Key Points
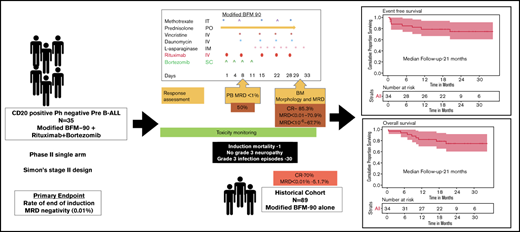
Integration of bortezomib and rituximab with modified BFM-90 induction therapy results in higher MRD-negative complete remission rates.
Bortezomib and rituximab with a pediatric-inspired regimen showed promising clinical activity with an acceptable safety profile.
Abstract
The expression of CD20 in precursor B-cell acute lymphoblastic leukemia (B-ALL) is associated with poor outcomes. The addition of rituximab to intensive chemotherapy in CD20+ ALL has led to improved outcomes in several studies. However, there is no clear evidence regarding the optimal number of doses and its benefit without an allogeneic stem cell transplant. Achieving measurable residual disease (MRD)-negative status postinduction would reduce the requirement for a transplant. Novel approaches are needed to induce a higher proportion of MRD-negative complete remission in patients with high-risk ALL. Given bortezomib’s activity in relapsed ALL and its synergism with rituximab in B-cell lymphomas, the addition of bortezomib to rituximab and chemotherapy may provide an incremental benefit in CD20+ precursor B-ALL. We conducted a phase 2 study to test the activity of bortezomib and rituximab in combination with a pediatric-inspired regimen during induction therapy in newly diagnosed adolescents and adults (aged >14 years) with CD20+, Philadelphia-negative precursor B-ALL; bone marrow MRD negativity at the end of induction was the primary end point. From December 2017 through August 2019, a total of 35 patients were enrolled. End-of-induction MRD-negative status was achieved in 70.9% of patients, as opposed to 51.7% in the historical cohort treated with chemotherapy alone. MRD-negative rates improved to 87.5% post-consolidation. At a median follow-up of 21 months, event-free survival and overall survival rates were 78.8% (95% confidence interval, 66-94) and 78.7% (95% confidence interval, 65.8-94), respectively. There was no significant increase in toxicity with bortezomib and rituximab compared with the historical cohort. The incidence of neuropathy was 26% (all less than grade 3). The combination of bortezomib, rituximab, and a pediatric-inspired ALL regimen was active and well tolerated in de novo CD20+ Philadelphia-negative precursor B-ALL. This trial was registered with the Clinical Trials Registry-India as CTRI/2017/04/008393(http://ctri.nic.in/Clinicaltrials).
Introduction
Treatment outcomes in de novo adult acute lymphoblastic leukemia (ALL) have plateaued at 25% to 40% in terms of long-term leukemia-free survival.1-3 Although the use of pediatric-inspired regimens in adolescents and young adults has improved outcomes, long-term event-free survival (EFS) rates have remained between 53% and 65%.4-6 Measurable residual disease (MRD) status at end-of-induction (EOI) and post-consolidation has emerged as the single most important prognostic factor.7,8 To achieve postinduction MRD-negative status in high-risk patients or overcome the negative prognostic impact of MRD-positive disease, current approaches include treatment intensification with chemotherapy and allogeneic stem cell transplant. However, adolescents and adults have poor tolerance to higher cumulative doses of non-myelosuppressive drugs and conventional cytotoxic agents. In resource-constrained settings, applicability of treatment intensification with chemotherapy or allogeneic stem cell transplant is limited due to excess infection-related mortality or morbidity, which negates the benefit of deeper responses. In addition, most of these patients do not have access to targeted therapies such as blinatumomab or inotuzumab for treatment of MRD-positive disease postinduction. Thus, there is a need to identify safe and active drugs for adolescent and adult patients with ALL that can increase the proportion of EOI MRD-negative responders and circumvent the need for treatment intensification, with either allogeneic stem cell transplant or multiagent intensive chemotherapy (high-risk blocks).
Approximately 40% to 50% of cases of precursor B-cell ALL (B-ALL) express CD20 at diagnosis and have poor outcomes compared with those without CD20 expression.9 Treatment with steroids further causes CD20 upregulation during induction, both in frequency and expression level.10 The addition of rituximab in combination with chemotherapy has shown improvement in outcomes in CD20+ Philadelphia-negative (Ph–) adult ALL.3,11,12 However, there are conflicting data on the impact of rituximab on postinduction MRD, complete remission (CR) rates, EFS, and overall survival (OS) across studies. The GMALL-R 07/2003 study showed similar CR rates (94% vs 91%) and better postinduction MRD negativity (57% vs 27%) and OS rates with the addition of rituximab in the standard-risk cohort. Conversely, the addition of rituximab failed to improve MRD-negative rates and CR rates postinduction (2 doses of rituximab given during induction) in the GRALL-R study but resulted in better EFS rates. Rituximab in combination with modified hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) led to better 3-year CR duration and MRD negativity rates (2 doses of rituximab given during induction) but did not improve OS. The UKALL14 trial used 4 doses of rituximab during induction without an improvement in EFS in the overall precursor B-ALL population and CD20+ subset.13 These studies used varied doses (range, 8-18 doses), schedule, and timing of rituximab (induction and consolidation with or without maintenance).
