TO THE EDITOR:
Primary mediastinal B-cell lymphoma (PMBCL) is a rare subtype of non-Hodgkin lymphoma (NHL) with poor outcomes in the relapsed setting.1 Axicabtagene ciloleucel (axi-cel) is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that is approved by the Food and Drug Administration for relapsed aggressive B-cell NHL, including PMBCL. Although patients with PMBCL were included in the phase 2 study of axi-cel in relapsed/refractory large B-cell lymphoma, very few patients with this histology were enrolled.2 Similarly, follow-up real-world studies have included a limited number of patients with PMBCL.3,4 The outcome of CAR T-cell therapy in this entity is therefore still poorly understood. Furthermore, unlike other aggressive B-cell NHL subtypes, PMBCLs often harbor genomic alterations of chromosome 9p24.1, including both copy gains and translocations, resulting in increased expression of the programmed death-1 (PD-1) ligands, PD-L1 and PD-L2.5,6 These and other changes facilitate immune evasion and support a central role for the PD-1 pathway in the pathogenesis of PMBCL.7-10 Pembrolizumab, a PD-1 inhibitor, is approved for the treatment relapsed/refractory PMBCL, based on the results of the phase 2 KEYNOTE-170 trial.11 The combination of nivolumab, a PD-1 inhibitor, and brentuximab, is also active in this setting.12 Although checkpoint blockade and CAR T-cell therapy can both be used in relapsed PMBCL, the optimal sequencing of these therapies remains unclear. In the present analysis, we examine outcomes of 33 patients with PMBCL who received axi-cel off trial. We explore the sequencing of axi-cel with checkpoint blockade and the impact of drug order on clinical outcome. To our knowledge, this is the largest series of patients with PMBCL who have received axi-cel therapy.
We performed a retrospective, multicenter study of adult patients with relapsed/refractory PMBCL who were treated with axi-cel outside of the setting of a clinical trial at 5 academic medical centers. This analysis was approved by the Virginia Commonwealth University Medical Center, Dana Farber/Harvard Cancer Center, MD Anderson Cancer Center, City of Hope National Medical Center, and Fred Hutchinson Cancer Research Center Institutional Review Boards. The study was conducted in accordance with the Declaration of Helsinki. Patients were treated between January 2018 and July 2019. Treatment selection and timing of response assessment followed institutional practices. Evaluation of bulky disease (defined as a mass >10 cm) and Eastern Cooperative Oncology Group performance status were performed prior to CAR T-cell therapy. Cytokine release syndrome (CRS) was graded according to the modified Lee criteria and per the American Society for Transplantation and Cellular Therapy (ASTCT) criteria once available.13,14 Neurotoxicity (NT) grading was per Common Terminology Criteria for Adverse Events version 4 and ASTCT criteria once available. Response was determined by positron emission tomography per Lugano criteria and was not centrally reviewed.
The baseline characteristics of the 33 patients are shown in Table 1. The median age at the time of axi-cel infusion was 32 years (range, 18-46 years). Patients received a median of 3 (range, 1-9) prior lines of therapy, and 30% of patients had received a prior autologous stem cell transplantation. Almost half (42%) of patients had bulky disease. Most patients received prior radiation (67%), including 2 patients who received bridging radiation. The median duration of follow-up was 13.8 months.
