Abstract
The objective of this study was to perform a systematic review of the literature on vaccine responsiveness in patients who have received anti-CD20 therapy. PubMed and EMBASE were searched up to 4 January 2021 to identify studies of vaccine immunogenicity in patients treated with anti-CD20 therapy, including patients with hematologic malignancy or autoimmune disease. The primary outcomes were seroprotection (SP), seroconversion (SC), and/or seroresponse rates for each type of vaccine reported. As the pandemic influenza vaccine (2009 H1N1) has standardized definitions for SP and SC, and represented a novel primary antigen similar to the COVID-19 vaccine, meta-analysis was conducted for SC of studies of this vaccine. Pooled estimates, relative benefit ratios (RBs), and 95% confidence intervals (CIs) were calculated using a random-effects model. Thirty-eight studies (905 patients treated with anti-CD20 therapy) were included (19 studies of patients with hematologic malignancies). Patients on active (<3 months since last dose) anti-CD20 therapy had poor responses to all types of vaccines. The pooled estimate for SC after 1 pandemic influenza vaccine dose in these patients was 3% (95% CI, 0% to 9%), with an RB of 0.05 (95% CI, 0-0.73) compared with healthy controls and 0.22 (95% CI, 0.09-0.56) compared with disease controls. SC compared with controls seems abrogated for at least 6 months following treatment (3-6 months post anti-CD20 therapy with an RB of 0.50 [95% CI, 0.24-1.06] compared with healthy and of 0.44 [95% CI, 0.23-0.84] compared with disease controls). For all vaccine types, response to vaccination improves incrementally over time, but may not reach the level of healthy controls even 12 months after therapy.
Introduction
Immunocompromised patients including those with hematologic malignancy are at increased risk of infections, including those that are vaccine preventable.1-3 COVID-19 is a viral infection with high mortality in immunocompromised patients.4,5 In healthy vaccine recipients, severe COVID-19 can be prevented; however, patients with immunocompromising conditions and/or those on immunosuppression were excluded from initial vaccine trials.6-9 With the advent of the COVID-19 vaccination rollout, clinicians are faced with questions regarding the effectiveness of vaccination in immunocompromised patients, particularly in those receiving anti-CD20 therapy.
Although anti-CD20 therapy is known to efficiently deplete peripheral B cells, these constitute only 2% of the total B-cell population.10 The effect of anti-CD20 treatment on B cells located in peripheral lymphoid tissues is less clear.11-16 Additionally, anti-CD20 therapy has less of an effect on memory B cells17 and long-lived plasma cells,18 which do not express the CD20 antigen. Furthermore, the peripheral blood B-cell compartment begins to recover ?6 to 9 months after therapy.10 There is limited and conflicting evidence on the ability of anti-CD20–treated patients to mount a vaccine response to both primary and recall vaccinations. Previous reports reviewing vaccine response following anti-CD20 therapy have been limited by their scope.19-21
As the COVID-19 pandemic continues and vaccinations begin, to help inform counseling, public health policies, and future research on vaccination in this vulnerable patient population, reviewing the literature regarding vaccine responsiveness of patients receiving anti-CD20 therapy is critical. The objective of this study was to perform a systematic review of the literature on vaccine responsiveness in patients receiving anti-CD20 therapy.
Methods
This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Definitions
Definitions used in this article are in accordance with the World Health Organization (WHO) Report on Biological Standardization.22 Immunological correlates of protection (ICP) have been established for a number of different vaccines. When an ICP has been established, the seroprotection (SP) rate is the percentage of subjects who achieve an antibody level at or above the ICP. The seroconversion (SC) rate is a predefined increase in serum antibody concentration or titer. In subjects without a detectable or quantifiable antibody (below the lower limit of detection or quantification) prior to vaccination, SC is usually defined as achieving a quantifiable antibody level postvaccination. In subjects with a quantifiable antibody prior to vaccination (due to previous exposure or vaccination), SC is commonly defined by a predefined fold increase from pre- to postvaccination.
SP and SC have been standardized for influenza vaccines.23 For other vaccines (eg, tetanus, diphtheria, pertussis, pneumovax conjugate and pneumovax polysaccharide, Haemophilus influenzae B, hepatitis B, hepatitis A, polio, varicella zoster), SP and SC definitions are not uniformly standardized. In our review, if an article used response criteria that did not meet the WHO definition for either SP or SC as indicated in this section, then the vaccination response rate was described as seroresponse (SR).
