal GA before therapy. Despite rising calls for GA incorporation into clinical trials and routine care for older patients with cancer across the board,12,17 this lack of GA use may reflect the paucity of data regarding how such measures would inform therapy in lymphoma patients, including cHL. Several cHL studies have incorporated at least some GA components, with the emergence of medical comorbidities and ADL status as the most straightforward predictors of treatment outcomes.9,10,14,15 The recently reported Elderly Prognostic Index (EPI), developed for older patients with DLBCL, highlights a potential framework to consider28 ; biologic and functional differences likely exist between older populations with DLBCL and cHL, and further study is needed to assess the utility of the EPI in the latter context. We compared the CIRS-G criteria used in the EPI and found the total score to be more predictive of severe toxicity, although histologies differ between studies, and our finding is potentially biased as a result of retrospective collection. Other factors such as gait speed, cognition, and nutrition have all been evaluated in geriatric hematology, if not specifically in the context of cHL,29 -32 and all are notably omitted from the EPI. Our data suggest that routine clinical practice at least in large academic centers in the United States rarely leverages these fitness markers at present to guide therapy for older cHL patients. This is a key area for future study, perhaps most importantly the study of how GA may help guide decisions on therapeutic intensity and which tests and scoring thresholds to use in order to optimize disease-related outcomes and tolerability with cHL-directed therapy.
This study had several important limitations, in part because of its retrospective design and the biases inherent in this approach. As mentioned, only 1 patient underwent comprehensive GA; we otherwise relied on retrospective collection of comorbidities, geriatric syndromes, and ADL impairment, which was dependent on provider documentation. As such, we suspect our study underestimates these geriatric measures by identifying only the most significant impairments and that more sensitive documentation and/or higher use of GA may produce different outcomes. Our cohort included low numbers of patients age >80 years, which is likely due to referral patterns of this patient population to academic medical centers in the United States. Regarding use of novel agents, this study was not powered to compare sequential vs concurrent administration of BV with AVD chemotherapy, although cross-trial comparisons suggest sequential administration may be better tolerated in older patients. We were also unable to adequately capture incidence, severity, and functional implications of treatment-related neuropathy; this remains of critical interest, especially with BV-based therapy, and warrants further study, especially with prolonged exposure. Given the already extensive data collection for each patient per site, we were unable to collect additional treatment details such as chemotherapy dose levels or growth factor use. Patients were all seen at academic medical centers; although a subset received at least some of their treatment at affiliated community sites, this does select for individuals willing and able to seek care at such locations.
Conventional chemotherapy (ABVD, AVD, or BV plus AVD) should be considered standard of care for fit older patients with cHL, irrespective of chronologic age. Risk of inferior outcomes resulting from TLT is higher in those with high medical comorbidities or a geriatric syndrome. Further prospective study is needed to define fitness in this context using GA and to evaluate the potential role of GA in guiding therapeutic intensity as frontline treatments evolve.
Acknowledgments
The authors acknowledge the contribution of Nolan Wages in developing the competing-risk regression model for this study, as well as the work of the University of Virginia Office of Grants and Contracts for coordinating this effort.
This study originated at the University of Virginia, with support from the University of Virginia Cancer Center (P30CA044579) (C.A.P.). Research reported in this publication was supported by the National Cancer Institute, National Institutes of Health (NIH), under award K12CA237806 from the Winship K12 Clinical Oncology Training Program (V.M.-O.) and by the National Center for Advancing Translational Sciences, NIH, under award UL1TR002378 from the Georgia Clinical and Translational Science Alliance (V.M.-O.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: V.M.-O., A.M.E., and C.A.P. designed the study; and all authors participated in the collection and analysis of data and in the writing/reviewing of the manuscript.