Patient demographics
| . | . | Checkpoint blockade . | . | |
|---|---|---|---|---|
| . | Total n = 33 (%) . | None/post-CAR T cell n = 18 (55) . | Prior to CAR T cell n = 15 (45) . | P . |
| Age, y | ||||
| Median (range) | 32 (18-46) | 30 (19-46) | 35 (18-40) | .73* |
| Stage | ||||
| I | 8 (24) | 5 (28) | 3 (20) | .68† |
| II | 8 (24) | 3 (17) | 5 (33) | |
| III | 7 (21) | 6 (33) | 1 (7) | |
| IV | 10 (30) | 4 (22) | 6 (40) | |
| Eastern Cooperative Oncology Group performance status | ||||
| 0 | 14 (42) | 8 (44) | 6 (40) | .61† |
| 1 | 17 (52) | 8 (44) | 9 (60) | |
| 2 | 1 (3) | 1 (6) | — | |
| 3 | 1 (3) | 1 (6) | — | |
| Bulky disease | ||||
| No | 19 (58) | 10 (56) | 9 (60) | >.99‡ |
| Yes | 14 (42) | 8 (44) | 6 (40) | |
| Prior radiation therapy | ||||
| No | 11 (33) | 8 (44) | 3 (20) | .27‡ |
| Yes | 22 (67) | 10 (56) | 12 (80) | |
| Prior auto | ||||
| No | 23 (70) | 15 (83) | 8 (53) | .13‡ |
| Yes | 10 (30) | 3 (17) | 7 (47) | |
| No. of prior lines of therapy | ||||
| Median (range) | 3 (1-9) | 3 (1-7) | 4 (3-9) | .04‡ |
| Restaging post-CAR T cell | ||||
| PD | 7 (21) | 5 (28) | 2 (13) | .29† |
| ORR | 25 (76) | 13 (72) | 12 (80) | |
| PR | 3 (9) | 2 (11) | 1 (7) | |
| CR | 22 (67) | 11 (61) | 11 (73) | |
| Missing | 1 (3) | 0 (0) | 1 (7) | |
| Response to checkpoint | ||||
| PD | 7 (21) | 1 (6) | 6 (40) | .23† |
| SD | 1 (3) | — | 1 (7) | |
| ORR | 9 (27) | 3 (17) | 6 (40) | |
| PR | 2 (6) | — | 2 (13) | |
| CR | 7 (21) | 3 (17) | 4 (27) | |
| Missing | 16 (48) | 14 (78) | 2 (13) | |
| CRS, any grade§ | ||||
| No | 4 (12) | 1 (6) | 3 (20) | .31‡ |
| Yes | 29 (88) | 17 (94) | 12 (80) | |
| CRS grade ≥3§ | ||||
| No | 31 (94) | 16 (89) | 15 (100) | .49‡ |
| Yes | 2 (6) | 2 (11) | — | |
| NT, any grade§ | ||||
| No | 20 (61) | 11 (61) | 9 (60) | >.99‡ |
| Yes | 13 (39) | 7 (39) | 6 (40) | |
| NT grade ≥3§ | ||||
| No | 24 (72) | 13 (72) | 11 (73) | >.99‡ |
| Yes | 9 (27) | 5 (28) | 4 (27) | |
| Immune mediated toxicity | ||||
| No | 26 (79) | 11 (61) | 15 (100) | >.99‡ |
| Yes | — | — | — | |
| Missing | 7 (21) | 7 (39) | 0 (0) | |
| . | . | Checkpoint blockade . | . | |
|---|---|---|---|---|
| . | Total n = 33 (%) . | None/post-CAR T cell n = 18 (55) . | Prior to CAR T cell n = 15 (45) . | P . |
| Age, y | ||||
| Median (range) | 32 (18-46) | 30 (19-46) | 35 (18-40) | .73* |
| Stage | ||||
| I | 8 (24) | 5 (28) | 3 (20) | .68† |
| II | 8 (24) | 3 (17) | 5 (33) | |
| III | 7 (21) | 6 (33) | 1 (7) | |
| IV | 10 (30) | 4 (22) | 6 (40) | |
| Eastern Cooperative Oncology Group performance status | ||||
| 0 | 14 (42) | 8 (44) | 6 (40) | .61† |
| 1 | 17 (52) | 8 (44) | 9 (60) | |
| 2 | 1 (3) | 1 (6) | — | |
| 3 | 1 (3) | 1 (6) | — | |
| Bulky disease | ||||
| No | 19 (58) | 10 (56) | 9 (60) | >.99‡ |
| Yes | 14 (42) | 8 (44) | 6 (40) | |
| Prior radiation therapy | ||||
| No | 11 (33) | 8 (44) | 3 (20) | .27‡ |
| Yes | 22 (67) | 10 (56) | 12 (80) | |
| Prior auto | ||||
| No | 23 (70) | 15 (83) | 8 (53) | .13‡ |
| Yes | 10 (30) | 3 (17) | 7 (47) | |
| No. of prior lines of therapy | ||||
| Median (range) | 3 (1-9) | 3 (1-7) | 4 (3-9) | .04‡ |
| Restaging post-CAR T cell | ||||
| PD | 7 (21) | 5 (28) | 2 (13) | .29† |
| ORR | 25 (76) | 13 (72) | 12 (80) | |
| PR | 3 (9) | 2 (11) | 1 (7) | |
| CR | 22 (67) | 11 (61) | 11 (73) | |
| Missing | 1 (3) | 0 (0) | 1 (7) | |
| Response to checkpoint | ||||
| PD | 7 (21) | 1 (6) | 6 (40) | .23† |
| SD | 1 (3) | — | 1 (7) | |
| ORR | 9 (27) | 3 (17) | 6 (40) | |
| PR | 2 (6) | — | 2 (13) | |
| CR | 7 (21) | 3 (17) | 4 (27) | |
| Missing | 16 (48) | 14 (78) | 2 (13) | |
| CRS, any grade§ | ||||
| No | 4 (12) | 1 (6) | 3 (20) | .31‡ |
| Yes | 29 (88) | 17 (94) | 12 (80) | |
| CRS grade ≥3§ | ||||
| No | 31 (94) | 16 (89) | 15 (100) | .49‡ |
| Yes | 2 (6) | 2 (11) | — | |
| NT, any grade§ | ||||
| No | 20 (61) | 11 (61) | 9 (60) | >.99‡ |
| Yes | 13 (39) | 7 (39) | 6 (40) | |
| NT grade ≥3§ | ||||
| No | 24 (72) | 13 (72) | 11 (73) | >.99‡ |
| Yes | 9 (27) | 5 (28) | 4 (27) | |
| Immune mediated toxicity | ||||
| No | 26 (79) | 11 (61) | 15 (100) | >.99‡ |
| Yes | — | — | — | |
| Missing | 7 (21) | 7 (39) | 0 (0) | |
*Wilcoxon rank-sum test.
