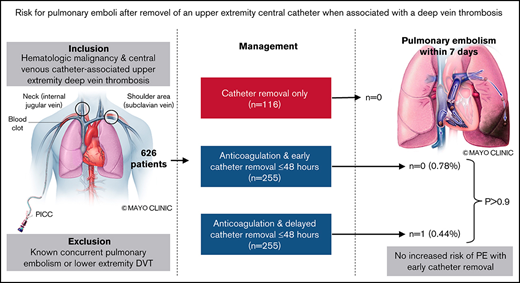
Key Points
In anticoagulated patients with UE-DVT, PE within 7 days occurred in 0.78% vs 0.44% with early vs delayed catheter removal.
Catheter removal within 48 hours of diagnosis was not associated with an increased risk of PE.
Abstract
Standard treatment of catheter-associated upper extremity deep vein thrombosis (UE-DVT) is anticoagulation, although catheters are often removed for this indication. The optimal time for catheter removal and whether the act and/or timing of catheter removal is associated with pulmonary embolism (PE) remain unknown. A retrospective cohort study was performed at 8 participating institutions through the Venous thromboEmbolism Network US. Patients with hematologic malignancies and central venous catheter (CVC)–associated UE-DVT were included from 1 January 2010 through 31 December 2016. The primary outcome was objectively confirmed PE within 7 days of UE-DVT diagnosis in anticoagulated patients comparing early (≤48 hours) vs delayed (>48 hours) catheter removal. A total of 626 patients were included, among whom 480 were treated with anticoagulation. Among anticoagulated patients, 255 underwent early CVC removal, while 225 had delayed or no CVC removal; 146 patients received no anticoagulation, among whom 116 underwent CVC removal alone. PE within 7 days occurred in 2 patients (0.78%) with early removal compared with 1 patient (0.44%) with delayed or no CVC removal (P > .9). PE or any cause of death within 7 days occurred in 3 patients in both the early removal (1.18%) and delayed/no removal (1.33%) groups (P > .9). In patients treated with CVC removal only (no anticoagulation), there were no PEs but 3 deaths within 7 days. In patients with hematological malignancy and CVC-associated UE-DVT, early removal of CVCs was not associated with an increased risk of PE compared with delayed or no removal.
Introduction
Upper extremity deep vein thrombosis (UE-DVT) is a well-described complication of central venous catheters (CVCs) that are necessary for the treatment of patients with hematologic malignancies. A systematic review of peripherally inserted central catheters (PICCs) noted that UE-DVT occurs in 6.7% (95% confidence interval, 4.7% to 8.6%) of cancer patients.1 Increased risk is also seen with centrally inserted catheters.2 Treating patients with hematologic malignancies and a CVC-associated UE-DVT can be challenging, because anticoagulation is often limited or contraindicated due to severe and prolonged thrombocytopenia and concern for associated bleeding complications.3‐5
Removal of a CVC is often considered when UE-DVT is diagnosed. Guidelines have advised leaving CVCs in place and treating with anticoagulation if the CVC remains functional and necessary for ongoing patient care. For patients who no longer need their CVC or who have another compelling reason for CVC removal (such as infection), the optimal timing for removal of the CVC is unknown.6 Clinicians fear triggering thrombus embolization with CVC removal, and this has not been examined in other studies of UE-DVT.2,7‐11 A guidance statement from the International Society on Thrombosis and Haemostasis regarding CVC-associated DVT in cancer patients suggests 3 to 5 days of anticoagulation before CVC removal.12 However, this recommendation is based on expert opinion and not high-quality evidence. Furthermore, it remains unknown if this is a safe and effective strategy or if it is even necessary.
We have previously demonstrated that, despite current guidelines, a large number of patients with hematologic malignancies who had CVC-associated UE-DVT had their CVCs removed in the absence of compelling reasons for CVC removal other than the DVT itself.13,14 Many of these patients never received anticoagulation. We sought to use the same patient cohort to evaluate the risk for symptomatic pulmonary embolism (PE) with different CVC management strategies.
