Key Points
Histone deacetylase inhibitor panobinostat with tacrolimus and sirolimus improved incidence of aGVHD to 18.4% for matched transplants.
Regimen is tolerable, with a 31.6% incidence of chronic GVHD and an overall survival of 89.5%.
Abstract
Immunomodulatory properties of histone deacetylase inhibitors represent a reasonable approach for acute graft-versus-host disease (aGVHD) prevention. We report a phase 2 trial evaluating panobinostat (PANO) administered over 26 weeks, starting on day −5 (5 mg orally 3 times a week) with tacrolimus initiated on day −3 plus sirolimus on day −1, with a median patient age of 58 years (range, 19-72 years) (n = 38). Donor source consisted of HLA 8/8–matched donors, related (n = 13) or unrelated (n = 25), using granulocyte colony-stimulating factor–stimulated peripheral blood stem cells. Myeloablative (n = 18) or reduced-intensity (n = 20) conditioning regimens were used for patients with acute myeloid leukemia (n = 17), myelodysplastic syndrome (n = 13), or other malignancies (n = 8). The cumulative incidence of aGVHD II-IV by day 100 was 18.4% (90% confidence interval [CI], 9.4% to 29.9%). Cumulative incidence of chronic GVHD at 1 year was 31.6% (90% CI, 19.5% to 44.3%). Adverse events related to PANO were thrombocytopenia (n = 5), leukopenia (n = 6), gastrointestinal toxicity (n = 3), rash (n = 4), renal failure/peripheral edema (n = 1), and periorbital edema (n = 1). At 1 year, overall survival was 89.5% (90% CI, 81.6% to 98.0%), relapse-free survival was 78.9% (90% CI, 68.8% to 90.6%), nonrelapse mortality was 2.6% (90% CI, 0.3% to 9.9%), and GVHD relapse-free survival was 60.5% (90% CI, 48.8% to 75.1%). PANO hits histone 3 as early as day 15 in CD8, CD4 and T regs. In conclusion, PANO combination met the primary study end point for aGVHD prevention and warrants further testing. This trial was registered at www.clinicaltrials.gov as #NCT02588339.
Introduction
Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic cell transplantation (HCT) and is associated with high morbidity and mortality.1,2 The reported incidence rate of acute GVHD (aGVHD) is ∼47% when using peripheral blood stem cells (PBSCs) for matched unrelated HCT3 using tacrolimus (TAC) and methotrexate (MTX) or cyclosporine and MTX. In an effort to minimize aGVHD, our group tested in a randomized phase 2 trial TAC and sirolimus (SIR) vs TAC and MTX for GVHD prophylaxis, reporting an aGVHD incidence rate of 43% with TAC/SIR.4 New immunosuppressive agents are needed to maximize aGVHD prevention and improve survival rates for patients after matched related or unrelated allogeneic HCT.
Nonselective pan-histone deacetylase inhibitors (HDACis) have several properties that are valuable for GVHD prevention, including reducing proinflammatory cytokines,5,6 increasing the number of and enhancing suppressive function of regulatory T cells (T-regs),7 immunomodulating human dendritic cells,8 and reducing human-derived dendritic cell inflammatory cytokines that are mediators of GVHD.9,10 The HDACi vorinostat has shown promising results in the prevention of aGVHD after allogeneic HCT.11,12 In addition, HDACis exhibit direct antitumor activity, thereby potentially minimizing relapse after HCT.13,14
Panobinostat (PANO), an HDACi that targets histone protein deacetylases classes III to IV, is a nonselective HDACi approved for the treatment of relapsed and relapsed/refractory multiple myeloma in combination with bortezomib and dexamethasone.15,16 PANO has been used for post-HCT maintenance for high-risk myelodysplastic syndrome (MDS),17 tested for epigenetic modulation of donor lymphocyte infusion after HCT,18 and used as a single agent as consolidation following suboptimal transplant outcomes in multiple myeloma.19 In this trial, we aimed to minimize aGVHD by adding PANO to TAC/SIR.4,20 We also built on our prior experience from a phase 1/2 trial using PANO in combination with glucocorticoids for primary GVHD treatment, which established the maximum tolerated dose used herein for GVHD prevention.21
The primary trial end point was to prospectively determine the cumulative incidence of aGVHD grades II to IV by day 100 using TAC and SIR in addition to PANO for GVHD prevention. We tested the hypothesis that the combination of PANO/TAC/SIR may enhance T-reg development and/or modulate antigen presentation, allowing for an immune-tolerant microenvironment; thereby, the combination can prevent GVHD.
Patients and methods
Overview of trial design
This single-arm phase 2 trial (NCT02588339) was designed to define the efficacy of PANO in combination with standard TAC/SIR for GVHD prevention. The primary objective of this trial was to prospectively evaluate the cumulative incidence of aGVHD grades II to IV by day 100. aGVHD score was adjudicated as per Glucksberg el al.22 Additional end points included cumulative incidence of chronic GVHD (cGHVD) based on National Institutes of Health consensus criteria on diagnosis and staging of cGVHD,23 engraftment, nonrelapse mortality, overall and relapse-free survival, and allied biologic correlative studies. Total planned follow-up for the trial was 12 months from HCT.