Eligibility criteria
All studies published on response to clinically available vaccinations in patients treated with anti-CD20 therapy were considered for inclusion, including noncancer and cancer populations. Only studies reporting SP, SC, and SR rates, and/or measurement of cellular (T-cell) immune response, were included. Case reports, conference proceedings, and studies with <10 patients who had received anti-CD20 therapy were excluded. Only English language reports were included. Full inclusion criteria are available in supplemental Table 1.
Information sources
PubMed and EMBASE databases were searched up to the week of 4 January 2021.
Search and study selection
The full search strategy is available in supplemental Table 2. Two authors (A.V. and I.G.) independently conducted the search strategy, and results were compared with ensure concordance. Differences in classification were discussed and resolved, with a third author (L.K.H.) available for resolution of disagreements. Titles and abstracts of articles were reviewed and any that were clearly irrelevant were excluded. Full texts of remaining articles were reviewed to find studies that met the inclusion criteria. Additionally, systematic reviews discovered in the search that focused on vaccine response in patients receiving immunosuppressive therapy were screened to identify additional references.
Data collection process and data items
A data extraction form was used to extract relevant information from the articles. Information was extracted on study design; geographic location; type of immunogenicity studies conducted (humoral vs cellular); the anti-CD20 drug used by patients; patient population; sample size; median/mean age; distribution by sex; type of vaccine administered; how response was defined; SP, SC, and SR rates; cellular response data if available; and safety data for patients with hematologic malignancies. Patients were considered to be on active treatment if the average interval between anti-CD20 therapy and vaccination was <12 weeks; otherwise, treatment durations were divided into average interval 3 to 6 months, 6 to 12 months, and >12 months. Data on controls (healthy and disease controls not receiving anti-CD20 therapy) in the study and their outcomes were also extracted.
Risk of bias in individual studies
To assess the quality of cohort studies and substudies of trials with a comparison group, the Newcastle-Ottawa assessment tool was used.24,25 For 1-arm cohort studies, the Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies was used.26 For randomized studies, the Cochrane Risk of Bias Scale was used.27 See supplemental Table 3 for details on each tool. Assessment was conducted by 2 authors (A.V. and I.G.), while a third author (L.K.H.) was available for differences of opinion.
Summary measures
The primary outcomes were the SP, SC, and/or SR rates to each vaccine. As the pandemic influenza vaccination has standardized definitions for SP and SC,23 this enabled meta-analysis of point estimates of SC to vaccination in patients treated with anti-CD20 therapy, as well as relative benefit ratios (RBs) comparing SC in anti-CD20–treated patients to healthy or disease controls. Secondary outcomes were cellular response to vaccinations, and safety data on vaccinations in patients with hematologic malignancies. Due to limitations in data, subgroup and sensitivity analyses were not performed.
Synthesis of results
The majority of the data synthesis was descriptive. For meta-analysis, the principal summary measures used were pooled estimates and RBs with 95% confidence intervals (CIs). Heterogeneity between estimates was assessed using the I2 statistic, and interpreted per the Cochrane Handbook recommendations: I2 0% to 40%, heterogeneity likely not substantial; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity.28 Pooled estimates were transformed using the Freeman-Tukey double arcsine method,29 and the final pooled results were back-transformed with a 95% CI for ease of interpretation. Meta-analysis was performed using a random-effects model (DerSimonian and Laird) using the MetaXL (www.epigear.com) add-in for Microsoft Excel, as well as Review Manager 5.4 (Cochrane Collaboration, 2020).
Results
Study selection
Figure 1 shows the flow diagram for study selection. A total of 38 peer-reviewed studies (31 cohort, 3 phase 3, 2 phase 3 substudies, 1 phase 1, 1 phase 1/2 substudy) comprising 905 anti-CD20–treated patients from North America, Europe, and Asia were included. Nineteen studies assessed vaccine responses in patients receiving anti-CD20 for hematologic malignancy; 19 studies assessed vaccine responses in patients receiving anti-CD20 for autoimmune diseases such as rheumatoid arthritis or multiple sclerosis.
