Key Points
Letermovir is highly effective at preventing CS-CMVi by day +98 after umbilical CBT.
Delayed-onset CS-CMVi after stopping letermovir mandates close monitoring and supports investigation of extended prophylaxis after CBT.
Abstract
Cytomegalovirus (CMV)-seropositive umbilical cord blood transplantation (CBT) recipients have a high incidence of CMV-associated complications. There are limited data regarding the efficacy of letermovir for preventing clinically significant CMV infection (CS-CMVi), and the impact of letermovir prophylaxis on delayed-onset CMV reactivation after letermovir discontinuation, in CBT recipients. We compared the cumulative incidence of CS-CMVi and CMV detection in 21 CMV-seropositive CBT recipients receiving letermovir prophylaxis with a historical cohort of 40 CBT recipients receiving high-dose valacyclovir prophylaxis. Letermovir was administered on day +1 up to day +98. The cumulative incidence of CS-CMVi was significantly lower by day 98 in the letermovir cohort (19% vs 65%). This difference was lost by 1 year due to a higher incidence of delayed-onset CMV reactivation in the letermovir cohort. No patients developed CMV disease in the letermovir cohort within the first 98 days compared with 2 cases (2.4%) in the high-dose valacyclovir cohort; 2 patients developed CMV enteritis after discontinuing letermovir. Median viral loads were similar in both cohorts. Thus, letermovir is effective at preventing CS-CMVi after CBT, but frequent delayed-onset infections after letermovir discontinuation mandate close monitoring and consideration for extended prophylaxis.
Introduction
Umbilical cord blood transplantation (CBT) recipients are at high risk for cytomegalovirus (CMV) reactivation and morbidity from the virus and its treatment.1-6 High-dose valacyclovir reduces the frequency and severity of CMV reactivation and associated complications in high-risk hematopoietic cell transplant (HCT) recipients,7 but most patients still require preemptive treatment.1,2,8-10 Prophylactic letermovir for the first 98 days after allogeneic HCT in CMV-seropositive adults ≥18 years old significantly reduces the incidence of clinically significant CMV infections (CS-CMVi).11 Although delayed reactivation that occurs in the subsequent 98 days after stopping letermovir was observed in the pivotal trial, there remains an overall reduction in the cumulative incidence of CS-CMVi.11 Similar findings of delayed reactivation occur in the setting of ganciclovir prophylaxis.12 This phenomenon may be in part due to reduced CMV-specific immune reconstitution resulting from lack of exposure to CMV antigens.13,14 CBT recipients may be at particularly increased risk for delayed-onset CMV given slower immune reconstitution compared with other cell sources.15-17 The efficacy of letermovir for CMV prophylaxis after CBT is not well described, and few were included in the phase 3 trial.11,18 We compared CMV reactivation and disease through 1 year after CBT among patients who received letermovir vs high-dose valacyclovir for CMV prophylaxis.
Methods
We analyzed consecutive CMV-seropositive CBT recipients ≥18 years old at Fred Hutchinson Cancer Research Center who received letermovir for CMV prophylaxis between October 2018 and December 2019. We compared these patients to a historical cohort of consecutive CMV-seropositive CBT recipients between 2014 and 2017 who received an optimized prophylactic strategy consisting of high-dose valacyclovir for CMV prophylaxis as previously described.10 Patients receiving treatment of CMV at the time of transplantation were excluded. Transplantation practices are described in the supplemental methods.
All patients were monitored for CMV reactivation with twice-weekly plasma CMV polymerase chain reaction testing through day +98 and weekly thereafter; test characteristics are described in the supplemental methods. In the letermovir cohort, letermovir was started on day +1 up to day +98. Preemptive therapy was started for CMV viral loads ≥150 IU/mL between days 1 and 98 and ≥500 IU/mL thereafter; no patients received letermovir beyond day +98 or for secondary prophylaxis. In the high-dose valacyclovir cohort, patients received 2 g of valacyclovir every 8 hours for the first 98 days, after which patients with prior CMV reactivation were recommended to take valganciclovir 900 mg once daily for 1 year; patients who could not tolerate valganciclovir continued high-dose valacyclovir. In these patients, preemptive therapy was started for any detectable CMV between days 1 and 98 or ≥250 IU/mL thereafter.
We compared the cumulative incidence of CS-CMVi, defined as CMV disease or CMV viremia leading to preemptive treatment, through 1-year post-HCT; death and second HCT were competing risks. Delayed-onset CMV was defined as CS-CMVi occurring after day 98. The study was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board; all participants provided informed consent.
Results and discussion
Twenty-four patients received a CBT during the study period; 3 were excluded due to CMV viremia at the time of CBT (n = 2) or for not receiving letermovir (n = 1). Patients received letermovir for a median of 97 days (interquartile range [IQR], 88-98). We compared this cohort to 40 CBT recipients who received high-dose valacyclovir for CMV prophylaxis as previously described.10 Characteristics are described in Table 1.