Inclusion and exclusion criteria
Included patients were adults aged ≥18 years with hematologic malignancies. Additional inclusion criteria were adequate vital organ function (left ventricular ejection fraction ≥ 45%; forced expiratory volume in one second, forced vital capacity, and diffusing capacity for carbon monoxide ≥ 50% of predicted values; aspartate transaminase or alanine transaminase <3 times the upper limit of normal values; and creatinine clearance ≥ 50 mL/min) and Karnofsky performance status ≥ 60%. Patients were excluded based on the following criteria: pregnancy; active infection that was uncontrolled with antimicrobial therapy; active HIV; hepatitis B or C infection; having received antithymocyte globulin or cyclophosphamide for GVHD prevention; impaired cardiac function or clinically significant cardiac diseases, including bifascicular block; myocardial infarction or unstable angina within ≤12 months; congenital long QT syndrome; sustained ventricular tachyarrhythmia (patients with controlled atrial arrhythmia were eligible) or any history of ventricular fibrillation or torsade de pointes; bradycardia (<45 beats per minute); having an average of 3 corrected QT intervals by Fridericia formula > 480 ms on a screening electrocardiogram; use of HDACis, deacetylases, heat-shock protein 90 inhibitors, or valproic acid within 30 days of HCT; receipt of cytochrome P450 3A4 inhibitors, with the exceptions of TAC, voriconazole, posaconazole, and cyclosporine, which were allowed; or HCT comorbidity index score ≥4.
Transplantation protocol and treatment design
Conditioning regimens, as per the treating physician’s choice, included fludarabine and busulfan targeted to an average daily area under the curve of >3500 µM/L × min per day or 5300 µM/L × min per day or fludarabine and melphalan 140 mg/m2. Eligible related or unrelated donors who were matched with the patient for HLA-A, -B, -C, and -DRB1 via high-resolution typing provided filgrastim-mobilized PBSCs for transplantation per standard practices (CD34 count of 5-10 × 106 cells/kg; a cell dose ≥ 2 × 106 CD34/kg was the minimal dose allowed).
All patients received TAC and SIR for GVHD prophylaxis. TAC was administered starting on day −3 as an IV formulation and then converted to oral therapy when tolerated, with taper recommended from day +50. TAC levels were maintained at a target range of 3 to 7 ng/mL. SIR was administered as an oral loading dose on day −1, followed by daily oral maintenance dosing to maintain levels of 5 to 14 ng/mL, with taper recommended from day +365.
We determined the PANO dose based on maximum tolerated dose established in our prior phase 1/2 GVHD treatment trial.21 Study patients began PANO therapy at 5 mg orally for 3 doses that were 48 hours apart in a 7-day period starting on day −5 or −6, and this dosing schedule was continued for 26 weeks with the goal of minimizing relapse post-HCT due to its effect on high-risk hematological malignancies.24 PANO treatment was discontinued as deemed necessary to investigator(s) or in the event of aGVHD, an adverse event (AE), patient’s noncompliance or consent withdrawal, prolongation of QT intervals by Fridericia formula, platelets < 20 × 103/μL, absolute neutrophil count < 0.5 × 103/μL, or death. Because of unknown interactions between conditioning regimens and PANO, as per protocol, PANO will be stopped for any nonhematological Common Terminology Criteria for Adverse Events (CTCAE) (version 4) grade 3 or higher toxicities unexpected for HCT seen between first dose of study drug and day +7 post-HCT, and hold parameters were provided for unexpected gastrointestinal toxicity depending of the severity. The accrual should be suspended if patient(s) experience CTCAE (version 4) grade 3 or higher toxicity unexpected with transplant.
Pharmacodynamics
Blood samples were collected from patients treated with TAC/SIR/PANO and compared with samples from control patients treated with TAC/SIR. These control patients had identical inclusion and exclusion criteria, except for not being treated with PANO, and signed specific consent at time of enrollment this trial
T-cell subsets and lymphocyte populations.
Fluorescence-activated cell-sorting analyses were performed on a BD Biosciences LSRII with 4 lasers (488 nm/405 nm/640 nm/561 nm) (BD Biosciences, San Jose, CA). T-cell subsets were determined using thawed cells collected on days +28 (± 3) and +90 (±15), incubated with the viability marker Live/Dead Aqua, and then stained with fluorochrome-labeled antibodies to CD3, CD4, CD8, CD45RA, CD27, CD31, CD279 (PD-1), CD357 (GITR), CD25, CD127, and Foxp3 (BD Biosciences). Lymphocyte populations were determined using thawed cells incubated with the viability marker Live/Dead Aqua and then stained with fluorochrome-labeled antibodies to CD3, CD56, CD19, CD14, CD16, and HLA-DR (BD Biosciences). Resultant data were analyzed on FlowJo V10.6.1.
Histone 3 acetylation of blood cell subsets.
Thawed cells collected on days 15 (± 3) and 28 (± 3) were incubated with the viability marker Live/Dead Near IR and then stained for CD3/CD4/FoxP3 using labeled antibodies. Cells were fixed with Fix buffer II (BD Biosciences) following the manufacturer’s suggested protocol. Fixed cells were permeabilized with 80% methanol and subsequently washed with fluorescence-activated cell sorting buffer (2% fetal bovine serum/phosphate-buffered saline) and stained for Alexa Flour 647 conjugate anti-acetylated histone 3 (Lys 9) (Cell Signaling Tech, Danvers, MA).
Cytokine and biomarker measurement.
Plasma samples collected on day 90 (± 15) were tested by Cytokine Bead Array Th1/Th2/Th17 (interleukin-2 [IL-2], IL-4, IL-6, IL-10, tumor necrosis factor α, interferon γ [IFN-γ], and IL-17a) using Q-Plex Array kits (Quansys Biosciences, Logan, UT). GVHD biomarkers (Elafin, ST2, and REG3A) were measured on day 28 (± 3) by enzyme-linked immunosorbent assay using methods previously described.25,26
Statistical methods
We used a phase 2 Simon’s minimax 2-stage design with 10% 1-sided type I error and 10% type II error rate; under these terms, the null hypothesis (incidence rate of 0.43 at day 100) would be rejected if there were ≤12 patients with grade II to IV aGVHD among the 38 evaluable patients. An evaluable patient for the primary end point was defined as a patient who received ≥1 dose of PANO per protocol and who neither relapsed nor died for any reason without experiencing grade II to IV aGVHD until day 100. For pharmacodynamic T-cell analysis, because the normality assumption by Shapiro-Wilk test was not met, the change from baseline was assessed by the Wilcoxon signed-rank test. HDAC enzymatic activity, cytokine levels, and biomarkers were tested in triplicate and analyzed using Kruskal-Wallis 1-way analysis of variance with Tukey’s multiple comparisons test. Prism software was used. P < .05 was considered significant.