Study characteristics
Tables 1 and 2 list the summary characteristics of the included studies. Supplemental Table 4 lists study definitions of vaccine response assessment.
Literature summary: humoral responses
| Study . | Design . | Location . | Anti-CD20 drug . | Population/ Control population . | N . | Median age, y* . | Sex, % F . | Interval between anti-CD20 and vaccination . | SP % . | SC % . | SR % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-cell–dependent vaccinations | |||||||||||
| Pandemic influenza | |||||||||||
| Bedognetti et al, 201244 | Cohort | Italy | RTX | NHL in CR; Healthy controls | 14; 14 | NR | 33 mo (range, 14-78 mo) | D1: 93, D2: 93; D1: 86, D2: 93 | D1: 36, D2: 64; D1: 71; D2: 79 | ||
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls with cancer on active treatment other than RTX | 13; 83 | 63† | 59† | All patients on active therapy | D1: 0, D2: 8; D1: 62, D2: 87 | D1: 0, D2: 0; D1: 62, D2: 84 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | B-cell malignancies | 12 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 33, D2: 42; 5 patients, <6 mo: D1: 0, D2: 0; 4 patients, 6-12 mo: D1: 50, D2: 50; 3 patients, >12 mo: D1: 66, D2: 100 | D1: 33, D2: 42; 5 patients, <6 mo: D1: 0, D2: 0; 4 patients, 6-12 mo: D1: 50, D2: 50; 3 patients, >12 mo: D1: 66, D2: 100 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls of B-cell malignancy not previously treated | 9 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 56, D2: 89 | D1: 78, D2: 78 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls postallotransplant | 22 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 46, D2: 73 | D1: 46, D2: 73 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls with chronic myeloid leukemia | 20 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 85, D2: 90 | D1: 80, D2: 95 | |
| Hottinger et al, 201231 | Cohort | Switzerland | RTX | Lymphoma on treatment; Healthy controls; Lymphoma on active treatment without RTX; Lymphoma not on active treatment | 11; 138; 51; 119 | 61† | 47† | On active therapy | D1: 0, D2: 27; 87; D1: 75, D2: 92; D1: 79, D2: 90 | D1: 0, D2: 18; 87; D1: 75, D2: 92; D1: 79, D2: 87 | |
| Ide et al, 201445 | Cohort | Japan | RTX | Lymphoma and acute leukemia | 11 | 59† | 60† | NR | D1: 0, D2: 0 | D1: 0, D2: 9 | |
| Issa et al, 201159 | Cohort | USA | RTX | Postallotransplant with RTX exposure in previous 1 y; Disease controls with no previous RTX exposure | 11; 71 | 55† | 51† | <12 mo | 9; 58 | ||
| Kapetanovic et al, 201432 | Cohort | Sweden | RTX | RA; Disease controls RA and spondyloarthropathy on non-RTX treatments | 10; 281 | 62 | 80 | On active therapy | 6 patients: 1D, 17, 4 patients: 2D, 25; 1D: 50, 2D: 65 | 6 patients: 1D, 0, 4 patients: 2D, 25; 1D: 45, 2D: 56 | |
| Kim et al, 201360 | Cohort | Korea | RTX | NMOSD; Healthy controls; Disease controls on MMF; Disease controls on IFN or azathioprine | 16; 8; 5; 14 | 39 | 81 | 20 wk (range, 1-45 wk) | 19; 100; 20; 86 | 38; 75; 40; 93 | |
| Mackay et al, 201133 | Cohort | Canada | RTX | Hematologic malignancy; Disease controls with hematologic malignancy on active therapy without RTX; Disease controls with solid tumor on active therapy | 11; 15; 20 | 53† | 35† | On active therapy | 9; 40; 50 | 0; 33; 45 | |
| Monkman et al, 201134 | Cohort | Canada | RTX | Hematologic malignancy; Disease controls who had never received RTX | 16; 46 | 67 | 47 | 12 patients: on active therapy; 4 patients: previously received (unknown length of time since last treatment) | Active RTX: 33 | Active RTX: 17 and previous RTX: 13; 24 | |