Methods
Study population
A multicenter retrospective cohort study was performed at 8 participating institutions of the Venous thromboEmbolism Network US (VENUS). Patients with hematologic malignancies and CVC-associated UE-DVT between 1 January 2010 and 31 December 2016 were identified using the International Classification of Diseases 9 or 10 codes (supplemental Table 1). Medical records of eligible patients from participating sites were searched and reviewed. Hematologic malignancies were confirmed by pathology, and UE-DVT was confirmed by objective radiologic imaging. Patients were excluded if a CVC (identified in the ipsilateral extremity) was not in place at the diagnosis of UE-DVT or if there was concurrent lower extremity DVT or PE. Isolated superficial vein thrombosis (cephalic and basilic vein thrombosis) was excluded. Types of upper extremity CVCs included were PICCs, ports, and other types of central lines (tunneled or nontunneled) placed in the jugular or subclavian veins. Institutional review board approval was obtained at each participating site, and data were deidentified and pooled for analysis. The study was conducted according to the Declaration of Helsinki.
Outcomes
Demographics, management of CVC-associated UE-DVT, duration of anticoagulation, and outcomes including recurrent venous thromboembolism (VTE) and bleeding events during anticoagulation were extracted from the electronic medical record. Recurrent VTE was defined as a DVT or PE involving new anatomic locations objectively confirmed by imaging. Anticoagulation start and finish dates, as well as the timing of the CVC removal, duration of follow-up, and death, were recorded. The primary outcome was PE objectively confirmed on imaging (computer tomography, pulmonary angiogram, or ventilation-perfusion scan) within 7 days after the index CVC-associated UE-DVT, and the secondary outcome was PE or death from any cause within 7 days.
Analysis
Patients started on therapeutic anticoagulation at the time of the index CVC-associated UE-DVT were divided into 2 groups: (1) anticoagulation with early CVC removal (≤48 hours) and (2) anticoagulation with delayed CVC removal (>48 hours after the diagnosis of UE-DVT) or no CVC removal. A third group, patients treated with CVC removal as their only treatment, was also assessed. The primary and secondary outcomes were compared statistically between groups 1 and 2 and numerically to group 3. Baseline characteristics were compared between groups using χ2 (or Fisher’s exact test) for categorical variables, 2-tailed t tests for continuous variables, and Wilcoxon rank-sum for nonparametric continuous variables. Fisher’s exact test was used to evaluate the primary and secondary outcomes.
Results
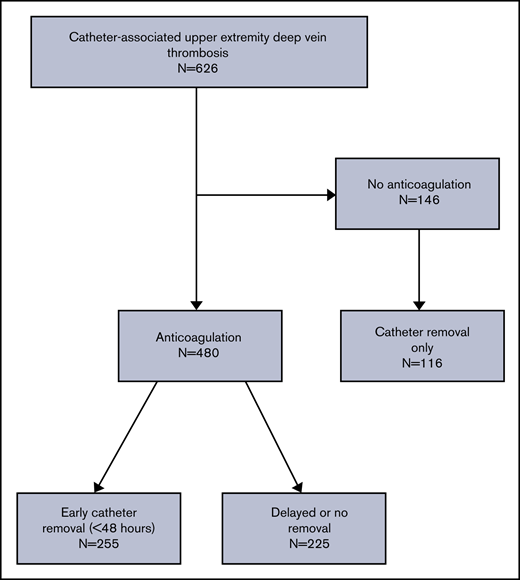
A total of 626 patients with active hematologic malignancy and CVC-associated UE-DVT were identified at the 8 participating centers. The majority (n = 480) of patients were treated with anticoagulation and the remainder did not receive anticoagulation (n = 146), of whom most (n = 116) underwent CVC removal as the only treatment (Figure 1). In patients treated with anticoagulation, 255 (43%) underwent early CVC removal (≤48 hours) and 225 (38%) had either delayed (>48 hours) or no CVC removal. In the early removal group, 225 patients (88%) had the catheter removed within 24 hours, and 30 patients (12%) had the catheter removed between 24 and 48 hours. In the delayed or no removal group, 62 patients (28%) had the catheter removed between days 3 and 7.