Data and safety monitoring.
The trial was conducted and approved by the Advarra Institutional Review Board. All patients signed informed consent forms. Serious AEs were reported to Advarra, the Moffitt Protocol Monitoring Committee, the Food and Drug Administration, and Novartis Pharmaceutical.
Results
Baseline patient characteristics
A total of 42 patients were enrolled; 2 enrolled subjects who completed screening were not transplanted due to disease relapse prior to conditioning regimen. Among the 40 remaining subjects, 2 were considered not evaluable as per protocol either because of mortality due to sinusoidal obstructive syndrome on day + 76 (n=1) who did not develop any GVHD; or consent withdrawal on day +26 (n = 1) with no GVHD at the time of trial withdraw, and, therefore, the trial had 38 patients evaluable for the primary end point of aGVHD. No other patients developed sinusoidal obstructive syndrome while treated on trial. Baseline patient characteristics are summarized in Table 1. Included patients mainly were diagnosed with AML (n=17), myelodysplastic syndrome (MDS; n=13), ALL (n=4), and other hematological malignancies (n=4). HCTs were from either a matched sibling (n=13) or 8/8 HLA-A, -B, -C, and -DRB1 allele–matched unrelated donors (n=25). All patients received filgrastim-mobilized PBSCs as per protocol with full donor marrow chimerism (>95% donor cells) when tested on day +30 (±7) post-HCT as per institutional standards. The median age of recipients was 58 years (range, 19-72 years), and 55% were male; patients were conditioned prior to HCT with either a myeloablative (n=18) or reduced-intensity (n=20) regimen as defined by Bacigalupo et al.27
Patient characteristics
| Characteristics . | Evaluable patients (n = 38) . |
|---|---|
| Age (y), median (range) | 58 (19-72) |
| Sex, no. (%) | |
| Female | 17 (44.7) |
| Male | 21 (55.3) |
| Race, no. (%) | |
| Asian | 1 (2.6) |
| Black or African American | 1 (2.6) |
| Unknown | 4 (10.5) |
| White | 32 (84.2) |
| Diagnosis, no. (%) | |
| ALL | 4 (10.5) |
| AML | 17 (44.7) |
| CLL | 1 (2.6) |
| CML | 2 (5.3) |
| MDS | 13 (34.2) |
| NHL T cell | 1 (2.6) |
| Chemotherapy regimen, no. (%) | |
| MAC BU 5300-FLU | 18 (47.4) |
| RIC BU 3500-FLU | 1 (2.6) |
| RIC MEL FLU | 19 (50.0) |
| Donor, no. (%) | |
| Related | 13 (34.2) |
| Unrelated | 25 (65.8) |
| Stem cell source, no. (%) | |
| PBSCs | 38 (100) |
| Characteristics . | Evaluable patients (n = 38) . |
|---|---|
| Age (y), median (range) | 58 (19-72) |
| Sex, no. (%) | |
| Female | 17 (44.7) |
| Male | 21 (55.3) |
| Race, no. (%) | |
| Asian | 1 (2.6) |
| Black or African American | 1 (2.6) |
| Unknown | 4 (10.5) |
| White | 32 (84.2) |
| Diagnosis, no. (%) | |
| ALL | 4 (10.5) |
| AML | 17 (44.7) |
| CLL | 1 (2.6) |
| CML | 2 (5.3) |
| MDS | 13 (34.2) |
| NHL T cell | 1 (2.6) |
| Chemotherapy regimen, no. (%) | |
| MAC BU 5300-FLU | 18 (47.4) |
| RIC BU 3500-FLU | 1 (2.6) |
| RIC MEL FLU | 19 (50.0) |
| Donor, no. (%) | |
| Related | 13 (34.2) |
| Unrelated | 25 (65.8) |
| Stem cell source, no. (%) | |
| PBSCs | 38 (100) |
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BU, busulfan; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; FLU, fludarabine; MAC, myeloablative; MDS, myelodysplastic syndrome; Mel, melphalan; NHL, non-Hodgkin lymphoma; RIC, reduced intensity.
Safety: engraftment, mucositis, AEs, and infectious complications
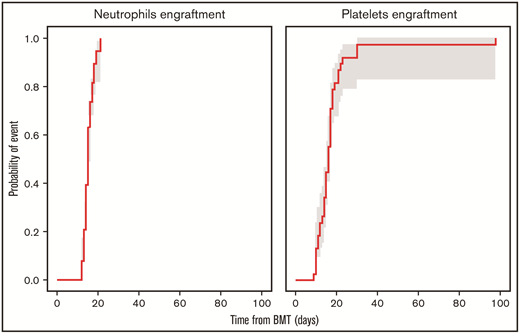
The median number of days from transplant to neutrophil engraftment was 15 (range, 12-21) days, and the median number of days from transplant to platelet engraftment was 16 (range, 9-98) (Figure 1). Mucositis was absent among 7 patients, and others developed grade 1 (n=13), grade 2 (n=8), or grade 3 (n=10) mucositis, with severity corresponding to conditioning regimen used. AEs probably/definitely related to PANO, per the investigator, are listed in Table 2. One patient developed a rash after 4 PANO doses, followed by angioedema after the sixth dose, after which PANO was discontinued. After a 14-day drug hold, PANO was resumed, and the patient developed within 1 hour a facial rash and periorbital edema, both of which resolved with supportive care; therefore, the AE was considered definitively related to PANO. AEs developed during the trial that were unrelated to PANO are included in Table 2.
Cumulative incidence of neutrophils and platelets engraftment at day 100 with 90% confidence interval. BMT, bone marrow transplant.
Cumulative incidence of neutrophils and platelets engraftment at day 100 with 90% confidence interval. BMT, bone marrow transplant.