Characteristics of CMV-seropositive patients undergoing CBT, stratified by CMV prevention strategy
| . | Letermovir (N = 21) . | High-dose valacyclovir (N = 40) . | P* . |
|---|---|---|---|
| Age, y, median (IQR) | 43.0 (39.2-55.5) | 25.8 (9.8-37.6) | <.001 |
| Sex | .19 | ||
| Female | 10 (48) | 26 (65) | |
| Male | 11 (52) | 14 (35) | |
| Transplantation year, range | 2018-2019 | 2014-2017 | |
| Number of donors | .21 | ||
| 1 | 5 (24) | 16 (40) | |
| 2 | 16 (76) | 24 (60) | |
| HLA disparity† | .875 | ||
| 4/6 | 16 (76) | 32 (80) | |
| 5/6 | 4 (19) | 6 (15) | |
| 6/6 | 1 (5) | 2 (5) | |
| Conditioning regimen | .27 | ||
| Myeloablative | 19 (90) | 39 (98) | |
| Nonmyeloablative | 2 (10) | 1 (3) | |
| Total nucleated cell dose (×107/kg), median (IQR) | 4.8 (4.0-5.4) | 7.2 (5.4-10.9) | .003 |
| Diagnosis | .005 | ||
| Acute lymphoblastic leukemia | 5 (24) | 21 (53) | |
| Acute myelogenous leukemia | 6 (29) | 13 (33) | |
| Myelodysplastic syndrome | 5 (24) | 0 (0) | |
| Other | 5 (24) | 6 (15) | |
| Acute graft-versus-host disease | .10 | ||
| Grades 0 to 1 | 9 (43) | 10 (25) | |
| Grades 2 to 4 | 11 (52) | 30 (75) | |
| Missing | 1 (5) | 0 (0) | |
| Absolute lymphocyte count (cells/mm3) at day +98, median (IQR)‡ | 650 (450-1008) | 645 (392-980) | .48 |
| Antiviral duration for CS-CMVi, d, median (IQR) | |||
| Day 0 to 98 period | 26 (21.5-33.5) | 52.5 (43-63.5) | .03 |
| Day 99 to 365 period | 28 (25-50) | 25.5 (20-40) | .2 |
| . | Letermovir (N = 21) . | High-dose valacyclovir (N = 40) . | P* . |
|---|---|---|---|
| Age, y, median (IQR) | 43.0 (39.2-55.5) | 25.8 (9.8-37.6) | <.001 |
| Sex | .19 | ||
| Female | 10 (48) | 26 (65) | |
| Male | 11 (52) | 14 (35) | |
| Transplantation year, range | 2018-2019 | 2014-2017 | |
| Number of donors | .21 | ||
| 1 | 5 (24) | 16 (40) | |
| 2 | 16 (76) | 24 (60) | |
| HLA disparity† | .875 | ||
| 4/6 | 16 (76) | 32 (80) | |
| 5/6 | 4 (19) | 6 (15) | |
| 6/6 | 1 (5) | 2 (5) | |
| Conditioning regimen | .27 | ||
| Myeloablative | 19 (90) | 39 (98) | |
| Nonmyeloablative | 2 (10) | 1 (3) | |
| Total nucleated cell dose (×107/kg), median (IQR) | 4.8 (4.0-5.4) | 7.2 (5.4-10.9) | .003 |
| Diagnosis | .005 | ||
| Acute lymphoblastic leukemia | 5 (24) | 21 (53) | |
| Acute myelogenous leukemia | 6 (29) | 13 (33) | |
| Myelodysplastic syndrome | 5 (24) | 0 (0) | |
| Other | 5 (24) | 6 (15) | |
| Acute graft-versus-host disease | .10 | ||
| Grades 0 to 1 | 9 (43) | 10 (25) | |
| Grades 2 to 4 | 11 (52) | 30 (75) | |
| Missing | 1 (5) | 0 (0) | |
| Absolute lymphocyte count (cells/mm3) at day +98, median (IQR)‡ | 650 (450-1008) | 645 (392-980) | .48 |
| Antiviral duration for CS-CMVi, d, median (IQR) | |||
| Day 0 to 98 period | 26 (21.5-33.5) | 52.5 (43-63.5) | .03 |
| Day 99 to 365 period | 28 (25-50) | 25.5 (20-40) | .2 |
Data are presented as no. (%) unless otherwise indicated.
χ2 test and Fisher's exact test were used for categorical variables, as appropriate; Wilcoxon rank-sum test was used to compare medians for continuous variables.
For recipients of 2 cord blood units, the HLA matching reflects the unit with the lowest match.