Given the evidence for upregulation of CD20 with steroid exposure and lack of clarity regarding the optimal dosing strategy for rituximab that can improve postinduction MRD-negative rates, we proposed using 5 doses of rituximab during induction. The continuation of rituximab beyond induction was not considered due to conflicting evidence on survival benefits with its longer duration, additional cost, and increased risk of infection. In addition to dose intensification of rituximab, bortezomib was combined with induction chemotherapy to improve postinduction MRD-negative rates, EFS, and OS. Bortezomib was selected based on its in vitro and in vivo synergism with steroids and anthracyclines in ALL and with rituximab in CD20+ lymphomas.14-16 Bortezomib has also shown activity in relapsed ALL with improvement in CR rates and OS.17-19
Based on this rationale, we evaluated the activity of this combination during induction in adolescents and adults with CD20+ Ph– precursor B-ALL to increase the proportion of patients with EOI MRD negativity and thus obviate the need for treatment intensification.
Methods
Study design
This study was a single-center, phase 2, nonrandomized open-label trial (Simon’s two-stage design) conducted between December 2017 and August 2019.
Study population
Patients with newly diagnosed Ph– precursor B-ALL expressing CD20 and aged >14 years were eligible for the study. CD20 positivity was defined as baseline CD20 antigen expression ≥20% on leukemic blasts using 10-color flow cytometry. Patients with organ dysfunction, history of hypersensitivity to boron-based compounds, active-uncontrolled infection, or active HIV, hepatitis B virus, and hepatitis C virus were excluded from the study.
Central nervous system (CNS) positivity was defined as CNS2 and CNS3 as per National Comprehensive Cancer Network guidelines or flow cytometric evidence of abnormal blasts in the cerebrospinal fluid (in the absence of hemorrhagic tap). High-risk patients were those who met any one of the following criteria: age ≥35 years, white blood cell count 30 × 109/L or higher as per MRC UKALL2; CNS involvement; hypodiploidy (<45 chromosomes) on karyotype; t(1;19) chromosomal translocation or TCF3-PBX1 fusion; or MLL lineage rearrangement, defined as t(4;11) chromosomal translocation, complex karyotype (≥5 chromosomal abnormalities as per Moorman et al20).
Study overview
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines, and it was approved by the Institutional Ethics Committee of Tata Memorial Centre. Written informed consent was obtained from all subjects at trial entry. An independent data safety monitoring committee reviewed the study for both safety and efficacy at predefined time points. The safety analysis was done after enrollment of the first 10 patients. The study was to be terminated if ≥3 of the first 10 patients died. The incidence of grade 3/4 neuropathy and infections was evaluated. The efficacy assessment was done after enrollment of the 14th patient (stage 1 of the study) to decide on the study’s continuation based on the predefined parameters.
Treatment and procedures
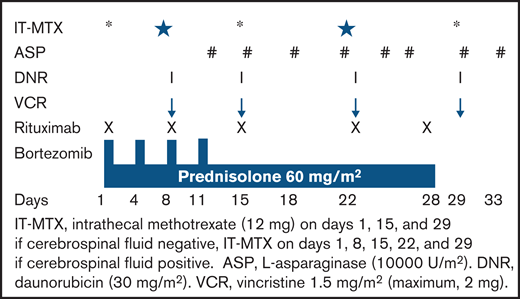
All patients received the modified BFM-90 protocol,21 with no high-risk blocks of chemotherapy. Rituximab was given as an intravenous infusion at a dose of 375 mg/m2 of body surface area, once weekly for 5 doses during induction (on days 1, 8, 15, 22, and 28).
Patients received acetaminophen and an antihistaminic drug (diphenhydramine) before each infusion. Bortezomib was administered subcutaneously at a dose of 1.3 mg/m2 for 4 doses (on days 1, 4, 8, and 11) during induction (Figure 1). All patients received acyclovir 200 mg twice daily from day 1 until day 42 as prophylaxis for varicella zoster infection.
Patients with high-risk disease at diagnosis, poor prednisolone response, induction failure, and postinduction MRD persistence were counseled for allogeneic stem cell transplant, as done in our routine practice. We serve patients mainly with a low socioeconomic status, and treatment expenditure is predominantly out of pocket, with limited support from government programs and philanthropic funding. Due to financial constraints and lack of an adequate number of transplant beds, our transplant rates are <5% for all transplant-eligible patients. All patients were given cranial radiation (12 Gy if CNS negative and 18 Gy if CNS positive).