The mean age and gender distribution were numerically similar across all groups (Table 1). A higher percentage (47% vs 37%) of patients with leukemia/MDS/MPNs and a lower percentage (34% vs 47%) of lymphoma patients were observed in the group who received anticoagulation and early CVC removal as compared with delayed or no CVC removal (P = .01). PICCs were observed more frequently (74% vs 49%) in the early removal group, and there was a lower frequency (5% vs 22%) of Port-a-caths in this group compared with the delayed or no removal group (P < .001). The leukemia/MDS/MPN group had the largest percentage of PICC lines (n = 215, 75%). The leukemia/MDS/MPN patients were predominately treated with anticoagulation and early catheter removal (n = 121), but they also constituted the largest percentage of the “CVC removal only” group (n = 83, 72%). This group also had a numerically lower median platelet count (30 × 109/L; IQR, 18-71) compared with the early removal groups (125 × 109/L; IQR, 61-208) and delayed or no removal (138 × 109/L; IQR, 72-207).
Baseline characteristics
| . | Early removal (n = 255) . | Delayed or no removal (n = 225) . | P . | Removal only (n = 116) . |
|---|---|---|---|---|
| Age, mean (SD) | 54 (16) | 51 (15) | .12* | 50 (18) |
| Male, n (%) | 143 (56) | 121 (54) | .61† | 63 (54) |
| Hematologic malignancy, n (%) | ||||
| Leukemia/MDS/MPN | 121 (47) | 83 (37) | .01† | 83 (72) |
| Lymphoma | 86 (34) | 106 (47) | 16 (14) | |
| Plasma cell dyscrasia | 48 (19) | 36 (16) | 17 (15) | |
| Catheter type, n (%) | ||||
| PICC | 189 (74) | 110 (49) | <.001† | 96 (83) |
| PORT-a-cath | 12 (5) | 49 (22) | 3 (2) | |
| Tunneled | 54 (21) | 66 (29) | 17 (15) | |
| DVT location, n (%) | ||||
| Brachial | 25 (10) | 13 (6) | <.001ठ| 25 (22) |
| Axillary | 58 (23) | 30 (13) | 33 (28) | |
| Subclavian | 108 (42) | 80 (36) | 40 (34) | |
| Internal jugular | 48 (19) | 80 (36) | 16 (14) | |
| Superior vena cava | 6 (2) | 4 (2) | 2 (2) | |
| Brachiocephalic | 8 (3) | 16 (7) | 0 | |
| Other | 2 (1) | 2 (1) | 0 | |
| Platelet count(× 109/L), median (IQR) | 125 (61-208) | 138 (72-207) | .25§ | 30 (18-71) |
| Anticoagulation, n (%) | ||||
| LMWH | 192 (75) | 178 (79) | .20‡ | NA |
| Bridge to VKA | 24 (9) | 10 (4) | ||
| UFH | 23 (9) | 24 (11) | ||
| Aspirin | 1 (0.4) | 0 | ||
| Other | 15 (6) | 13 (6) |
| . | Early removal (n = 255) . | Delayed or no removal (n = 225) . | P . | Removal only (n = 116) . |
|---|---|---|---|---|
| Age, mean (SD) | 54 (16) | 51 (15) | .12* | 50 (18) |
| Male, n (%) | 143 (56) | 121 (54) | .61† | 63 (54) |
| Hematologic malignancy, n (%) | ||||
| Leukemia/MDS/MPN | 121 (47) | 83 (37) | .01† | 83 (72) |
| Lymphoma | 86 (34) | 106 (47) | 16 (14) | |
| Plasma cell dyscrasia | 48 (19) | 36 (16) | 17 (15) | |
| Catheter type, n (%) | ||||
| PICC | 189 (74) | 110 (49) | <.001† | 96 (83) |
| PORT-a-cath | 12 (5) | 49 (22) | 3 (2) | |
| Tunneled | 54 (21) | 66 (29) | 17 (15) | |
| DVT location, n (%) | ||||
| Brachial | 25 (10) | 13 (6) | <.001ठ| 25 (22) |
| Axillary | 58 (23) | 30 (13) | 33 (28) | |
| Subclavian | 108 (42) | 80 (36) | 40 (34) | |
| Internal jugular | 48 (19) | 80 (36) | 16 (14) | |
| Superior vena cava | 6 (2) | 4 (2) | 2 (2) | |
| Brachiocephalic | 8 (3) | 16 (7) | 0 | |
| Other | 2 (1) | 2 (1) | 0 | |
| Platelet count(× 109/L), median (IQR) | 125 (61-208) | 138 (72-207) | .25§ | 30 (18-71) |
| Anticoagulation, n (%) | ||||
| LMWH | 192 (75) | 178 (79) | .20‡ | NA |
| Bridge to VKA | 24 (9) | 10 (4) | ||
| UFH | 23 (9) | 24 (11) | ||
| Aspirin | 1 (0.4) | 0 | ||
| Other | 15 (6) | 13 (6) |
P values reported are for delayed or no removal vs early removal. Early removal was defined as removal within 48 hours of UE-DVT diagnosis. DVT location refers to most proximal location of the thrombus.