Data included for patients from each cohort with results within 7 d of day +98 (n = 19 and 38, respectively). Among the 18 individuals in the letermovir cohort with CMV monitoring data beyond day +98, absolute lymphocyte count values were similar among those who subsequently developed CS-CMVi (n = 9; 830 cells/mm3; IQR, 610-1230) compared with those who did not (n = 9; 500 cells/mm3; 325-895).
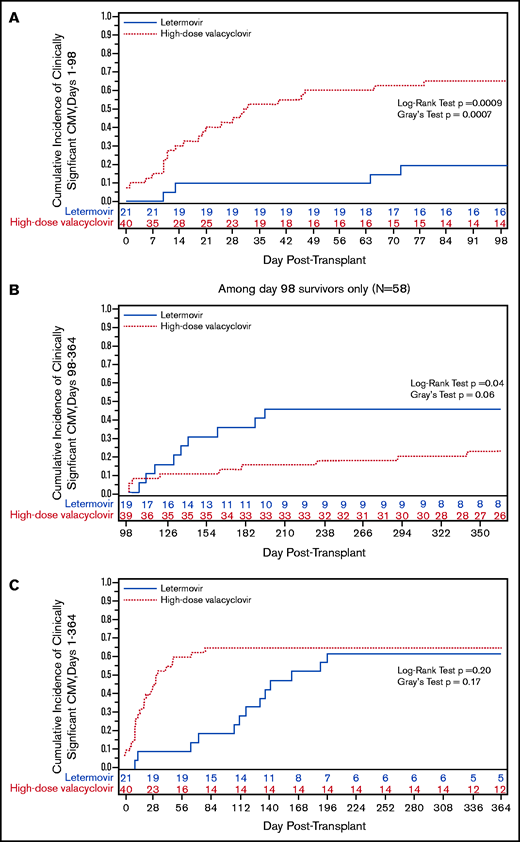
The cumulative incidence of CS-CMVi was significantly lower by day +98 in the letermovir cohort (Figure 1A). Only 4 patients (19%) were treated for CMV in the letermovir cohort compared with 26 patients (65%) in the high-dose valacyclovir cohort, a 71% reduction in CMV-specific antiviral therapy. Treatment was prompted by viremia with low-peak viral loads (range, 150-880 IU/mL) in all 4 letermovir patients, 2 of whom were receiving corticosteroids. However, there was a higher incidence of delayed-onset CS-CMVi in the letermovir cohort in the first 3 months after stopping letermovir (Figure 1B), resulting in a similar incidence in both groups by 1 year (Figure 1C).
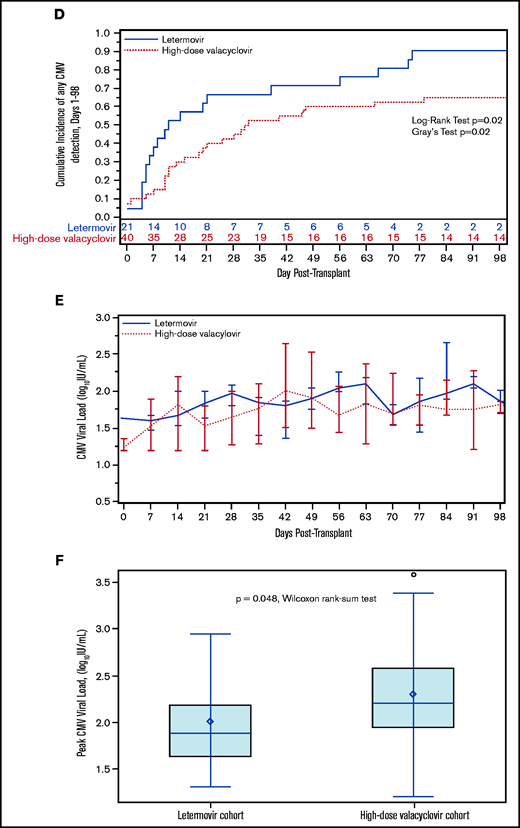
We also explored the incidence and kinetics of plasma CMV detection. The cumulative incidence of CMV detection in the first 98 days appeared to be higher in the letermovir cohort (Figure 1D) with similar median time to first CMV detection in the letermovir (10 days; IQR, 5-38) and high-dose valacyclovir cohorts (17 days; IQR, 10-31). Median weekly viral loads were similar in both cohorts (Figure 1E). Median peak viral loads (Figure 1F) were lower in the letermovir cohort, despite more permissive viral load thresholds before starting preemptive therapy.