Due to the high incidence of quinolone resistance in the local population, we do not practice levofloxacin prophylaxis in our patients. The incidence of fungal infections (invasive pulmonary aspergillosis) in patients with prolonged neutropenia, including acute leukemias, is significantly high at our center.22,23 There are several limitations to the use of prophylactic oral antifungal agents in our patient population. These include fluconazole resistance among the Candida and Aspergillus species and adverse events due to drug interaction of vinca alkaloids with posaconazole and voriconazole. In our prior experience, use of azoles during induction therapy led to a high incidence of symptomatic hyponatremia (seizures and altered sensorium requiring admission) and peripheral neuropathy, resulting in either omission or dose modification of vincristine. The use of parenteral prophylaxis with non-azoles would severely compromise the ability to provide care to the large number of patients with ALL who are treated at our center.
Criteria for response and end points
The primary end point of the study was the rate of EOI MRD negativity, which was defined as <1 × 10–4 (<0.01%) residual leukemic cells. Secondary end points were the rate of peripheral blood MRD <1% on day 8, CR rates at the end of induction, complete remission duration (CRD), EFS, OS, the incidence of grade 2 or higher peripheral neuropathy, and grade 2 or higher infections.
Day 8 peripheral blood MRD negativity was defined as <1 × 10–2 (<1%) of residual leukemic cells on 10-color flow cytometry assay in a peripheral blood sample collected on day 8 of induction. Complete remission (CR) was defined as <5% blasts in normocellular bone marrow with absolute neutrophil counts ≥1 × 109/L, platelets ≥100 × 109/L, and no organomegaly or lymphadenopathy. Complete remission duration (CRD) was the time from CR until relapse or last follow-up. EFS was calculated from the date of enrollment in the study to any event or last follow-up. Relapse, progression, induction failure, or death due to any cause were considered events. Induction failure was defined as persistence of leukemic blasts in the bone marrow at the end of induction (week 4), defined as marrow with >5% blasts. OS was calculated from the date of enrollment to death due to any cause.
Flow cytometry–based MRD assessment
Flow cytometry–based MRD assessment was performed by using day 8 peripheral blood samples and bone marrow samples at EOI days 29 to 33 (EOI). MRD was repeated in bone marrow samples at the end-of-consolidation week 16 for patients who had persistent MRD positivity at EOI. MRD was performed by using a high-sensitivity (10–6) 10-color antibody panel as published elsewhere.24
Statistical analysis
The sample size was based on Simon’s two-stage design. With α = 0.05 and power of 80% in the first stage, 14 patients were to be enrolled, and if ≤6 patients (<50%) achieved MRD (day 28) <10–4, the study was to then be terminated (NO GO). If ≥7 patients achieved the MRD end point (GO GO), 11 more patients were enrolled. The combination was to be considered effective for further evaluation if a total of ≥16 patients achieved MRD <10–4. To account for nonevaluability for MRD on day 28 due to technical reasons or losses to follow-up and death, a total of 35 subjects were to be included in the study. All patients who received 1 dose of study-related treatment were analyzed for efficacy and safety. The percentage of patients achieving MRD negativity and the CR rate were calculated by using descriptive statistics. The denominator was the total number of patients who received at least 1 dose of trial-related therapy.
The EOI MRD rates and CR rates were compared with a historical cohort of 89 patients treated with modified BFM-90 chemotherapy alone. Survival distributions were estimated by using Kaplan-Meier methodology and were compared between groups by using the log-rank test. Statistical analysis was done by using SPSS version 25 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Results
Characteristics of patients
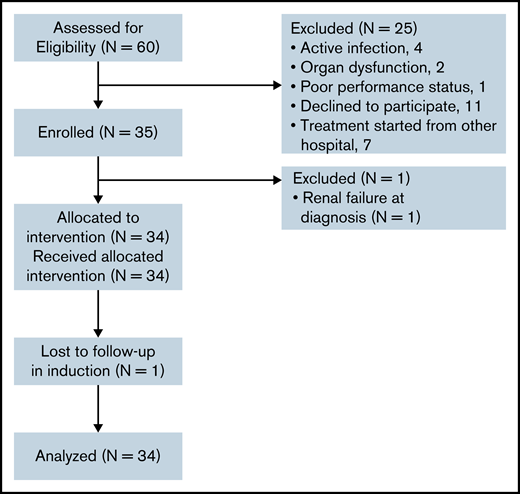
A total of 147 Ph– precursor B-ALL patients in the group aged 15 to 57 years were seen between December 2017 and August 2019. Of these, 60 patients were CD20+ (irrespective of the percentage of CD20). From this subset, 35 patients with CD20 expression >20% were enrolled in the study. The details of screening failure are listed in Figure 2. Many patients who register at our center are from other states and find it difficult to stay for the entire duration of ALL therapy. The same is reflected in the reasons for screen failures, as they declined to participate in the study (11 patients) or started treatment at another center (7 patients).
Pretreatment baseline characteristics for the patients enrolled in the study are shown in Table 1. The median age was 20 years (range, 15-52 years). A total of 76.4% of patients were male, a pattern observed due to gender bias in referral. None of the patients had testicular involvement. CNS involvement, as detected by cytomorphology and flow cytometry, was seen in 29.4% at diagnosis. High-risk features were seen in 59% of patients.