Total AEs
| Toxicity category . | CDUS/CTCAE code . | AE grade . | All grades of AE . | Possibly-definitively related to PANO? . | |||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||
| Gastrointestinal disorders* | Diarrhea | — | — | 2 (5.3) | — | 2 (5.3) | Yes |
| Nausea | — | — | 1 (2.6) | — | 1 (2.6) | Yes | |
| General disorders and administration site conditions* | Edema limbs | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Investigations* | Neutrophil count decreased | — | — | 2 (5.3) | 4 (10.5) | 6 (15.8) | Yes |
| Platelet count decreased | — | — | 1 (2.6) | 4 (10.5) | 5 (13.2) | Yes | |
| Renal and urinary disorders* | Acute kidney injury | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Skin and subcutaneous tissue disorders* | Periorbital edema | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Rash maculo-papular | 1 (2.6) | — | 3 (7.9) | — | 4 (10.5) | Yes | |
| Blood and lymphatic system disorders | Leukocytosis | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Cardiac disorders | Atrial fibrillation | — | 2 (5.3) | — | — | 2 (5.3) | No |
| Eye disorders | Floaters | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Gastrointestinal disorders | Diarrhea | — | — | 2 (5.3) | — | 2 (5.3) | No |
| Nausea | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| General disorders and administration site conditions | Edema limbs | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Fever | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Non-cardiac chest pain | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Immune system disorders | Immune system disorders—other, specify | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Infections and infestations | Appendicitis | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Encephalitis infection | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Lung infection | — | — | 1 (2.6) | 1 (2.6) | 2 (5.3) | No | |
| Papulopustular rash | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Sepsis | — | — | — | 1 (2.6) | 1 (2.6) | No | |
| Sinusitis | — | 1 (2.6) | 1 (2.6) | — | 2 (5.3) | No | |
| Investigations | Neutrophil count decreased | — | — | 1 (2.6) | 3 (7.9) | 4 (10.5) | No |
| Platelet count decreased | — | — | 2 (5.3) | 2 (5.3) | No | ||
| Weight loss | 1 (2.6) | — | — | 1 (2.6) | No | ||
| Metabolism and nutrition disorders | Anorexia | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Hypocalcemia | 4 (10.5) | — | — | — | 4 (10.5) | No | |
| Nervous system disorders | Akathisia | 1 (2.6) | — | 1 (2.6) | — | 2 (5.3) | No |
| Headache | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Myelitis | 2 (5.3) | — | — | — | 2 (5.3) | No | |
| Syncope | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Psychiatric disorders | Confusion | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Hallucinations | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Renal and urinary disorders | Acute kidney injury | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Respiratory, thoracic and mediastinal disorders | Cough | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Pleural effusion | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Pneumonitis | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Pulmonary edema | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Skin and subcutaneous tissue disorders | Palmar-plantar erythrodysesthesia syndrome | — | 1 (2.6) | — | — | 1 (2.6) | No |
| Periorbital edema | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Rash maculo-papular | — | 1 (2.6) | — | 1 (2.6) | No | ||
| Vascular disorders | Hypertension | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Toxicity category . | CDUS/CTCAE code . | AE grade . | All grades of AE . | Possibly-definitively related to PANO? . | |||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||
| Gastrointestinal disorders* | Diarrhea | — | — | 2 (5.3) | — | 2 (5.3) | Yes |
| Nausea | — | — | 1 (2.6) | — | 1 (2.6) | Yes | |
| General disorders and administration site conditions* | Edema limbs | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Investigations* | Neutrophil count decreased | — | — | 2 (5.3) | 4 (10.5) | 6 (15.8) | Yes |
| Platelet count decreased | — | — | 1 (2.6) | 4 (10.5) | 5 (13.2) | Yes | |
| Renal and urinary disorders* | Acute kidney injury | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Skin and subcutaneous tissue disorders* | Periorbital edema | 1 (2.6) | — | — | — | 1 (2.6) | Yes |
| Rash maculo-papular | 1 (2.6) | — | 3 (7.9) | — | 4 (10.5) | Yes | |
| Blood and lymphatic system disorders | Leukocytosis | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Cardiac disorders | Atrial fibrillation | — | 2 (5.3) | — | — | 2 (5.3) | No |
| Eye disorders | Floaters | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Gastrointestinal disorders | Diarrhea | — | — | 2 (5.3) | — | 2 (5.3) | No |
| Nausea | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| General disorders and administration site conditions | Edema limbs | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Fever | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Non-cardiac chest pain | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Immune system disorders | Immune system disorders—other, specify | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Infections and infestations | Appendicitis | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Encephalitis infection | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Lung infection | — | — | 1 (2.6) | 1 (2.6) | 2 (5.3) | No | |
| Papulopustular rash | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Sepsis | — | — | — | 1 (2.6) | 1 (2.6) | No | |
| Sinusitis | — | 1 (2.6) | 1 (2.6) | — | 2 (5.3) | No | |
| Investigations | Neutrophil count decreased | — | — | 1 (2.6) | 3 (7.9) | 4 (10.5) | No |
| Platelet count decreased | — | — | 2 (5.3) | 2 (5.3) | No | ||
| Weight loss | 1 (2.6) | — | — | 1 (2.6) | No | ||
| Metabolism and nutrition disorders | Anorexia | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Hypocalcemia | 4 (10.5) | — | — | — | 4 (10.5) | No | |
| Nervous system disorders | Akathisia | 1 (2.6) | — | 1 (2.6) | — | 2 (5.3) | No |
| Headache | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Myelitis | 2 (5.3) | — | — | — | 2 (5.3) | No | |
| Syncope | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Psychiatric disorders | Confusion | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Hallucinations | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Renal and urinary disorders | Acute kidney injury | — | — | 1 (2.6) | — | 1 (2.6) | No |
| Respiratory, thoracic and mediastinal disorders | Cough | 1 (2.6) | — | — | — | 1 (2.6) | No |
| Pleural effusion | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Pneumonitis | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Pulmonary edema | — | — | 1 (2.6) | — | 1 (2.6) | No | |
| Skin and subcutaneous tissue disorders | Palmar-plantar erythrodysesthesia syndrome | — | 1 (2.6) | — | — | 1 (2.6) | No |
| Periorbital edema | 1 (2.6) | — | — | — | 1 (2.6) | No | |
| Rash maculo-papular | — | 1 (2.6) | — | 1 (2.6) | No | ||
| Vascular disorders | Hypertension | — | — | 1 (2.6) | — | 1 (2.6) | No |
CDUS, Clinical Data Update System.