| Muller et al, 201343 | Cohort | Switzerland | RTX | Autoimmune rheumatic diseases | 16 | 45 | 88 | Median, 6 mo (range, 1-36 mo) | D1: 38, D2: 44 | ||
| Villa et al, 201337 | Phase 3 | Canada | RTX | Lymphoid malignancies on active chemotherapy or within 1 y following ASCT; Disease controls who had not received RTX | 14; 26 | 49-57† | 60† | On active therapy, therapy within last 3 mo, or ASCT within past year | D1: 14, D2: 21; D1: 35, D2: 46 | D1: 0, D2: 14; D1: 27, D2: 39 | |
| Yri et al, 201161 | Cohort | Norway | RTX | Lymphoma; Healthy controls | 67; 51 | 63 | 43 | Median, 21 d (range, 0-171 d) | 0; 82 | ||
| Seasonal influenza | |||||||||||
| Arad et al, 201139 | Cohort | Israel | RTX | RA; Healthy controls; Disease controls on DMARDs | 29; 16; 17 | 62 | 79 | 16 patients: <5 mo; 13 patients: >5 mo | 58-97; 88-100; 88-100 | 14-35 (avg. 26); 38-44 (avg. 42); 61-78 (avg. 68) | |
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls on IFN or no treatment | 68; 34 | 40 | 66 | 10-18 wk | 56-80; 75-97 | 10-60; 53-89 | |
| Bedognetti et al, 201152 | Cohort | Italy | RTX | NHL in CR; Healthy controls | 31; 34 | 66 | 61 | 29 mo (range, 7-65 mo) | 23-74; 44-94 | 3-29; 29-53 | |
| Bedognetti et al, 201244 (conjugated seasonal influenza) | Cohort | Italy | RTX | NHL in CR; Healthy controls | 14; 14 | 65† | NR | 33 mo (range, 14-78 mo) | 57-79; 86-100 | 29-43; 43-79 | |
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls of patients with cancer on active treatment | 13; 83 | 63† | 59† | All patients on active treatment | 8-17; 59-70 | 0; 42-50 | |
| Cho et al, 201763 | Cohort | USA | RTX | Autoimmune blistering skin diseases; Healthy controls | 23; 28 | 51 | 65 | 11 mo (range, 5-24 mo) | 69-77; 64-100 | ||
| Eisenberg et al, 201342 | Cohort | USA | RTX | Rheumatologic disease; Healthy controls | 17; 15 | 49 | 94 | 7-9 mo | 17; 67 | ||
| Oren et al, 200851 | Cohort | Israel | RTX | RA; Healthy controls; Disease controls treated with DMARDs | 14; 21; 29 | 53 | 76 | 7 patients: <6 mo; All patients: <18 mo; no difference in interval between responders and non-responders | 21-36; 40-45; 30-67 | ||
| Richi et al, 201964 | Cohort | Spain | RTX | Autoimmune inflammatory diseases (AIRD, psoriasis, or IBD) | 20 | 49† | 59† | NR | 40-55; ≥12 wk: 80; <12 wk: 25 | ||
| van Assen et al, 201035 | Cohort | The Netherlands | RTX | RA; Healthy controls; Disease controls on MTX | 23; 29; 20 | 56 | 70 | 11 patients: 4-8 wk; 12 patients: 6-10 mo | 17-26; 48-86; 25-65 | 4-8 wk: 0; 6-10 mo: 25 | |
| Tetanus | |||||||||||
| Albert et al, 200865 | Cohort | USA | RTX | SLE | 14 | 43 | 93 | 7 mo | 36 | ||
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls on IFN or no treatment | 68; 34 | 40 | 66 | 10 wk | 100; 100 | 24; 55 | |
| Binghamet al, 201066 | Phase 3 | USA | RTX | Active RA; Disease controls on MTX | 68; 32 | 50 | 78 | 22-26 wk | 39; 42 | ||
| Bühler et al, 201967 | Cohort | Switzerland | RTX | RA and vasculitis; Healthy controls | 11; 253 | 52† | 57† | 4.9 mo (IQR, 3.7-5.6 mo) | 73; 100 | 9; 47 | |
| Colucci et al, 201953 | Cohort | Italy | RTX and OFA | Frequently relapsing/ steroid- dependent pediatric idiopathic nephrotic syndrome | 11 | 19† | 33† | 36 mo (range, 10-82 mo) | 9 | 9 | |
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and -consolidation RTX | 22 | 51† | 34† | 6-9 mo | 64-68 | ||
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 93 | 7 | |
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; Disease controls treated with placebo | 46; 29 | 20 | 37 | 44 wk | 67; 83 | ||