No patients developed CMV disease in the letermovir cohort by day +98 compared with 2 patients (2.4%) in the high-dose valacyclovir cohort. Among 18 patients in the letermovir cohort with data beyond day +98, 9 patients (50%) developed CS-CMVi and 2 patients (11%) developed CMV enteritis, all within 3 months after discontinuing letermovir. None of these patients had CS-CMVi prior to day +98, but 6 patients (67%) had low-level viremia (<150 IU/mL). In comparison, 8 of 36 patients (22%) in the high-dose valacyclovir cohort with data beyond day +98 had CS-CMVi, and 2 patients (2.4%) developed CMV enteritis. CMV treatment was longest when started during days 0 to 98 in the high-dose valacyclovir cohort (Table 1). There were no CMV-attributable deaths in either cohort.
We found that CMV-seropositive CBT recipients receiving letermovir prophylaxis had a decreased incidence of CS-CMVi in the first 98 days post-CBT compared with patients receiving high-dose valacyclovir. However, this benefit was lost within the subsequent 3 months after discontinuing letermovir, and the overall incidence of CS-CMVi was similar in both cohorts by day 180. This increase in delayed-onset CMV reactivation following letermovir discontinuation was more pronounced than observed in the phase 3 trial, which only randomized 12 CBT recipients.11 These findings support an extended duration of monitoring, and ideally prophylaxis, after CBT.
This is one of the first studies to report the incidence of CS-CMVi following discontinuation of letermovir prophylaxis in CBT recipients. Two studies with similar numbers of CBT recipients also demonstrated a decrease in CS-CMVi in patients receiving letermovir vs high-dose valacyclovir, and 1 study with follow-up through day 180 suggested higher delayed-onset reactivation in the letermovir cohort.18,19 In these studies and ours, the letermovir cohorts had lower median peak viral loads compared with controls, and no patients in the letermovir cohorts developed CMV disease in the first 98 days.
Delayed onset of CS-CMVi in patients receiving prophylaxis has been demonstrated in HCT and solid organ transplant recipients receiving prophylactic ganciclovir, a finding associated with decreased CMV-specific T-cell responses, perhaps due to reduced viral exposure.12,13,20,21 A recent study of HCT recipients receiving letermovir (N = 56, including 10 CBT who overlap with this study) vs historical controls (N = 93, 1 CBT) demonstrated that day +90 polyfunctional CMV-specific T-cell responses were decreased in letermovir recipients, and increased CMV-specific T-cell polyfunctionality was associated with a lower risk of subsequent CS-CMVi.14 These concepts are supported by our finding that none of the individuals with delayed-onset CS-CMVi after letermovir discontinuation had CS-CMVi by day +98. Thus, monitoring CMV-specific immunity prior to stopping letermovir in CBT recipients may be important for risk-stratification.22
In conclusion, we demonstrate that letermovir is highly effective at preventing CS-CMVi after CBT, but frequent delayed-onset infections after letermovir discontinuation mandate close monitoring. These data underscore the importance of determining the utility of extended letermovir prophylaxis, and a randomized trial is underway (#NCT03930615).23 Until those data are available, CBT recipients require continued weekly surveillance for CS-CMVi after stopping letermovir. Extended letermovir prophylaxis through day +365 can be considered with close monitoring for toxicities; if unavailable, we recommend high-dose valacyclovir during this period.
Acknowledgments
The authors acknowledge Chris Davis and Jessica Morris for help obtaining data from electronic databases.
This work was supported by NIH/NCI Cancer Center Support Grant P30CA0087-48.
Authorship
Contribution: J.A.H., M.B., and F.M. designed the study; J.A.H., H.X., and W.M.L. analyzed the data and created the figures; J.A.H., F.M., L.A.T., S.A.P., A.D., C.D., and D.Z. collected the data; J.A.H wrote the first draft and all authors contributed to the writing and revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: J.A.H. received consulting fees from Gilead Sciences, Amplyx, Allovir, Allogene Therapeutics, CRISPR Therapeutics, and Takeda and research funding from Takeda, Allovir, Karius, and Gilead Sciences. M.B. received research funding from Merck and Gilead and received consulting fees from SymBio Pharmaceuticals, Merck, Gilead, Evrys Bio (also ownership options), Helocyte (also ownership options), and AlloVir. C.D. receives salary from Deverra Therapeutics and has equity in Deverra Therapeutics. S.A.P. reports grant support from Global Life Technologies, Inc, participates in research trials with Chimerix, Inc and Merck & Co, and currently participates in a clinical trial sponsored by NIAID (U01-AI132004); vaccines for this trial are provided by Sanofi-Aventis, all outside of this submitted work. The remaining authors declare no competing financial interests.
Correspondence: Joshua Aiden Hill, Assistant Professor, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail Stop E-400, Seattle, WA 98109; e-mail: jahill3@fredhutch.org.
References
Author notes
For original data, please contact jahill3@fredhutch.org.
The full-text version of this article contains a data supplement.