†Cochran-Armitage test.
‡Fisher's exact test.
§CRS grading by Lee criteria and NT grading by Common Terminology Criteria for Adverse Events in 51%, and by ASTCT in the remaining patients.
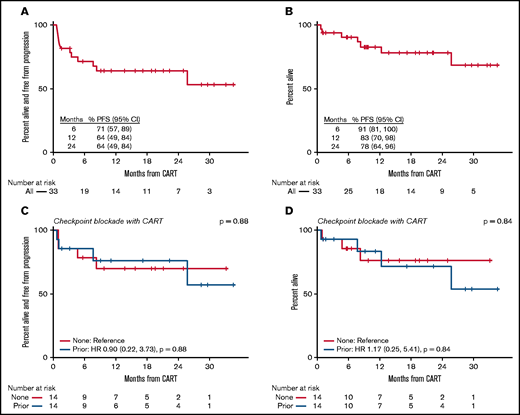
Of the 33 patients who were treated with axi-cel, 97% of patients were evaluable for response. One patient died because of sepsis during their admission for CAR T-cell infusion and did not have a response assessment. Among evaluable patients, the overall response rate (ORR) was 78% with a 69% complete response (CR) rate. In the intent-to-treat population who had received CAR T-cell therapy, the ORR was 76%, with a 67% CR rate. The median time to best response was 29 days (range, 20-492 days). Among all 33 patients, the 24-month (intent-to-treat) progression-free survival (PFS) was 64% (95% confidence interval [CI], 49-84), and the 24-month overall survival (OS) was 78% (95% CI, 64-96) (Figure 1). CRS of any grade was seen in 88% of patients. Grade 3 or higher CRS occurred in 6% of patients. Neurologic toxicity of any grade was observed in 39% of patients, with 27% grade 3 or higher toxicity.
(A) PFS in patients with PMBCL treated with axi-cel. (B) OS in patients with PMBCL treated with axi-cel. (C) PFS among patients who received no checkpoint blockade or checkpoint blockade prior to axi-cel. (D) OS among patients who received no checkpoint blockade or checkpoint blockade prior to axi-cel .
(A) PFS in patients with PMBCL treated with axi-cel. (B) OS in patients with PMBCL treated with axi-cel. (C) PFS among patients who received no checkpoint blockade or checkpoint blockade prior to axi-cel. (D) OS among patients who received no checkpoint blockade or checkpoint blockade prior to axi-cel .
Nineteen patients were also treated with checkpoint blockade, either before (n = 14), after (n = 4), or before and after (n = 1) axi-cel therapy. Two patients received checkpoint blockade as bridging therapy. Of the 15 patients who received checkpoint blockade prior to axi-cel infusion, the best overall response to checkpoint blockade was 40%, with a 27% CR rate. Among the 4 patients who received checkpoint blockade after axi-cel, best overall response to checkpoint blockade was 75%, with all responding patients achieving CR. In these cases, checkpoint blockade was given within 100 days of CAR T-cell therapy in 3 patients, and ∼1 year following CAR T-cell therapy in 1 patient. Of note, there was 1 patient who did not respond to nivolumab prior to axi-cel infusion but achieved a CR when treated with pembrolizumab in the post-CAR T-cell setting, ∼100 days following administration of CAR T-cell therapy. One patient did not respond to pembrolizumab following axi-cel but did ultimately achieve response when treated with brentuximab and nivolumab after allogeneic stem cell transplantation. In this series, no patient experienced immune-mediated toxicity following checkpoint blockade.
