TO THE EDITOR:
Central nervous system (CNS) relapses are rare events in diffuse large B-cell lymphoma (DLBCL) that typically occur very early in the disease course, leading to poor survival rates.1-3 Several identified risk factors include number and location of extranodal sites2,4; factors identified by the 2016 CNS International Prognostic Index (CNS-IPI)3; biologically, MYC gene rearrangements and double-/triple-hit lymphoma,5-7 and more recently hypothesized specific genomic subtypes (eg, MYD88L265P and CD79B mutations).8
Prophylaxis for CNS relapses currently employs methotrexate (MTX), a blood–brain barrier–penetrating drug,9 although the efficacy of intrathecal (IT) MTX is debated.10,11 High-dose intravenous (HDIV) MTX seems more effective, especially in younger patients, but with significant toxicity/intolerability, notably in older patients with complex comorbidities and/or impaired renal function.12-14 Identification of alternative agents that lower the incidence of CNS relapses is needed.15
Lenalidomide (LEN) is a blood–brain barrier–penetrating immunomodulatory agent16 with demonstrated efficacy in relapsed DLBCL17 and primary CNS lymphoma18-20 but disparate results when combined upfront with R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone).21,22 Here, we analyzed the role of LEN maintenance post-R-CHOP, with the objectives of assessing the risk of CNS relapse, the role of CNS prophylaxis, and risk factors associated with CNS relapse.
We present a post hoc analysis of the multicenter, double-blinded, randomized, phase 3 REMARC trial (NCT01122472).23 Previously untreated patients with DLBCL were aged 60 to 80 years, Eastern Cooperative Oncology Group 0 to 2, Ann Arbor stage II to IV, and age-adjusted IPI ≥1. All randomized patients with a partial response (PR) or complete response (CR) after first-line R-CHOP received maintenance LEN 25 mg/day (days 1-21/28) vs placebo (PBO) for 2 years. Prophylactic IT MTX 15 mg (4-6 cycles) was given per local practice/investigator discretion. Two cycles of HDIV MTX 1.5 g/m2 were allowed 3 and 6 weeks after day 1 of the last R-CHOP cycle. CNS relapses were documented upon occurrence of CNS symptoms and localized after standardized staging evaluation per central radiological and/or pathological review. Cell-of-origin (COO) was determined by immunohistochemistry and molecular testing. Statistical evaluations (using SAS 9.3) and additional methods are provided in supplemental Methods. This study was approved by all relevant institutional review boards and/or ethics committees, and all human participants gave written informed consent.
Overall, 323 out of 650 REMARC patients (50%) had >1 extranodal site involved (for baseline characteristics, see supplemental Table 1), including sites commonly associated with CNS relapses (5% renal, 4% adrenal, 3% testis, 2% breast, and 0.6% ovarian). CNS-IPI risk groups included 5% low risk, 61% intermediate risk, and 35% high risk. Of 650 randomized patients, 333 (51%) received CNS prophylaxis (n = 324 IT MTX [n = 164 LEN; n = 160 PBO], n = 6 HDIV MTX [n = 4 LEN; n = 2 PBO], and n = 3 PBO patients both IT and HDIV MTX); 67% of patients received 4 IT MTX administrations (median, 4; range, 1-8).
With a median 81.1-month follow-up (6.75 years), 221 patients (34%) relapsed, including 22 out of 221 patients (10%) with CNS-specific relapse (n = 12 parenchymal site only, n = 2 parenchymal and meningeal site, and n = 8 unspecified CNS site). CNS was the only site of relapse for 13 patients, but part of systemic relapse in 9 patients. Median time from diagnosis to CNS occurrence was 19.9 months (95% confidence interval [CI], 13.3-101.4). At the time of CNS relapse, 8 out of 22 patients were on maintenance therapy (n = 6 LEN; n = 2 PBO). At the end of R-CHOP, 5 patients were in PR, and 17 patients were in CR.
Baseline characteristics for 22 patients with CNS relapse were 96% stage III to IV, 64% elevated lactate dehydrogenase (LDH), 64% high age-adjusted IPI 2 or 3, 64% >1 extranodal site involved, and 55% intermediate-risk or 45% high-risk CNS-IPI (supplemental Table 1). Where available, COO classification was 10 out of 16 patients (63%) with activated B-cell (ABC)-like DLBCL subtype, 6 out of 11 (55%) MYC and 9 out of 11 (82%) BCL2 positive expressions, and 5 out of 11 (45%) MYC/BCL2 double expressors (no detectable MYC/BCL2 rearrangements).