IQR, interquartile range; LMWH, low molecular weight heparin; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; SD, standard deviation; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Student t test.
χ2 test.
Fisher’s exact test.
Mann-Whitney U test.
The primary outcome of PE within 7 days of the index UE-DVT was observed in 2 patients (0.78%) treated with anticoagulation and early CVC removal (both occurred on day 1) compared with 1 patient (0.44%) treated with anticoagulation and delayed CVC removal (occurred on day 5) (P > .9; Table 2). The secondary outcome of PE or death within 7 days of UE-DVT diagnosis was observed in 3 patients (1.18%) treated with anticoagulation and early CVC removal only compared with 3 patients (1.33%) treated with anticoagulation and delayed CVC removal (P > .9). In the group of patients treated with CVC removal only, there were no PEs but 3 deaths (2.59%) within 7 days. All 3 patients diagnosed with PE within 7 days of UE-DVT diagnosis had PICC lines and leukemia/MDS and the site of most proximal DVT involvement was brachiocephalic veins (2 patients) and subclavian vein (1 patient). None of the patients with recurrent PE had a brachial vein DVT; thus, a sensitivity analysis of removing the patients with brachial vein DVT did not affect the outcomes. If brachiocephalic or superior vena cava thrombosis were excluded, no patients in either group had PE within 7 days.
Outcomes of PE and death within 7 d by treatment group
| . | n . | PE within 7 d, n (%) . | P . | PE or death within 7 d, n (%) . | P . |
|---|---|---|---|---|---|
| Early removal (≤48 hours) | 255 | 2 (0.78) | 3 (1.18) | >.9* | |
| Delayed or no removal | 225 | 1 (0.44) | >.9* | 3 (1.33) | |
| Removal only | 116 | 0 | 3 (2.59) |
| . | n . | PE within 7 d, n (%) . | P . | PE or death within 7 d, n (%) . | P . |
|---|---|---|---|---|---|
| Early removal (≤48 hours) | 255 | 2 (0.78) | 3 (1.18) | >.9* | |
| Delayed or no removal | 225 | 1 (0.44) | >.9* | 3 (1.33) | |
| Removal only | 116 | 0 | 3 (2.59) |
Fisher’s exact test.
Discussion
To our knowledge, this multi-institutional retrospective cohort study is the largest study to this date of CVC-associated UE-DVT in patients with hematologic malignancies. Detailed information on the timing of CVC removal, anticoagulation administration, and imaging-confirmed PE allowed us the opportunity to perform a robust evaluation of the outcomes related to the timing of CVC removal, a commonly encountered clinical management issue. In patients treated with anticoagulation, removal of CVCs within 48 hours was not associated with an increased risk of PE within 7 days compared with delayed or no removal of the CVC. For patients treated with CVC removal alone, no PE were found within 7 days of removal. To account for potentially unrecognized significant PE resulting in death, we evaluated the secondary outcome of PE or death within 7 days and it was similar among all groups.