Consider probably related to PANO.
Nine out of 38 patients (23%) completed 26 weeks of PANO treatment without interruptions. PANO was held temporarily for 29 patients (77%) because of diarrhea on day +5 (n=1), mucositis (n=2), or other AEs (neutropenia, thrombocytopenia, nausea, rash, QT corrected for heart rate prolongation, gastroesophageal reflux disease, akathisia, dysesthesia, appendicitis, or acute kidney injury). PANO was discontinued because of GVHD (n=6), patient’s refusal to reinitiate PANO after AE hold (n=4), relapse (n=2), rash (n=1), or acute kidney injury (n=1).
Planned PANO dose intensity was 390 mg administered over 180 days at an intended dose of 5 mg 3 times per week. Nine patients completed intended dose with no interruptions. Among 7 patients with GVHD, the median PANO dose intensity was 387.8 mg (342.3-390 mg) corresponding to 99.4% (87.7% to 100%) of intended dose intensity. Among evaluable patients with no GVHD, the median PANO dose intensity was 339 mg (32.5-385.6 mg) corresponding to a median 86.9% (8.33% to 98.8%) of intended dose. Total infections or viral reactivations that were noted during the trial are described in Table 3. Stopping rules were not met, as no patient experienced CTCAE (version 4) grade ≥3 or toxicity unexpected with transplant.
Infections
| Site . | Organism . | Grade . | No. patients . |
|---|---|---|---|
| Upper respiratory | |||
| RSV | 2 | 3 | |
| Parainfluenza 3 | 1 | 1 | |
| Influenza A | 2 | 1 | |
| Rhinovirus | 1 | 3 | |
| Coronavirus | 1 | 3 | |
| Moraxella catarrhalis | 2 | 1 | |
| Sinusitis | 2 | 1 | |
| Unknown | 1 | 3 | |
| Pulmonary | Pneumonia | 2 | 3 |
| Oral cavity | Candida | 1 | 5 |
| Skin | Hidradenitis suppurativa | 1 | 1 |
| Fusarium | 3 | 1 | |
| Staphylococcus | 3 | 1 | |
| Line-associated | 3 | 1 | |
| Genitourinary | UTI due to E. coli | 2 | 1 |
| UTI due to Pseudomonas | 2 | 1 | |
| UTI (unknown organism) | 2 | 1 | |
| BK Hemorrhagic Cystitis | 3 | 1 | |
| Epididymitis | 2 | 1 | |
| Viral reactivations | Cytomegalovirus | 2 | 2 |
| Epstein-Barr Virus | 3 | 1 | |
| Sinus | Fusarium | 3 | 1 |
| Unknown | 2 | 1 | |
| Fever | Neutropenic fever | 3 | 3 |
| Fever | 1 | 1 | |
| Gut | Clostridium difficile | 1 | 10 |
| Site . | Organism . | Grade . | No. patients . |
|---|---|---|---|
| Upper respiratory | |||
| RSV | 2 | 3 | |
| Parainfluenza 3 | 1 | 1 | |
| Influenza A | 2 | 1 | |
| Rhinovirus | 1 | 3 | |
| Coronavirus | 1 | 3 | |
| Moraxella catarrhalis | 2 | 1 | |
| Sinusitis | 2 | 1 | |
| Unknown | 1 | 3 | |
| Pulmonary | Pneumonia | 2 | 3 |
| Oral cavity | Candida | 1 | 5 |
| Skin | Hidradenitis suppurativa | 1 | 1 |
| Fusarium | 3 | 1 | |
| Staphylococcus | 3 | 1 | |
| Line-associated | 3 | 1 | |
| Genitourinary | UTI due to E. coli | 2 | 1 |
| UTI due to Pseudomonas | 2 | 1 | |
| UTI (unknown organism) | 2 | 1 | |
| BK Hemorrhagic Cystitis | 3 | 1 | |
| Epididymitis | 2 | 1 | |
| Viral reactivations | Cytomegalovirus | 2 | 2 |
| Epstein-Barr Virus | 3 | 1 | |
| Sinus | Fusarium | 3 | 1 |
| Unknown | 2 | 1 | |
| Fever | Neutropenic fever | 3 | 3 |
| Fever | 1 | 1 | |
| Gut | Clostridium difficile | 1 | 10 |
RSV, respiratory syncytial virus; UTI, urinary tract infection.
aGVHD and cGVHD
aGVHD organ staging and overall grades are presented in Table 4. Five of 7 patients who experienced aGVHD received all intended PANO doses, and 2 patients with aGVHD had their course interrupted temporarily because of AEs. These 2 patients only received 30% and 45% of intended PANO doses. Median time to aGVHD onset was 38 (range, 18-66) days. PANO was discontinued in 6 out of 7 patients who experienced aGVHD, as they required corticosteroid treatment. Of note, 1 patient who developed aGVHD had received ruxolitinib before HCT, and he responded to reinstitution of ruxolitinib. Another patient developed upper gastrointestinal symptoms upon narcotic withdrawal and demonstrated grade 1 GVHD on upper gastrointestinal biopsies. A third was noncompliant to immune suppression. Four out of 7 subjects required systemic glucocorticoids for GVHD treatment. No patient developed late aGVHD.