Conflict-of-interest disclosure: N.L.B. reports research funding from Celgene; consultancy for ADC Therapeutics; and research funding from ADC Therapeutics, Affimed, Merck, Janssen, Immune Design, Gilead, Millennium, Pfizer, Pharmacyclics, Seattle Genetics, Forty Seven, Genentech, and Bristol-Myers Squibb. N.S.G. reports consultancy for Seattle Genetics. N.N.B. reports advisory board membership for Adicet Bio, Seattle Genetics, Purdue Pharma, and Kite Pharma and research funding from Bristol-Myers Squibb. B.T.H. reports consultancy for Kite Pharma, Gilead, Pharmacyclics, AstraZeneca, Celgene, Seattle Genetics, AbbVie, and Genentech; honoraria from Kite Pharma, Gilead, Pharmacyclics, AstraZeneca, Celgene, Seattle Genetics, and AbbVie; board of directors or advisory committee membership for Gilead, Pharmacyclics, AstraZeneca, Celgene, Seattle Genetics, and AbbVie; and research funding from Pharmacyclics, Celgene, Takeda, Amgen, TG Therapeutics, AbbVie, and Genentech. R.H.A. reports consultancy for Takeda, Gilead Sciences, Kite Pharma, Autolus, Bayer, Celmed, Roche/Genentech, Kyowa Kirin Pharmaceutical Developments, Seattle Genetics, Cell Medica, AstraZeneca, and Pharmacyclics; board of directors or advisory committee membership for Takeda, Gilead Sciences, Kite Pharma, Autolus, Bayer, Bristol-Myers Squibb, Celmed, Roche/Genentech, AstraZeneca, and Pharmacyclics; research funding from Millennium, Kura, Janssen, Merck, Regeneron, Infinity Pharma, Bristol-Myers Squibb, Celgene, Forty Seven, Roche/Genentech, Agensys, Seattle Genetics, and Pharmacyclics; and employment by and equity ownership in Stanford University. J.S. reports consultancy for AstraZeneca, Pharmacyclics, Kyowa, Bristol-Myers Squibb, and Seattle Genetics and research funding from Celgene, Incyte, Pharmacyclics, Merck, Bristol-Myers Squibb, and Seattle Genetics. G.M. reports speakers bureau participation for Tevan Oncology. T.A.F. reports honoraria from Takeda, Celgene, Seattle Genetics, AbbVie, Pharmacyclics, Janssen, Kite Pharma, and Bayer; speakers bureau participation for Celgene, Seattle Genetics, AbbVie, Pharmacyclics, Janssen, and Kite Pharma; research funding from Celgene, Amgen, Cell Medica, Roche, Corvus, Eisai, Kyowa Hakko Kirin, Pfizer, Portola Pharma, Trillium, and Viracta; consultancy for Seattle Genetics and Bayer; travel expenses from Seattle Genetics, AbbVie, Pharmacyclics, and Kite Pharma; and board of directors or advisory committee membership for Bayer. J.B.C. reports research funding from Astra Zeneca, American Society of Hematology, Unum, Hutchison, Takeda, Lymphoma Research Foundation, LAM Therapeutics, Seattle Genetics, Bristol-Meyers Squibb, and Genentech and consultancy for Gilead Sciences, Kite Pharma, Seattle Genetics, Janssen, and Genentech. A.M.E. reports consultancy for Seattle Genetics, Epizyme, Verastem, and Pharmacyclics; honoraria from Seattle Genetics, Epizyme, Verastem, and Pharmacyclics; and research funding from Seattle Genetics and Tesaro. C.A.P. reports research funding from AbbVie, Genentech, BeiGene, Kite Pharma, Acerta/AstraZeneca, TG Therapeutics, Xencor, Roche/Genentech, and Infinity; consultancy for Pharmacyclics, Janssen, Genentech, Amgen, Bayer, BeiGene, and Kite Pharma. The remaining authors declare no competing financial interests.
Correspondence: Victor M. Orellana-Noia, Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, 1365B Clifton Rd, Number 4011, Atlanta, GA 30322; e-mail: orellana-noia@emory.edu.
References
Author notes
Presented in part at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
Qualified researchers may request nonconfidential data from the corresponding author at orellana-noia@emory.edu.
The full-text version of this article contains a data supplement.