Baseline characteristics
| Characteristic . | All patients (N = 34) . |
|---|---|
| Age, median (range), y | 20 (15-52) |
| Age 15-25 y | 24 |
| Age <35 y | 28 |
| Age >35 y | 6 |
| Risk group | |
| Standard risk | 14 (41%) |
| High risk | 20 (59%) |
| Male:female sex | 26:8 |
| ECOG performance status >1 | 6 (17.6%) |
| White blood cell count ≥30 × 109/L | 7 (20.6%) (range, 30-70 × 109/L) |
| CNS involvement | 10 (29.4%) |
| Flow cytometry alone | 10 (29.4%) |
| Cytomorphology and flow cytometry | 5 (14.7%) |
| CD20%, median (range) | 75% (20%-100%) |
| Cytogenetic features | |
| Diploid | 12 (34%) |
| Hyperdiploidy | 9 (25%) |
| Hypodiploidy | 6 (17%) |
| Ploidy not possible due to absence of metaphases | 7 (20%) |
| Endoreduplication/masked hypodiploid | |
| t (1:19) | 1 (3%) |
| TCF3-PBX1 | 1 (3%) |
| Characteristic . | All patients (N = 34) . |
|---|---|
| Age, median (range), y | 20 (15-52) |
| Age 15-25 y | 24 |
| Age <35 y | 28 |
| Age >35 y | 6 |
| Risk group | |
| Standard risk | 14 (41%) |
| High risk | 20 (59%) |
| Male:female sex | 26:8 |
| ECOG performance status >1 | 6 (17.6%) |
| White blood cell count ≥30 × 109/L | 7 (20.6%) (range, 30-70 × 109/L) |
| CNS involvement | 10 (29.4%) |
| Flow cytometry alone | 10 (29.4%) |
| Cytomorphology and flow cytometry | 5 (14.7%) |
| CD20%, median (range) | 75% (20%-100%) |
| Cytogenetic features | |
| Diploid | 12 (34%) |
| Hyperdiploidy | 9 (25%) |
| Hypodiploidy | 6 (17%) |
| Ploidy not possible due to absence of metaphases | 7 (20%) |
| Endoreduplication/masked hypodiploid | |
| t (1:19) | 1 (3%) |
| TCF3-PBX1 | 1 (3%) |
ECOG, Eastern Cooperative Oncology Group.
Outcomes
All enrolled patients except one (who developed renal failure before the start of study intervention) were included in the efficacy analysis (Table 2). Bone marrow MRD at EOI (week 4) was evaluable in 31 patients (not evaluable in 3 patients [due to loss to follow-up in 1 induction, death in 1, and uninterpretable in 1]). The percentage of MRD negativity (<1 × 10–4) at EOI was 70.9%. A total of 67.7% of patients had no detectable MRD at the end of induction, as assessed by high-sensitivity flow (1 in 10–6). In 9 patients who had EOI MRD >0.01%, MRD was repeated at the end of phase b (week 8). Of these 9 patients, bone marrow MRD was negative in 5 patients, resulting in MRD-negative rates of 88% post-consolidation. In the historical cohort, the EOI MRD negativity rates were 51.7%. Peripheral blood MRD on day 8 was available for 28 patients. The percentage of patients with peripheral blood MRD levels lower than 1 × 10−2 on day 8 of induction was 50%. Of 34 evaluable patients, 29 (85.3%) achieved CR with treatment. In the historical cohort, the CR rates were 70%.
Efficacy
| . | n/N (%) . |
|---|---|
| Early response to therapy MRD < 10–4 bone marrow blasts | |
| Poor peripheral blood day 8 blast clearance | 2/34 (5.9%) |
| Day 8 peripheral blood MRD <10−2 | 14/28 (50%) |
| Response to induction | |
| CR | 29/34 (85.3%) (3 induction failures, 1 induction death, and 1 lost to follow-up) |
| Induction failure | 3 (8.8%) |
| After induction phase 1a (week 4) | 22/31 (70.9%) (could not be evaluated in 3 patients [1 death, 1 lost to follow-up, and 1 uninterpretable]) |
| After induction phase 1a plus 1b (week 8) | 27/32 (84.3%) |
| After induction plus consolidation (week 16) | 28/32 (87.5%) |
| . | n/N (%) . |
|---|---|
| Early response to therapy MRD < 10–4 bone marrow blasts | |
| Poor peripheral blood day 8 blast clearance | 2/34 (5.9%) |
| Day 8 peripheral blood MRD <10−2 | 14/28 (50%) |
| Response to induction | |
| CR | 29/34 (85.3%) (3 induction failures, 1 induction death, and 1 lost to follow-up) |
| Induction failure | 3 (8.8%) |
| After induction phase 1a (week 4) | 22/31 (70.9%) (could not be evaluated in 3 patients [1 death, 1 lost to follow-up, and 1 uninterpretable]) |
| After induction phase 1a plus 1b (week 8) | 27/32 (84.3%) |
| After induction plus consolidation (week 16) | 28/32 (87.5%) |
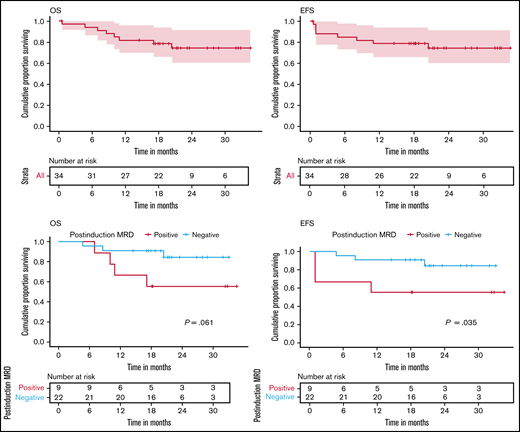
As of October 2020, the median follow-up was 21 months. Among these patients, the median CRD has not been achieved. At 21 months, CRD was 93.6%. At 21 months, EFS and OS were 78.8% (95% confidence interval [CI], 64.6-94) and 78.7% (95% CI, 65.8-94), respectively (Figure 3A-B). None of the patients underwent an allogeneic stem cell transplant.