Prior studies evaluating the optimal timing of CVC removal in patients with CVC-associated UE-DVT have been limited by their sample size and lack of pertinent details. In a retrospective review of 112 cancer patients who had CVC-associated UE-DVT between 1992 and 1995, most received anticoagulation (n = 61, 55%), among whom 9 had additional CVC removal and 13 underwent CVC removal and replacement.15 Among patients not anticoagulated, 20 underwent CVC removal, 12 underwent CVC removal followed by replacement, and 10 received other/no therapy. Within 2 weeks of the diagnosis of the CVC-associated UE-DVT, the study reported no symptomatic PE or unexplained death. Details on the timing of CVC removal and the type or duration of anticoagulation before CVC removal were not reported. Another retrospective study compared 62 patients treated with a strategy of PICC line removal only compared with 21 patients treated with PICC removal and anticoagulation.16 There was no progressive or new VTE in patients who received anticoagulation. In patients with CVC removal only, 1 PE, 3 new DVTs, and 5 episodes of DVT extension occurred, but the timing of these VTEs as related to the timing of CVC removal was not reported. Another study focusing on thrombus resolution of CVC-associated UE-DVT included 101 patients, 43 of whom underwent removal of the CVC within 48 hours, and there was no PE reported.17
UE-DVT can be associated with concurrent PE in 9% to 36% of patients.9‐11,18,19 In our study, we excluded patients who had known PE at the time of their UE-DVT diagnosis. The difference in the incidence of PE associated with UE-DVT in the literature is likely due to differences between baseline patient characteristics as well as the frequency of radiologic imaging. Routine radiographic surveillance for PE was not performed in our study; therefore, it is possible that some of the PEs identified in our study occurred before anticoagulation or CVC removal. This means that the small number of PEs may represent an overestimation of the number that occur with CVC removal. In some patients, both PE and UE-DVT may have been initially suspected, but imaging for PE may have been delayed or deferred, especially in anticoagulated patients. Since the primary outcome in this study was a safety end point, the most conservative definition of new PE was used and may be an overestimation.
Data from registries have shown that 32% to 41% of UE-DVTs were associated with CVCs, and in cases when they were not, malignancy was a provoking risk factor in 50% of patients.18,19 Therefore, the patient population in this study, active hematologic malignancy with CVCs, may not be generalizable to all CVC-associated UE-DVT. However, this group is considered to be at high risk for adverse outcomes, and therefore, we believe our data showing a low rate of PE could be reassuring to other lower-risk groups. This group of patients also has unique risk factors for bleeding, including prolonged and severe thrombocytopenia, making anticoagulation challenging. Indeed, we observed that a strategy of no anticoagulation with CVC removal was common, mostly due to perceived contraindication to anticoagulation because of severe and prolonged thrombocytopenia and concern for bleeding complications. Whether CVC removal only was an adequate treatment cannot be fully determined in this retrospective study.
Although our study showed a low risk of PE even in patients not treated with anticoagulation, it is not an endorsement of treatment with CVC removal only without anticoagulation in this patient population. The number of patients included in the nonanticoagulated group was smaller, and only the short-term (7 days) outcome of PE was evaluated. Previously reported data from this cohort13,14 demonstrated that patients treated with CVC removal only without anticoagulation ultimately had a higher rate of VTE recurrence and mortality. An additional consideration is that anticoagulation could help to reduce the risk of postthrombotic syndrome in the upper extremities that can occur in 15% to 25% of cases.20 Therefore, in patients with CVC-associated UE-DVT, we believe that anticoagulation, whenever tolerable and safe, remains the standard of care.
Our analysis has several limitations and strengths. Inherent biases are present with retrospective data from which this analysis was based, and the unadjusted comparison between each group could be effected by measured and unmeasured confounding. The reasons why anticoagulation was not prescribed were not obtained from the medical record. The majority of patients were treated with heparins in this study, which might not reflect current practice patterns of increasing utilization of direct oral anticoagulants in this population. Also, since this was a retrospective study, not all patients had imaging to look for potentially asymptomatic PE. The data were collected from multiple centers to allow the collection of different treatment practices and patient populations. Although the number of events for this analysis was limited, pooling of patient data from several centers allowed a bigger population to evaluate this commonly encountered clinical question.
Conclusions
The risk of symptomatic PE after removal of a CVC in patients with CVC-associated UE-DVT is low and did not differ significantly based on the timing of CVC removal (<48 hours or >48 hours) in patients treated with anticoagulation. Our data do not provide a compelling reason to delay CVC removal for the concern of PE.
Acknowledgments
The above work was performed through VENUS.
Statistical support was provided through funding by Versiti.
Authorship
Contribution: All authors were involved in the conception and design or analysis and interpretation of the data, drafting of the manuscript, or revising it critically, and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Damon E. Houghton, Division of Vascular Medicine, Department of Cardiovascular Diseases & the Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic, 200 1st St, Rochester, MN 55905; e-mail: houghton.damon@mayo.edu.
References
Author notes
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
For data sharing, please contact the corresponding author at houghton.damon@mayo.edu.
The full-text version of this article contains a data supplement.