Individual aGVHD organ staging and overall aGVHD grade at time of diagnosis
| Patient no. . | GVHD onset day+ . | PANO % dose prior to GVHD onset . | Skin stage . | Gut stage . | Liver stage . | Overall grade . | GVHD treatment with systemic steroids? (> 1 mg/kg) . |
|---|---|---|---|---|---|---|---|
| 1* | 41 | 100 | 1 | 1 | 0 | II | Yes |
| 2 | 53 | 100 | 2 | 1 | 0 | II | Yes |
| 3 | 27 | 30† | 2 | 1 | 0 | II | Yes |
| 4 | 22 | 100 | 0 | 1 | 0 | II | No‡ |
| 5§ | 66 | 97 | 0 | 1 | 0 | II | No |
| 6ǁ | 18 | 100 | 0 | 1 | 0 | II | No |
| 7¶ | 38 | 45# | 0 | 3 | 0 | III | Yes |
| Patient no. . | GVHD onset day+ . | PANO % dose prior to GVHD onset . | Skin stage . | Gut stage . | Liver stage . | Overall grade . | GVHD treatment with systemic steroids? (> 1 mg/kg) . |
|---|---|---|---|---|---|---|---|
| 1* | 41 | 100 | 1 | 1 | 0 | II | Yes |
| 2 | 53 | 100 | 2 | 1 | 0 | II | Yes |
| 3 | 27 | 30† | 2 | 1 | 0 | II | Yes |
| 4 | 22 | 100 | 0 | 1 | 0 | II | No‡ |
| 5§ | 66 | 97 | 0 | 1 | 0 | II | No |
| 6ǁ | 18 | 100 | 0 | 1 | 0 | II | No |
| 7¶ | 38 | 45# | 0 | 3 | 0 | III | Yes |
Noncompliance with immunosuppression prior to GVHD.
PANO held due to rash prior to GVHD.
GVHD resolved with TAC/SIR levels adjustment.
Upper gastrointestinal symptoms suspected due to narcotic withdraw with upper gastrointestinal histology GVHD grade 1. Nausea resolved with supportive care.
Suspected delayed chemotherapy toxicity with upper gastrointestinal histology grade 1 GVHD. Symptoms resolved with supportive care.
Ruxolitinib discontinued within 30 days before HCT and GVHD resolution with steroids and ruxolitinib after HCT.
#PANO held due to AEs (confusion/prolonged QT corrected for heart rate/pneumonia).
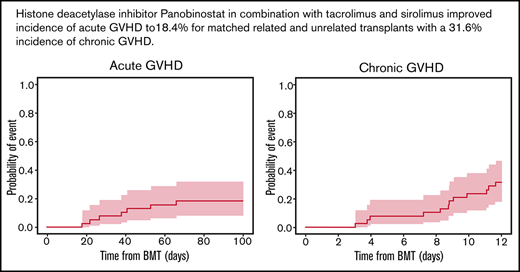
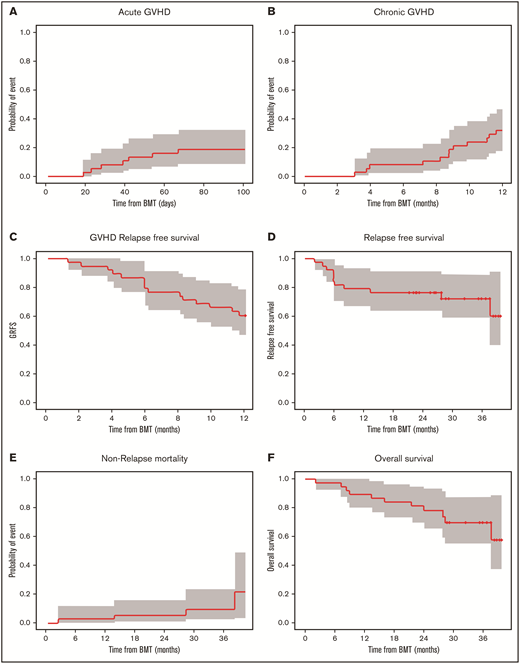
The day 100 cumulative incidence of grade II to IV aGVHD was 18.4% (90% confidence interval [CI], 9.4% to 29.9%) (Figure 2A). The cumulative incidence of any grade of cGVHD was 31.6% (90% CI, 19.5% to 44.3%) (n = 12/35 patients). cGVHD was mild (n = 6), moderate (n = 4), or severe (n = 2) (Figure 2B). Median time from HCT to any grade of cGVHD was 8.8 (range, 3.0-11.6) months, and time from HCT to moderate to severe cGVHD diagnosis was 9.5 (range, 3.9-11.6) months. Maximum severity for cGVHD individual organ staging and overall grades are presented in Table 5.
Cumulative incidence of aGVHD at day 100, cGVHD at 12 months, and GVHD relapse-free survival, relapse-free survival, nonrelapse mortality, and overall survival at 36 months. Kaplan-Meier plots of aGVHD (A) and cGVHD (B), GVHD relapse-free survival (C), relapse-free survival (D), nonrelapse mortality (E), and overall survival (F) are presented. CIs shown are 90% intervals.