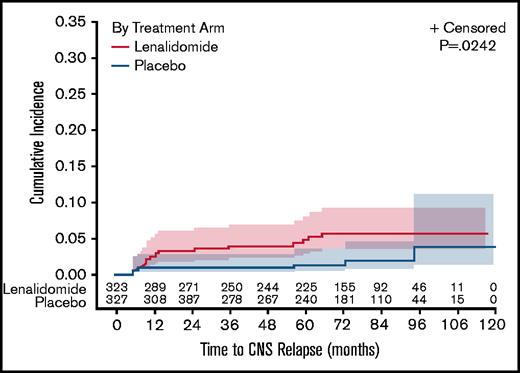
Based on maintenance treatment, the CNS relapse incidence was 16/323 (5%) with LEN and 6/327 (2%) with PBO (P = .0242; Figure 1). Median time to CNS relapse was 12.2 months for LEN (95% CI, 8-36) and 32 months for PBO (95% CI, 5-79). Estimated 2-year CNS relapse rates were 3.3% for LEN vs 0.9% for PBO.
Estimated risk of CNS relapse according to the maintenance treatment arm: LEN compared with PBO.
Estimated risk of CNS relapse according to the maintenance treatment arm: LEN compared with PBO.
According to CNS-IPI, the estimated 2-year CNS relapse risk was 0% for low risk, 1.9% (95% CI, 0.9-4.0) for intermediate risk, and 3% (95% CI, 1.4-6.6) for high risk (P = .5109; supplemental Figure 1A). In the LEN arm, estimated 2-year CNS relapse rates were 0%, 3.2%, and 4.1% for the respective groups (P = .6552; supplemental Figure 1B). In the PBO arm, estimated 2-year CNS relapse rates were 0%, 0.6%, and 1.9% for the respective groups (P = .7312; supplemental Figure 1C).
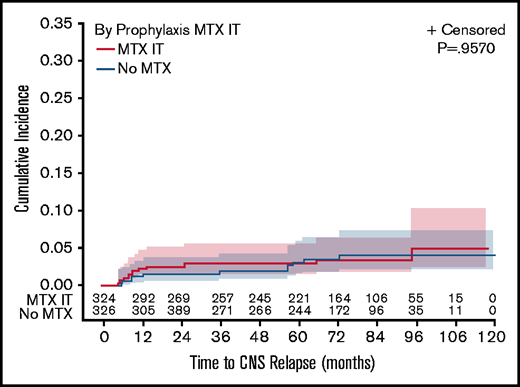
According to CNS prophylaxis use, CNS relapse risk was no different between patients receiving CNS prophylaxis and those receiving none overall (3.3% vs 3.5%, P = .9331), LEN-treated patients (4.8% vs 5.2%, P = .9332), or those receiving PBO (1.8% vs 1.9%, P = .9211). Estimated 2-year CNS relapse rates were 2.6% for patients receiving IT MTX only and 1.6% for those receiving no CNS prophylaxis (P = .9570; Figure 2). Estimated 2-year CNS relapse rate was 2.5% for patients receiving any CNS prophylaxis (IT or IV) and 1.6% for patients receiving none (P = .9331; supplemental Figure 2).
Estimated risk of CNS relapse according to the CNS prophylaxis. CNS relapse risk was evaluated based on the use of IT MTX only compared with no CNS prophylaxis.
Estimated risk of CNS relapse according to the CNS prophylaxis. CNS relapse risk was evaluated based on the use of IT MTX only compared with no CNS prophylaxis.
Focusing on 85 patients with ≥1 involved extranodal site, estimated 2-year CNS relapse risk was 2.6% vs 2.0% for those with no extranodal involvement (P = .0253; supplemental Figure 3). The estimated 2-year CNS relapse risk showed no significance based on treatment (4.8% for LEN vs 0% for PBO; P = .1589). CNS prophylaxis was given in 56 of 85 patients; estimated 2-year CNS relapse risk was 2.1% for those receiving CNS prophylaxis vs 3.6% for patients receiving none (P = .5542).
Based on univariate analysis, an increased risk of CNS occurrence was associated with IPI 3 to 5 (hazard ratio [HR], 4.016), elevated LDH (HR, 3.194), ABC-like DLBCL (by NanoString; HR, 3.875), and extranodal involvement (HR, 0.358; supplemental Table 2). Neither LEN maintenance nor CNS prophylaxis reduced CNS relapse risk. By multivariable analysis, only ABC-DLBCL subtype (HR, 3.519; P = .0367) was independently predictive of CNS relapse occurrence. In a selected multivariate model including LDH and GCB/ABC profile, only ABC-DLBCL subtype remained associated with significantly higher CNS relapse risk, independent of treatment or CNS prophylaxis (HR, 3.813; P = .024)
Median progression-free survival (PFS) was not reached in the low-risk group, comprising only 30 patients (supplemental Figure 4A). Median PFS was 87.7 and 67.2 months in the intermediate- and high-risk groups, respectively. Median overall survival was not reached in any CNS-IPI group (supplemental Figure 4B). Patients with high CNS-IPI vs low-CNS-IPI had significantly worse PFS (HR, 2.09 [95% CI, 1.1-4.1]; P = .035) and overall survival (HR, 2.70 [95% CI, 1.1-6.7]; P = .032).