Clinical outcomes. (A) Rate of OS. At 21 months, OS was 78.7% (95% CI, 65.8-94). (B) Rate of EFS. At 21 months, EFS was 78.8% (95% CI, 66-94). (C) Rate of OS at 21 months based on postinduction (week 4) MRD status: 55.6% (95% CI, 31-99.7) in MRD-positive group vs 90.9% (95% CI, 79.7-100) in the MRD-negative group. (D) Rate of EFS at 21 months based on postinduction (week 4) MRD status: 50% (95% CI, 31-99.7) in the MRD-positive group vs 90.9% (95% CI, 79.7-100) in the MRD-negative group. Censoring of data are indicated by vertical bars.
Clinical outcomes. (A) Rate of OS. At 21 months, OS was 78.7% (95% CI, 65.8-94). (B) Rate of EFS. At 21 months, EFS was 78.8% (95% CI, 66-94). (C) Rate of OS at 21 months based on postinduction (week 4) MRD status: 55.6% (95% CI, 31-99.7) in MRD-positive group vs 90.9% (95% CI, 79.7-100) in the MRD-negative group. (D) Rate of EFS at 21 months based on postinduction (week 4) MRD status: 50% (95% CI, 31-99.7) in the MRD-positive group vs 90.9% (95% CI, 79.7-100) in the MRD-negative group. Censoring of data are indicated by vertical bars.
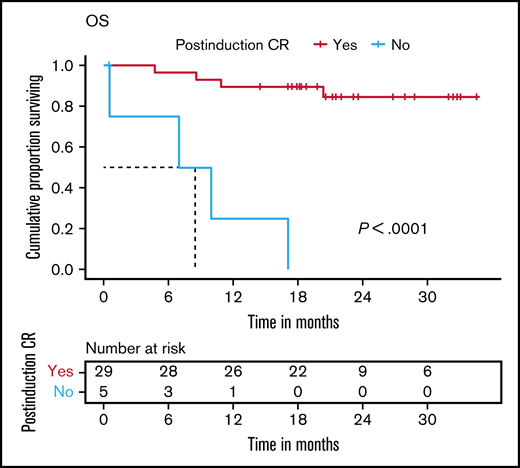
Postinduction persistent MRD was associated with inferior EFS and OS (Figure 3C-D). Failure to achieve CR at the end of induction was associated with inferior OS, with a median OS of 8.3 months (Figure 4). A total of 8 patients (23%) experienced an event. These included 3 induction failures (9%), 1 death during induction (3%), 1 relapse during maintenance (3%), and 3 deaths (9%) in remission (sepsis during reinduction, n = 1; meningitis, n = 1; and likely upper gastrointestinal bleed due to underlying portal hypertension, which could not be evaluated due to the restrictions during the COVID-19 pandemic, n = 1).
OS. At 21 months, OS was 89.7% (95% CI, 79.2-100) in patients who achieved CR postinduction (week 4) vs 0% in the patients who did not achieve CR. Censoring of data are indicated by vertical bars.
OS. At 21 months, OS was 89.7% (95% CI, 79.2-100) in patients who achieved CR postinduction (week 4) vs 0% in the patients who did not achieve CR. Censoring of data are indicated by vertical bars.
Toxicity
All 34 patients enrolled were evaluated for toxicity. The details of adverse events are shown in Table 3. Infections were the most common adverse events. Thirty grade 3/4 infectious episodes were documented during induction in 23 patients. Eleven patients developed probable fungal pneumonia as per European Organization for Research and Treatment of Cancer Mycoses study group criteria. These lesions responded to antifungal therapy as confirmed by computed tomography thorax imaging on follow-up. Microbiologically documented infections occurred in 10 patients (gram negative, n = 7; gram positive, n = 2; candidemia, n = 1). Febrile neutropenia without clinically documented infection and microbiologically documented infection was observed in 8 patients. Only 1 patient died during induction compared with 7 (7.9%) of 89 deaths in the historical cohort. Twenty-six percent of patients experienced neuropathy, but none developed grade 3/4 neuropathy. There was no grade 3/4 infusion reaction with the administration of rituximab. A total of 32% of patients experienced cytomegalovirus viremia during late intensification and maintenance therapy.