| Puissant-Lubrano et al, 201050 | Cohort | France | RTX | Renal transplant; Disease controls who had not received RTX | 13; 26 | 55 | 23 | Median, 9 mo (IQR, 4-11.5 mo) | 92 96 | 31 61 | |
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 58; 65 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 25 | ||
| Diphtheria | |||||||||||
| Bühler et al, 201967 | Cohort | Switzerland | RTX | RA and vasculitis; Healthy controls | 11; 253 | 52† | 57† | Median, 4.9 mo (IQR, 3.7-5.6 mo) | 64; 84-88 | 43 | |
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 67 | 20 | |
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; Type 1 DM treated with placebo | 46; 29 | 20 | 37 | 44 wk | 69; 76 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 75; 70 | ||
| Pertussis | |||||||||||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 60; 66 | ||
| Small et al, 200947 | Cohort | USA | RTX | Post-ASCT RTX for NHL | 17 | 45† | NR | Median, 31 mo in all (pre-transplant RTX and post) | 0 | 0 | |
| Haemophilus influenza B | |||||||||||
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and consolidation RTX | 22 | 51† | 34† | 6-9 mo | D1: 73, D2: 77 | ||
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP; Disease controls treated with placebo | 17; 7 | 40 | 71 | 6 mo | 29; 83 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 85; 89 | ||
| Hepatitis B | |||||||||||
| Avivi et al, 201971 | Cohort | Israel | RTX | NHL who met criteria for “minimal B-cell recovery”; Healthy controls, ≤35 y; Healthy controls, ≥55 y | 22; 8; 17 | 65 | NR | 38 mo (range, 14-56 mo) | 64; 100; 59 | ||
| Jaffe et al, 200654 | Cohort | USA | RTX | Postallotransplant; Postallotransplant who had not received RTX | 25; 244 | 24† | 38† | Median, 17 mo | 56 | 56; 65 | |
| Richi et al, 202072 | Cohort | Spain | RTX | Autoimmune inflammatory diseases (AIRD, psoriasis, or IBD); Healthy controls; Autoimmune inflammatory diseases on other biological DMARDs; Autoimmune inflammatory diseases on synthetic DMARDs | 14; 49; 173; 48 | 56 | 61† | NR | 29; 98; 86; 94 | 29; 98; 86; 94 | |
| Hepatitis A virus | |||||||||||
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; T1DM treated with placebo | 46; 29 | 20 | 37 | 44 wk | 47; 67 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 0 | ||
| Polio virus | |||||||||||
| Jaffe et al, 200654 | Cohort | USA | RTX | Postallotransplant; Postallotransplant who had not received RTX | 25; 244 | 24† | 38† | NR; HBV in trial given after median 16.6 mo | 91; 91 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 62; 80 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 20 | ||
| Pneumococcal conjugate (PCV-7) | |||||||||||
| Kapetanovic et al, 201373 | Cohort | Sweden | RTX | RA; Disease controls on abatacept; Disease controls on tocilizumab; Disease controls on MTX; Alternate disease controls with spondyloarthropathy on anti-inflammatories and analgesics | 55; 17; 16; 85; 86 | 65 | 67 | 86 d (range, 0-894 d) | 5; 18; 50; 21; 48 | ||
| T-cell–independent vaccinations | |||||||||||
| Pneumococcal polysaccharide (PCV-23) | |||||||||||
| Albert et al, 200865 | Cohort | USA | RTX | SLE | 14 | 43 | 93 | 7 mo | 29 | ||
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls: relapsing MS on IFN or no treatment | 68; 34 | 40 | 66 | 14 wk | 37% to ≥12 serotypes; 97% to ≥12serotypes | ||
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls with cancer on active treatment other than RTX | 13; 83 | 63† | 59† | All patients on active treatment | 25; 68 | 0; 42 | |