Cumulative incidence of aGVHD at day 100, cGVHD at 12 months, and GVHD relapse-free survival, relapse-free survival, nonrelapse mortality, and overall survival at 36 months. Kaplan-Meier plots of aGVHD (A) and cGVHD (B), GVHD relapse-free survival (C), relapse-free survival (D), nonrelapse mortality (E), and overall survival (F) are presented. CIs shown are 90% intervals.
cGVHD scoring according to National Institutes of Health consensus criteria: individual organ severity and global severity score
| . | Evaluable patients no. (%) . |
|---|---|
| Skin | |
| 0 | 30 (85.7) |
| 1 | 2 (5.7) |
| 2 | 3 (8.6) |
| 3 | 0 (0.0) |
| Mouth | |
| 0 | 32 (91.4) |
| 1 | 2 (5.7) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Eye | |
| 0 | 30 (85.7) |
| 1 | 4 (11.4) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Lung | |
| 0 | 33 (94.3) |
| 1 | 1 (2.9) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Gastrointestinal | |
| 0 | 33 (94.3) |
| 1 | 2 (5.7) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Liver | |
| 0 | 28 (80.0) |
| 1 | 5 (14.3) |
| 2 | 1 (2.9) |
| 3 | 1 (2.9) |
| Genital | |
| 0 | 35 (100.0) |
| 1 | 0 (0.0) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Joint/fascia | |
| 0 | 32 (91.4) |
| 1 | 3 (8.6) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Overall grade | |
| 0 | 23 (65.7) |
| 1 | 6 (17.1) |
| 2 | 4 (11.4) |
| 3 | 2 (5.7) |
| . | Evaluable patients no. (%) . |
|---|---|
| Skin | |
| 0 | 30 (85.7) |
| 1 | 2 (5.7) |
| 2 | 3 (8.6) |
| 3 | 0 (0.0) |
| Mouth | |
| 0 | 32 (91.4) |
| 1 | 2 (5.7) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Eye | |
| 0 | 30 (85.7) |
| 1 | 4 (11.4) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Lung | |
| 0 | 33 (94.3) |
| 1 | 1 (2.9) |
| 2 | 1 (2.9) |
| 3 | 0 (0.0) |
| Gastrointestinal | |
| 0 | 33 (94.3) |
| 1 | 2 (5.7) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Liver | |
| 0 | 28 (80.0) |
| 1 | 5 (14.3) |
| 2 | 1 (2.9) |
| 3 | 1 (2.9) |
| Genital | |
| 0 | 35 (100.0) |
| 1 | 0 (0.0) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Joint/fascia | |
| 0 | 32 (91.4) |
| 1 | 3 (8.6) |
| 2 | 0 (0.0) |
| 3 | 0 (0.0) |
| Overall grade | |
| 0 | 23 (65.7) |
| 1 | 6 (17.1) |
| 2 | 4 (11.4) |
| 3 | 2 (5.7) |
Malignancy relapse and mortality
Overall survival at 12 months was 89.5% (90% CI, 81.6% to 98.0%), relapse-free survival was 78.9% (90% CI, 68.8% to 90.6%), nonrelapse mortality was 2.6% (90% CI, 0.3% to 9.9%), and GVHD relapse-free survival was 60.5% (90% CI, 48.8% to 75.1%). At 36 months, relapse-free survival was 72.1% (90% CI: 60.5% to 85.9%), nonrelapse mortality was 9.5% (90% CI, 2.9% to 20.8%), and overall survival was 69.7% (90% CI: 57.2% to 84.9%) for the follow-up period (Figure 2C-F). Among the 38 evaluable patients, deaths were due to infection (n = 1) and relapse (n = 7) during the follow-up period. Disease relapsed occurred in AML (n = 4), ALL (n = 1), or MDS (n = 2) patients who were transplanted with either matched unrelated donor (n = 5) or matched related donor (n = 2) cells.
Pharmacodynamic studies on T-cell subsets
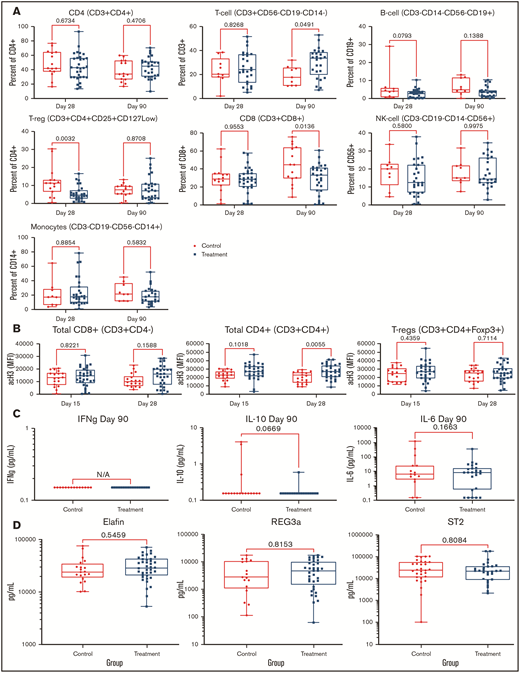
PANO therapy had no significant effect on lymphocyte (CD3, CD4, or CD8), B cell (CD19), monocyte (CD14), T-reg (CD3+/CD4+/CD25+/CD127low), or natural killer cell (CD56) populations compared with that of control patients as measured by flow cytometry on day 90 (±15) and day 30 (±3) (Figure 3A). Acetylation studies showed a trend of increased histone 3 acetylation in CD8, CD4, and T-regs among PANO-treated patients (Figure 3B). Among inflammatory cytokines tested in serum at day 90 (± 3), we were only able to detect IFN-γ, IL-10, and IL-6, with no significant differences observed (Figure 3C). Examining validated GVHD serum biomarkers (REG3α, ST2, and elafin) analyzed by enzyme-linked immunosorbent assay on day 28 (± 3), we found no difference among PANO-treated patients compared with control patients (Figure 3D). In summary, we observed some evidence of on-target effect of the drug combination using PANO with increased trend in histone 3 acetylation in CD8-CD4 and T-regs of PANO-treated subjects vs controls with the understanding that further studies are needed to elucidate mechanism of action.
Pharmacodynamics studies. Data presented among patients treated with PANO/TAC/SIR (squares) compared with control patients (circles) treated with TAC/SIR only. (A) Immune cell populations (CD4, T cells, B cells, CD8, natural killer cells, monocytes, and T-regs) were measured by flow cytometry on days 28 (± 3) and 90 (± 3). (B) Histone 3 acetylation on lymphocyte subsets (CD8+, CD4+, and T-regs) on days 15 (±3) and 28 (±3). (C) IFN plasma, IL-10, and IL-6 levels on day 90 (±15). (D) GVHD biomarkers elafin, REG-3α, and ST2 on day 28 (±3). NK, natural killer.