Adverse events in induction
| Event . | All patients (N = 34) . |
|---|---|
| Grade 3/4 infections | 23 patients 30 episodes MDI, n = 10 (gram negative, n = 7*; gram positive, n = 2†; candidemia, n = 1) Febrile neutropenia without MDI or CDI, n = 8 Probable fungal pneumonia, n = 11 Bacterial pneumonia, n = 1‡ |
| Pneumothorax | 1 |
| Pancreatitis | 1 |
| Grade 3/4 neuropathy | 0 |
| Cardiac arrhythmia | 1 |
| Septic cardiomyopathy | 1 |
| CNS bleed | 1 |
| GI bleed | 1 |
| Intestinal obstruction | 1 |
| Portal vein thrombosis | 1 (died during maintenance due to upper GI bleed) |
| Infusion reactions (rituximab-related) grade 3/4 | 0 |
| Tumor lysis syndrome | 3 |
| Death during induction (due to gram-negative sepsis) | 1 |
| Event . | All patients (N = 34) . |
|---|---|
| Grade 3/4 infections | 23 patients 30 episodes MDI, n = 10 (gram negative, n = 7*; gram positive, n = 2†; candidemia, n = 1) Febrile neutropenia without MDI or CDI, n = 8 Probable fungal pneumonia, n = 11 Bacterial pneumonia, n = 1‡ |
| Pneumothorax | 1 |
| Pancreatitis | 1 |
| Grade 3/4 neuropathy | 0 |
| Cardiac arrhythmia | 1 |
| Septic cardiomyopathy | 1 |
| CNS bleed | 1 |
| GI bleed | 1 |
| Intestinal obstruction | 1 |
| Portal vein thrombosis | 1 (died during maintenance due to upper GI bleed) |
| Infusion reactions (rituximab-related) grade 3/4 | 0 |
| Tumor lysis syndrome | 3 |
| Death during induction (due to gram-negative sepsis) | 1 |
CDI, clinically documented infection; GI, gastrointestinal; MDI, microbiologically documented infection.
Klebsiella pneumoniae, n = 2; Escherichia coli, n = 2; Pseudomonas aeruginosa, n = 2; Burkholderia cepacia, n = 1.
Group A Streptococcus.
Psuedomonas aeruginosa.
Discussion
This study evaluated the activity of bortezomib in combination with rituximab and chemotherapy in CD20+ Ph– precursor B-ALL cases to improve EOI MRD-negative rates.
The EOI MRD-negative response of 70.9% in the current study reflect an improvement over the other studies using pediatric-inspired chemotherapy alone or in combination with rituximab and chemotherapy intensification in patients with CD20+ precursor B-ALL.
In the CALGB 10403 study, which used pediatric-inspired chemotherapy alone, only 44% of the evaluated adolescent and young adult ALL patients achieved undetectable MRD.25 Similarly, in our historical cohort of CD20+ precursor B-ALL patients treated with modified BFM-90 alone, only 51.7% of patients could achieve MRD negativity (<1 × 10–4) postinduction.
The addition of rituximab to chemotherapy in CD20+ B-ALL was evaluated in Group for Research on Adult Acute Lymphoblastic Leukemia (GRALL) - 2005 and German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) - 07/2003 studies. Two doses of rituximab during induction in GRALL-R12 did not improve the postinduction MRD-negative responses compared with chemotherapy alone (65% vs 61%) in patients evaluated for MRD (40% of the trial population). The study used intensified anthracycline and cyclophosphamide during induction. The GMALL 07/2003-R study reported postinduction MRD-negative rates only in the standard-risk group. The addition of 1 dose of rituximab resulted in MRD-negative rates of 57% vs 27% with chemotherapy alone.11 The induction chemotherapy used dexamethasone and cyclophosphamide along with anthracycline intensification. In contrast to these studies, which used intensification of chemotherapy backbone, our study used dose-dense rituximab in combination with bortezomib and could achieve better and deeper MRD negativity (70.9% MRD-negative, with 67.7% of patients with undetectable MRD <1 × 10–6). As opposed to other studies, which evaluated MRD only in a proportion of patients enrolled in the trial and thus introduced the possibility of selection bias, the current study assessed MRD in all evaluable patients.
The post-consolidation MRD-negative rates (87.5%) in our study seem comparable to those of the other 2 studies, which reported week 16 MRD-negative rates of 90% and 91%.11,12 The CR rate in our study was 85.3%, as opposed to 70% in the historical cohort treated with modified BFM-90 alone. The CR rate in the current study, which included 59% high-risk patients, compares favorably to that reported in the high-risk subgroup of GMALL07/2003 (81%)11 and CALGB10403 (237 of 295 patients [80.3%]).25 The GRALL-R and modified hyper-CVAD and rituximab regimen reported higher CR rates than our study. This may be the result of chemotherapy intensification in these studies.