| Bingham et al, 201066 | Phase 3 | USA | RTX | Active RA; Disease controls: active RA on MTX alone | 68; 32 | 50 | 78 | 22-26 wk | 19% to ≥6 serotypes; 61% to ≥6 serotypes | ||
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and consolidation RTX | 22 | 51† | 34† | 6-9 mo | 41 | ||
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 33% to ≥7 serotypes | 20 | |
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP ITP treated with placebo | 17; 7 | 40 | 71 | 6 mo | 21; 67 |
| Study . | Design . | Location . | Anti-CD20 drug . | Population/ Control population . | N . | Median age, y* . | Sex, % F . | Interval between anti-CD20 and vaccination . | SP % . | SC % . | SR % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-cell–dependent vaccinations | |||||||||||
| Pandemic influenza | |||||||||||
| Bedognetti et al, 201244 | Cohort | Italy | RTX | NHL in CR; Healthy controls | 14; 14 | NR | 33 mo (range, 14-78 mo) | D1: 93, D2: 93; D1: 86, D2: 93 | D1: 36, D2: 64; D1: 71; D2: 79 | ||
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls with cancer on active treatment other than RTX | 13; 83 | 63† | 59† | All patients on active therapy | D1: 0, D2: 8; D1: 62, D2: 87 | D1: 0, D2: 0; D1: 62, D2: 84 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | B-cell malignancies | 12 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 33, D2: 42; 5 patients, <6 mo: D1: 0, D2: 0; 4 patients, 6-12 mo: D1: 50, D2: 50; 3 patients, >12 mo: D1: 66, D2: 100 | D1: 33, D2: 42; 5 patients, <6 mo: D1: 0, D2: 0; 4 patients, 6-12 mo: D1: 50, D2: 50; 3 patients, >12 mo: D1: 66, D2: 100 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls of B-cell malignancy not previously treated | 9 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 56, D2: 89 | D1: 78, D2: 78 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls postallotransplant | 22 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 46, D2: 73 | D1: 46, D2: 73 | |
| de Lavallade et al, 201140 | Cohort | UK | RTX | Disease controls with chronic myeloid leukemia | 20 | 57† | 40† | 19 mo (range, 2-83 mo) | D1: 85, D2: 90 | D1: 80, D2: 95 | |
| Hottinger et al, 201231 | Cohort | Switzerland | RTX | Lymphoma on treatment; Healthy controls; Lymphoma on active treatment without RTX; Lymphoma not on active treatment | 11; 138; 51; 119 | 61† | 47† | On active therapy | D1: 0, D2: 27; 87; D1: 75, D2: 92; D1: 79, D2: 90 | D1: 0, D2: 18; 87; D1: 75, D2: 92; D1: 79, D2: 87 | |
| Ide et al, 201445 | Cohort | Japan | RTX | Lymphoma and acute leukemia | 11 | 59† | 60† | NR | D1: 0, D2: 0 | D1: 0, D2: 9 | |
| Issa et al, 201159 | Cohort | USA | RTX | Postallotransplant with RTX exposure in previous 1 y; Disease controls with no previous RTX exposure | 11; 71 | 55† | 51† | <12 mo | 9; 58 | ||
| Kapetanovic et al, 201432 | Cohort | Sweden | RTX | RA; Disease controls RA and spondyloarthropathy on non-RTX treatments | 10; 281 | 62 | 80 | On active therapy | 6 patients: 1D, 17, 4 patients: 2D, 25; 1D: 50, 2D: 65 | 6 patients: 1D, 0, 4 patients: 2D, 25; 1D: 45, 2D: 56 | |
| Kim et al, 201360 | Cohort | Korea | RTX | NMOSD; Healthy controls; Disease controls on MMF; Disease controls on IFN or azathioprine | 16; 8; 5; 14 | 39 | 81 | 20 wk (range, 1-45 wk) | 19; 100; 20; 86 | 38; 75; 40; 93 | |
| Mackay et al, 201133 | Cohort | Canada | RTX | Hematologic malignancy; Disease controls with hematologic malignancy on active therapy without RTX; Disease controls with solid tumor on active therapy | 11; 15; 20 | 53† | 35† | On active therapy | 9; 40; 50 | 0; 33; 45 | |