Pharmacodynamics studies. Data presented among patients treated with PANO/TAC/SIR (squares) compared with control patients (circles) treated with TAC/SIR only. (A) Immune cell populations (CD4, T cells, B cells, CD8, natural killer cells, monocytes, and T-regs) were measured by flow cytometry on days 28 (± 3) and 90 (± 3). (B) Histone 3 acetylation on lymphocyte subsets (CD8+, CD4+, and T-regs) on days 15 (±3) and 28 (±3). (C) IFN plasma, IL-10, and IL-6 levels on day 90 (±15). (D) GVHD biomarkers elafin, REG-3α, and ST2 on day 28 (±3). NK, natural killer.
Discussion
The immune modulation potential of HDACis in stem cell transplantation led us to explore the combination of PANO with TAC/SIR for aGVHD prevention in the setting of matched related or unrelated HCTs using PBSCs. We report an incidence of grade II to IV aGVHD at day 100 lower than the prespecified limit of 31.6% and lower than the rate previously reported in our study using standard immune prophylaxis (43%).4 The study met the primary end point, with only 7 of 38 patients (18.4%) with aGVHD grade II (n = 6) or grade III (n = 1). Although limited by the single-arm design and population skewed to mostly white patients, we are encouraged by the low incidence of grade II to IV aGVHD recorded. These results are similar those using vorinostat with TAC and MTX for GVHD prevention, with a 22% rate of aGVHD grade II though IV in matched related and reduced-intensity conditioning HCT,11,28 therefore solidifying HDACi-class drug contributions in GVHD management.
In our study, we used PANO for 6 months, with the goal of providing extended drug benefit and minimizing relapse post-HCT, as PANO has been shown to improve outcomes for patients with high-risk acute myeloid leukemia/MDS24 and is currently being furthered explored as an epigenetic modulator (NCT04326764). We observed clinically relevant reduction in aGVHD, with an 18% incidence of relapse, suggesting that PANO either contributes to optimizing the graft-versus-leukemia effect or provides a direct antileukemic effect, although any such mechanism remains unknown. An additional benefit of long-term PANO use is prevention of cGVHD, with a reported a cumulative incidence of any grade cGVHD of 31.6% in this study, which compares favorably to historical control of 53%.4
The PANO-mediated prevention strategy resulted in sustained engraftment for 100% of patients and no cases of graft failure. Mucositis was not worsened by the intervention, and observed infections seem acceptable for this patient population. The AEs observed in this trial were largely the expected complications of HCT. AEs possibly related to PANO consisted of reversible thrombocytopenia (n = 5) or leukopenia (n = 6), gastrointestinal toxicity (n = 3), rash (n = 4), renal failure/peripheral edema (n = 1), and periorbital edema (n = 1), all of which have been previously described.29,30
Pharmacodynamic studies demonstrated that PANO seems to hit histone 3 specifically in CD8, CD4, or T-regs as early as day 15 with no major changes in subset populations when compared with control. Because patients had few T-regs shortly after HCT, we could not test the suppressive function of T-regs in our cohort. Furthermore, we did not observe changes in measured plasma biomarkers of GVHD (REG3A, ST2, or elafin) or proinflammatory cytokines measured in serum.
In conclusion, PANO combined with standard GVHD prophylaxis for patients undergoing matched HCT is safe and feasible. This regimen resulted in a low cumulative incidence of clinically significant aGVHD without major AEs. Further randomized trials are warranted to extend these findings. Testing HDACis in the more stringent setting of HLA-mismatched transplantation remains to be explored.
Acknowledgments
The authors thank Maria Urdiales and Jermaine Castro for trial coordination and data acquisitions.
Novartis Pharmaceuticals Corporation supported this trial and provided PANO. Editorial assistance was provided by the Moffitt Cancer Center’s Scientific Editing Department by Paul Fletcher & Daley Drucker. No compensation was given beyond their regular salaries.
Authorship
Contribution: L.P. and C.A. contributed to conceptualization and research design; L.P. contributed to funding acquisition, supervision, and writing the original draft; A.A., J. Powers, and E.S. assisted with and performed the experiments; L.P., H.F., M.K.-D., F.K., B.B., A.M., E.A., F.L.L., L.O.-B., M.N., and J. Pidala performed the clinical trial; R.T. and X.W. analyzed the data; and H.F., M.K.-D., F.K., B.B., A.M., E.A., F.L.L., L.O.-B., M.N., J. Pidala, A.A., J. Powers, E.S., R.T., X.W., and C.A. reviewed and edited the manuscript.
Conflict-of-interest disclosure: F.L.L. has a scientific advisory role with Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, GammaDelta Therapeutics, Iovance, Janssen, Legend Biotech, Novartis, and Wugen; received research funding from Kite Pharma (Institutional), Allogene (Institutional), and Novartis (Institutional); has a consulting role with Cellular Biomedicine Group, Cowen, EcoR1, Emerging Therapy Solutions, and Gerson Lehrman Group; reports education or editorial activity with the American Society of Hematology, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and Society of Immunotherapy of Cancer; and has several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy. The remaining authors declare no completing financial interests.
The current affiliation for E.A. is the Malignant Hematology & Cellular Therapy Department, Mayo Clinic, Jacksonville FL.
The current affiliation for H.F. is the Malignant Hematology & Cellular Therapy Department, Memorial Healthcare System, Pembroke Pines, FL
The current affiliation for M.K.-D. is the Malignant Hematology & Cellular Therapy Department, Mayo Clinic, Jacksonville, FL.
The current affiliation for B.B. is the Cellular Therapy Department of the Masonic Cancer Center, Minneapolis, MN.
References
Author notes
The data that support the findings of this study are available from the corresponding author upon reasonable request (lia.perez@moffitt.org). Deidentified patient data will be made available upon reasonable request.