There was no apparent difference in response or toxicity to axi-cel in patients who received checkpoint blockade prior to axi-cel infusion (Figure 1). In patients who had received prior checkpoint blockade, the ORR and CR rate following axi-cel was 80% and 73%, respectively, vs 72% and 61% for those without prior checkpoint blockade. PFS following axi-cel was similar among patients treated with and without prior checkpoint blockade (hazard ratio: 0.9; 95% CI, 0.22-3.73; P = .88). OS also appeared similar among patients who had or had not received checkpoint blockade prior to axi-cel infusion (hazard ratio: 1.17; 95% CI, 0.25-5.41; P = .84). Rates of CRS and NT were similar among patients regardless of prior treatment with checkpoint blockade (Table 1). Of note, 1 patient who received checkpoint blockade as bridging therapy 14 days prior to CAR T-cell therapy developed grade 3 NT with acute, irreversible, paraplegia, on day 7 following CAR T-cell infusion, which was thought to be cytokine mediated. The patient did not have known central nervous system or spinal disease and had not received spinal radiation, although the patient did receive radiation to a site of refractory disease in the kidney 6 months prior.
There are limited data on the response and toxicity of CAR T-cell therapy in patients with relapsed/refractory PMBCL. Furthermore, there appears to be no apparent deleterious (or beneficial) impact on the efficacy or safety of CAR T-cell therapy when patients receive prior checkpoint blockade. Interestingly, 1 patient who had progressive disease (PD) following checkpoint blockade and axi-cel therapy went on to achieve a CR when re-treated with checkpoint blockade in the post CAR T-cell setting. Although our findings are limited by their retrospective nature and small sample size, they raise the question of whether checkpoint blockade can result in enhanced activity following treatment with axi-cel. Studies have demonstrated increased PD-1 expression following CAR T-cell therapy, suggesting a potential mechanism of immune escape.3,15 The combination of checkpoint blockade and axi-cel has been examined in ZUMA-6, the phase 1/2 study of axi-cel combined with the anti–PD-L1 antibody, atezolizumab, in patients with diffuse large B-cell lymphoma.16 Although the combination was safe, efficacy outcomes of axi-cel combined with atezolizumab appeared to be similar to those of patients treated with axi-cel alone in preliminary results. The combination of checkpoint blockade and CAR T-cell therapy, however, has not been examined in lymphomas known to have increased sensitivity to PD-1 inhibition, where the synergistic potential might be greatest.
In conclusion, axi-cel is an active therapy for patients with relapsed/refractory PMBCL with an efficacy toxicity profile similar to that seen in prior studies in large B-cell lymphomas.2-4 Additional prospective studies exploring the combination of checkpoint blockade and CAR T-cell therapy are warranted.
Contribution: J.L.C., L.J.N., P.A., and G. Simmons designed the research, analyzed the data, and wrote the manuscript; R.R. analyzed the data; K.T., G. Shouse, A.F.H., V.A.C., M.S., O.C.-P., A.S., and C.A.J. collected and analyzed data and provided critical review of the manuscript.
Conflict-of-interest disclosure: J.L.C. has received research support from Bayer and Abbvie; consulting fees from Incyte and Karyopharm. L.J.N. has received honorarium from ADC Therapeutics, Bayer, BMS/Celgene, Epizyme, Genentech, Janssen, KITE/Gilead, Morphosys, Novartis, Pfizer, and TG Therapeutics; research support from BMS/Celgene, Caribou, Epizyme, Genentech, Janssen, Lam Therapeutics, Novartis, Takeda, and TG Therapeutics. G. Shouse has received honoraria from Kite Pharmaceuticals. A.F.H. has received research funding from BMS, Merck, Genentech/F. Hoffmann-La Roche, Gilead Sciences, Seattle Genetics, Immune Design, AstraZeneca, and Pharmacyclics; consultant fees from BMS, Merck, Genentech/F. Hoffmann-La Roche, Gilead Sciences, Seattle Genetics, and Karyopharm; and travel, accommodation, and expense compensation from BMS. V.A.C. has received research funding from AstraZeneca. M.S. reports consulting, advisory boards, steering committees, or data safety monitoring committees from Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, and Atara Biotherapeutics; research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, and Atara Biotherapeutics. C.A.J. has received consulting fees from Kite/Gilead, Novartis, BMS/Celgene, Nkarta, Precision Biosciences, Lonza, Bluebird Bio, and Abbie; research funding from Pfizer and Kite. P.A. received consulting fees from Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, Astra Zeneca, Genentech; research funding from Merck, BMS, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, IGM, Kite; honoraria from Merck and BMS. The remaining authors declare no competing financial interests.
Correspondence: Jennifer Crombie, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: Jennifer_Crombie@dfci.harvard.edu.
References
Author notes
Deidentified participant data that underlie the reported results can be requested by contacting the corresponding author.
J.L.C. and L.J.N. contributed equally to this study.
P.A. and G. Simmons contributed equally to this study.