| Monkman et al, 201134 | Cohort | Canada | RTX | Hematologic malignancy; Disease controls who had never received RTX | 16; 46 | 67 | 47 | 12 patients: on active therapy; 4 patients: previously received (unknown length of time since last treatment) | Active RTX: 33 | Active RTX: 17 and previous RTX: 13; 24 | |
| Muller et al, 201343 | Cohort | Switzerland | RTX | Autoimmune rheumatic diseases | 16 | 45 | 88 | Median, 6 mo (range, 1-36 mo) | D1: 38, D2: 44 | ||
| Villa et al, 201337 | Phase 3 | Canada | RTX | Lymphoid malignancies on active chemotherapy or within 1 y following ASCT; Disease controls who had not received RTX | 14; 26 | 49-57† | 60† | On active therapy, therapy within last 3 mo, or ASCT within past year | D1: 14, D2: 21; D1: 35, D2: 46 | D1: 0, D2: 14; D1: 27, D2: 39 | |
| Yri et al, 201161 | Cohort | Norway | RTX | Lymphoma; Healthy controls | 67; 51 | 63 | 43 | Median, 21 d (range, 0-171 d) | 0; 82 | ||
| Seasonal influenza | |||||||||||
| Arad et al, 201139 | Cohort | Israel | RTX | RA; Healthy controls; Disease controls on DMARDs | 29; 16; 17 | 62 | 79 | 16 patients: <5 mo; 13 patients: >5 mo | 58-97; 88-100; 88-100 | 14-35 (avg. 26); 38-44 (avg. 42); 61-78 (avg. 68) | |
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls on IFN or no treatment | 68; 34 | 40 | 66 | 10-18 wk | 56-80; 75-97 | 10-60; 53-89 | |
| Bedognetti et al, 201152 | Cohort | Italy | RTX | NHL in CR; Healthy controls | 31; 34 | 66 | 61 | 29 mo (range, 7-65 mo) | 23-74; 44-94 | 3-29; 29-53 | |
| Bedognetti et al, 201244 (conjugated seasonal influenza) | Cohort | Italy | RTX | NHL in CR; Healthy controls | 14; 14 | 65† | NR | 33 mo (range, 14-78 mo) | 57-79; 86-100 | 29-43; 43-79 | |
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls of patients with cancer on active treatment | 13; 83 | 63† | 59† | All patients on active treatment | 8-17; 59-70 | 0; 42-50 | |
| Cho et al, 201763 | Cohort | USA | RTX | Autoimmune blistering skin diseases; Healthy controls | 23; 28 | 51 | 65 | 11 mo (range, 5-24 mo) | 69-77; 64-100 | ||
| Eisenberg et al, 201342 | Cohort | USA | RTX | Rheumatologic disease; Healthy controls | 17; 15 | 49 | 94 | 7-9 mo | 17; 67 | ||
| Oren et al, 200851 | Cohort | Israel | RTX | RA; Healthy controls; Disease controls treated with DMARDs | 14; 21; 29 | 53 | 76 | 7 patients: <6 mo; All patients: <18 mo; no difference in interval between responders and non-responders | 21-36; 40-45; 30-67 | ||
| Richi et al, 201964 | Cohort | Spain | RTX | Autoimmune inflammatory diseases (AIRD, psoriasis, or IBD) | 20 | 49† | 59† | NR | 40-55; ≥12 wk: 80; <12 wk: 25 | ||
| van Assen et al, 201035 | Cohort | The Netherlands | RTX | RA; Healthy controls; Disease controls on MTX | 23; 29; 20 | 56 | 70 | 11 patients: 4-8 wk; 12 patients: 6-10 mo | 17-26; 48-86; 25-65 | 4-8 wk: 0; 6-10 mo: 25 | |
| Tetanus | |||||||||||
| Albert et al, 200865 | Cohort | USA | RTX | SLE | 14 | 43 | 93 | 7 mo | 36 | ||
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls on IFN or no treatment | 68; 34 | 40 | 66 | 10 wk | 100; 100 | 24; 55 | |
| Binghamet al, 201066 | Phase 3 | USA | RTX | Active RA; Disease controls on MTX | 68; 32 | 50 | 78 | 22-26 wk | 39; 42 | ||
| Bühler et al, 201967 | Cohort | Switzerland | RTX | RA and vasculitis; Healthy controls | 11; 253 | 52† | 57† | 4.9 mo (IQR, 3.7-5.6 mo) | 73; 100 | 9; 47 | |
| Colucci et al, 201953 | Cohort | Italy | RTX and OFA | Frequently relapsing/ steroid- dependent pediatric idiopathic nephrotic syndrome | 11 | 19† | 33† | 36 mo (range, 10-82 mo) | 9 | 9 | |