At a median follow-up of 21 months, the current study found EFS and OS rates of 78.8% (95% CI, 64.6-94) and 78.7% (95% CI, 65.8-94), respectively. Although the follow-up is short, these results compare favorably with other studies (Table 4), given that no patient in the current study received risk-stratified treatment intensification with high-risk blocks or allogeneic stem cell transplant. A total of 34% of patients treated with rituximab in GRAALL-2005 and 69% of high-risk patients in GMALL-07/2003 underwent transplant in CR1. These results were achieved with a fewer number of rituximab courses (ie, 5 doses in the current study, as opposed to 8-18 doses in other studies).
Outcomes in CD20+ precursor B-ALL treated with chemoimmunotherapy
| Parameter . | R-GMALL study (N = 181) . | Thomas et al study (N = 97) . | GRAALL-R study (N = 105) . | Our study (N = 34) . |
|---|---|---|---|---|
| Median age (range), y | NA (15-55) | 41 (13-83) | 40 (25-51) | 20 (15-52) |
| CNS positivity | NA | 16% | 7% | 29% |
| Chemotherapy, backbone and additional drug | GMALL with rituximab (comparator-GMALL chemotherapy) | Modified hyper-CVAD with rituximab (comparator-modified hyper-CVAD) | GRAALL-2005 regimen with rituximab (comparator-GRALL-2005 chemotherapy) | BFM-90 with rituximab and bortezomib (historical cohort treated with modified BFM-90 alone as comparator) |
| Total no. of rituximab infusions in induction | 1 | 2 | 2 | 5 |
| Total no. of rituximab infusions | 8 (induction and consolidation) | 12 (induction, intensification, and maintenance) | 16-18 All phases of treatment | 5 |
| Postinduction CR rate | 94% 81% in high-risk group (comparator arm- 91% in standard-risk and 88% in high-risk) | 92%-100% based on use of intensification (comparator arm, 94%) | 90% (89% in comparator arm) | 85.3% (70% in historical cohort) |
| Postinduction MRD level <10−4; evaluable patients/total) | 57% in standard-risk; Not reported (comparator arm- 27% in standard risk; Not reported); not reported for high-risk | 81% (at CR); 57/97 (compared with CD20– subset, 58%) | 65%; 49/105 (comparator arm- 61%; 36/104) | 70.9%; 31/34 (51.7%; 85/89 in historical cohort) |
| Day of bone marrow EOI MRD assessment | Day 24 | Day 21 | Day 29 | Day 29 |
| Post-consolidation MRD level <10−4 | 90% | NA | 91% | Postinduction plus consolidation, 87.5% |
| Consolidation time point | Week 16 | NA | Weeks 12-14 | Week 16 |
| MRD detection method | NA | Four-color MFC-sensitivity <1 × 10−4 | Multiplex real-time quantitative PCR Sensitivity at least 10−4 | 10-color MFC sensitivity 1 in 10−6 |
| Early death | 7% | NA | 7% | 3% |
| CR duration (%) | 5-year CRD, 80% | 3-year CRD, 67% | NA | 21-month CRD, 93.6% |
| 2-year EFS | NA | NA | 65% | Estimated 2-year EFS, 71.4% |
| OS (%) | 5 years, 71% | 3 years, 61% | 71% | Estimated 2-year OS, 71.4% |
| Grade 3/4 neuropathy | NA | NA | 4% (4/105) | 0 |
| Parameter . | R-GMALL study (N = 181) . | Thomas et al study (N = 97) . | GRAALL-R study (N = 105) . | Our study (N = 34) . |
|---|---|---|---|---|
| Median age (range), y | NA (15-55) | 41 (13-83) | 40 (25-51) | 20 (15-52) |
| CNS positivity | NA | 16% | 7% | 29% |
| Chemotherapy, backbone and additional drug | GMALL with rituximab (comparator-GMALL chemotherapy) | Modified hyper-CVAD with rituximab (comparator-modified hyper-CVAD) | GRAALL-2005 regimen with rituximab (comparator-GRALL-2005 chemotherapy) | BFM-90 with rituximab and bortezomib (historical cohort treated with modified BFM-90 alone as comparator) |
| Total no. of rituximab infusions in induction | 1 | 2 | 2 | 5 |
| Total no. of rituximab infusions | 8 (induction and consolidation) | 12 (induction, intensification, and maintenance) | 16-18 All phases of treatment | 5 |
| Postinduction CR rate | 94% 81% in high-risk group (comparator arm- 91% in standard-risk and 88% in high-risk) | 92%-100% based on use of intensification (comparator arm, 94%) | 90% (89% in comparator arm) | 85.3% (70% in historical cohort) |
| Postinduction MRD level <10−4; evaluable patients/total) | 57% in standard-risk; Not reported (comparator arm- 27% in standard risk; Not reported); not reported for high-risk | 81% (at CR); 57/97 (compared with CD20– subset, 58%) | 65%; 49/105 (comparator arm- 61%; 36/104) | 70.9%; 31/34 (51.7%; 85/89 in historical cohort) |
| Day of bone marrow EOI MRD assessment | Day 24 | Day 21 | Day 29 | Day 29 |
| Post-consolidation MRD level <10−4 | 90% | NA | 91% | Postinduction plus consolidation, 87.5% |
| Consolidation time point | Week 16 | NA | Weeks 12-14 | Week 16 |
| MRD detection method | NA | Four-color MFC-sensitivity <1 × 10−4 | Multiplex real-time quantitative PCR Sensitivity at least 10−4 | 10-color MFC sensitivity 1 in 10−6 |
| Early death | 7% | NA | 7% | 3% |
| CR duration (%) | 5-year CRD, 80% | 3-year CRD, 67% | NA | 21-month CRD, 93.6% |
| 2-year EFS | NA | NA | 65% | Estimated 2-year EFS, 71.4% |
| OS (%) | 5 years, 71% | 3 years, 61% | 71% | Estimated 2-year OS, 71.4% |
| Grade 3/4 neuropathy | NA | NA | 4% (4/105) | 0 |
hyper-CVAD, hyper-fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; MFC, multicolor flow cytometer; NA, data not available; PCR, polymerase chain reaction.