CNS relapse in DLBCL remains a rare but dramatic, often-fatal event in the era of modern immunochemotherapy. The incidence of CNS relapse was 3% here (5% LEN; 2% PBO), corresponding with expected rates in this patient population.1-3,24 Although CNS relapses typically occur earlier in the DLBCL disease course,25 here, the lack of effect with LEN may be reflective of weak activity (limited durability) due to its late administration as maintenance or LEN dosing discontinuations/reductions to manage toxicity, or perhaps it lost clinical benefit over time. In this report, few CNS relapses occurred late, perhaps also due to the selection of responding patients to R-CHOP and exclusion of primary refractory disease. Limitations of this study are based on the inherent nature of post-hoc analyses in a small number of patients with CNS relapse (as well as in additional subgroup analyses), thus making interpretation of these results challenging.
In summary, LEN maintenance given for 2 years after R-CHOP in older patients with DLBCL and ≥1 IPI risk factor was not associated with lower rates of CNS relapses. Our study results failed to establish a clear benefit from IT or HDIV MTX in de novo DLBCL, and we acknowledge that randomized, prospective analyses are needed to best define the effect of these and other prophylactic strategies. Among risk factors associated with CNS relapses, high CNS-IPI and ABC-like subtype were independently associated with higher CNS relapse risk, as previously shown in GOYA.5 These analyses support inclusion of CNS-IPI and further validation of COO in assessing patient risk for developing CNS relapse.
Acknowledgments
The authors thank all the patients and families; the Lymphoma Study Association and Lymphoma Academic Research Organization REMARC team, including physicians, statisticians Samuel Griolet and Loic Chartier, pathologists, and biologists; and Celgene for the support and Bio Connections. This study was conducted in 9 countries (Australia, Austria, Belgium, France, Israel, Poland, Portugal, Spain, and Switzerland) and sponsored by the Lymphoma Academic Research Organization of France and Australasian Leukaemia and Lymphoma Group of Australia. Administrative support was provided by the Lymphoma Study Association Recherche Clinique.
Funding was provided by Celgene Corporation, Summit, NJ.
Authorship
Contribution: C.T. and S.B. contributed to conception and design; C.T. and S.B. contributed to collection and assembly of data; and all authors provided study material or patients, contributed to data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: S.B., Janssen received travel compensation; H.G., Celgene, Gilead, Janssen, and Roche is a member of the advisory board; R.-O.C., AbbVie, Amgen, Gilead, MSD, Roche, and Takeda received honoraria, is a member of the advisory board and received travel compensation; M.G.d.S., AbbVie, Gilead, Janssen, and Roche is a member of the advisory board and received travel compensation; P.F., AbbVie, AstraZeneca, Gilead, Janssen, and Roche received honoraria, is a member of the advsiory board and received travel compensation; F.M., AbbVie, Celgene, Epizyme, Genentech, Gilead, Janssen, Roche, and Servier received honoria, is a memeber of the advisory board and received travel compensation; J.T., Celgene, BeiGene, PCYC, Roche, and Takeda received honoria, is a member of the advisory board and received travel compensation; R.G., AbbVie, AstraZeneca, BMS, Celgene, Gilead, Janssen, MSD, Novartis, Roche, and Takeda received honoraria, is a member of the advisory board and received travel compensation; A.M.G.-S. received honoraria from Celgene/BMS, Gilead, Janssen, Roche, Servier, and Takeda and consulting fees from Celgene/BMS, Clinigen, Eusa Pharma, Gilead, Kyowa Kirin, Morphosys, Novartis, Roche, and Servier received honoraria, is a member of the advisory board and received travel compensation; C.H., Amgen, Celgene, Gilead, Incyte, Janssen, Milteny, Novartis, Roche, Servier, and Takeda received honoraria, is a member of the advisory board and received travel compensation; C.T., AbbVie, Amgen, Bayer, BMS, Celgene, Cellectis, Hospira, Incyte, Kite, Novartis received honoraria, is a member of the advisory board and received travel compensation, and Roche; S.Griolet, S. Grosicki, K.V.E., L.R., and C.C.B. declare no competing financial interests.
Correspondence: Catherine Thieblemont, Hôpital Saint-Louis - Service d’Hémato-Oncologie, 1, Avenue Claude Vellefaux, 75010 Paris, France; e-mail: catherine.thieblemont@aphp.fr.
References
Author notes
Data requests may be submitted to the Lymphoma Study Association to the direction of Loic Chartier (e-mail: loic.chartier@lysarc.org) and must include a description of the research proposal.
The full-text version of this article contains a data supplement.