Peripheral blood MRD <1% by day 8 has been used as one of the prognostic factors in pediatric ALL studies.26,27 Our study used both bortezomib and rituximab during induction, using the drug synergy in the initial phase of therapy to achieve early and deep responses. This end point was used as an indicator of the in vivo activity of this combination in the first 7 days of induction, as no drug other than prednisolone was administered during this period. Achievement of peripheral blood MRD <1% by day 8 in 50% of patients further supports this hypothesis.
There were a higher number of grade 3/4 infection episodes in the current study. Eleven patients developed probable fungal pneumonia. Although these are much higher than other studies using rituximab in ALL, a very similar trend has been seen in our patients undergoing induction therapy for acute leukemia and adolescent and young adult ALL.18,21,22 Higher infection rates compared with the reported literature did not increase induction mortality than the reported literature (3%-7%).11,12,25 We observed no grade 3 or higher neuropathy despite combining 2 potentially neurotoxic drugs. This finding was likely due to the subcutaneous administration of bortezomib and the avoidance of concurrently using these drugs except on day 8 of induction.
The incidence of rituximab infusion reactions was 82% with the first dose without any grade 3/4 reactions. The incidence of infusion reaction was negligible in subsequent doses, suggesting an association with the disease burden.
In our study, cytomegalovirus viremia developed in 32% of patients, predominantly during the re-intensification and maintenance phase. The incidence of cytomegalovirus reactivation was higher in the current study than in our earlier study: 32% vs 11%, respectively.28 The higher incidence may be due to the T-cell immunosuppressive effects of bortezomib.29
This study, which enrolled a uniform cohort of patients and performed MRD assessment in all evaluable patients, could achieve its primary end point of improving the MRD rates postinduction. There was a trend toward improvement in survival; however, given the phase 2 design, it needs to be explored further. A limitation of this study is the inability to differentiate the benefit of bortezomib and rituximab combination over rituximab alone due to the lack of a comparator arm with rituximab alone.
The results of the current study are relevant to the resource-constrained setting in which the feasibility of treatment intensification or targeted therapy (eg, blinatumomab, inotuzumab) in patients with postinduction MRD positivity is limited, either due to high risk of treatment-related morbidity and mortality or poor access and affordability. Use of the proposed regimen can improve postinduction MRD-negative rates without increasing toxicity and can translate into better long-term outcomes. We have planned to further evaluate the combination in a phase 3 trial with rituximab and chemotherapy as the comparator for the CD20+ subset of B-ALL.
In summary, the combination of bortezomib and rituximab with a pediatric-inspired regimen is active in CD20+ Ph– B-ALL without any new safety concerns.
Acknowledgments
The authors acknowledge the help of Mangesh Kadam in study conduct and data capturing.
This study was supported by intramural funding from Tata Memorial Centre Research Administrative Council (TRAC/0917/1731/001).
Authorship
Contribution: H.J., M.S., and P.T. designed and implemented the study; H.J., M.S., J.T., P.T., A.B., L.N., P.G.S., B.B., N.S., H.G., N.P., and S.G. contributed to gathering the data, documentation, and the follow-up process; H.J., M.S., V.B.G., J.T., P.T., D.S., A.B., L.N., P.G.S., N.P., and S.G. planned and conducted data analysis; P.T., P.G.S., D.S., N.P., and S.G. headed the conduct of laboratory tests and interpretation of the tests; H.J., M.S., V.B.G., J.T., P.T., D.S., A.B., L.N., P.G.S., and B.B. drafted the manuscript, with important contributions from N.P. and S.G.; and H.J., M.S., V.B.G., J.T., P.T., D.S., A.B., L.N., P.G.S., N.P., and S.G. contributed to critical revision of the manuscript; and all authors reviewed the manuscript and approved the final version for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manju Sengar, Adult Hematolymphoid Unit, Department of Medical Oncology, Tata Memorial Centre, Affiliated to Homi Bhabha National University, E Borges Road, Mumbai, 400 012, Maharashtra, India; e-mail: manju.sengar@gmail.com.
References
Author notes
Requests for data sharing may be submitted to the corresponding author (Manju Sengar; e-mail: manju.sengar@gmail.com).