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and -consolidation RTX | 22 | 51† | 34† | 6-9 mo | 64-68 | ||
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 93 | 7 | |
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; Disease controls treated with placebo | 46; 29 | 20 | 37 | 44 wk | 67; 83 | ||
| Puissant-Lubrano et al, 201050 | Cohort | France | RTX | Renal transplant; Disease controls who had not received RTX | 13; 26 | 55 | 23 | Median, 9 mo (IQR, 4-11.5 mo) | 92 96 | 31 61 | |
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 58; 65 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 25 | ||
| Diphtheria | |||||||||||
| Bühler et al, 201967 | Cohort | Switzerland | RTX | RA and vasculitis; Healthy controls | 11; 253 | 52† | 57† | Median, 4.9 mo (IQR, 3.7-5.6 mo) | 64; 84-88 | 43 | |
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 67 | 20 | |
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; Type 1 DM treated with placebo | 46; 29 | 20 | 37 | 44 wk | 69; 76 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 75; 70 | ||
| Pertussis | |||||||||||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 60; 66 | ||
| Small et al, 200947 | Cohort | USA | RTX | Post-ASCT RTX for NHL | 17 | 45† | NR | Median, 31 mo in all (pre-transplant RTX and post) | 0 | 0 | |
| Haemophilus influenza B | |||||||||||
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and consolidation RTX | 22 | 51† | 34† | 6-9 mo | D1: 73, D2: 77 | ||
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP; Disease controls treated with placebo | 17; 7 | 40 | 71 | 6 mo | 29; 83 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 85; 89 | ||
| Hepatitis B | |||||||||||
| Avivi et al, 201971 | Cohort | Israel | RTX | NHL who met criteria for “minimal B-cell recovery”; Healthy controls, ≤35 y; Healthy controls, ≥55 y | 22; 8; 17 | 65 | NR | 38 mo (range, 14-56 mo) | 64; 100; 59 | ||
| Jaffe et al, 200654 | Cohort | USA | RTX | Postallotransplant; Postallotransplant who had not received RTX | 25; 244 | 24† | 38† | Median, 17 mo | 56 | 56; 65 | |
| Richi et al, 202072 | Cohort | Spain | RTX | Autoimmune inflammatory diseases (AIRD, psoriasis, or IBD); Healthy controls; Autoimmune inflammatory diseases on other biological DMARDs; Autoimmune inflammatory diseases on synthetic DMARDs | 14; 49; 173; 48 | 56 | 61† | NR | 29; 98; 86; 94 | 29; 98; 86; 94 | |
| Hepatitis A virus | |||||||||||
| Pescovitz et al, 201170 | Phase 3 substudy | USA | RTX | Type 1 DM; T1DM treated with placebo | 46; 29 | 20 | 37 | 44 wk | 47; 67 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 0 | ||
| Polio virus | |||||||||||
| Jaffe et al, 200654 | Cohort | USA | RTX | Postallotransplant; Postallotransplant who had not received RTX | 25; 244 | 24† | 38† | NR; HBV in trial given after median 16.6 mo | 91; 91 | ||
| Shah et al, 201546 | Cohort | USA | RTX | Postallogeneic CBT for heme malignancy, disease free 6 mo posttransplant; Disease controls who had not received RTX | 13; 48 | 34 | NR | Median, 15 mo (range, 3-35 mo) | 62; 80 | ||
| van der Kolk et al, 200236 | Phase 1/2 substudy | The Netherlands | RTX | Relapsed low-grade lymphoma | 11 | 53 | NR | 4 wk | 20 | ||
| Pneumococcal conjugate (PCV-7) | |||||||||||
| Kapetanovic et al, 201373 | Cohort | Sweden | RTX | RA; Disease controls on abatacept; Disease controls on tocilizumab; Disease controls on MTX; Alternate disease controls with spondyloarthropathy on anti-inflammatories and analgesics | 55; 17; 16; 85; 86 | 65 | 67 | 86 d (range, 0-894 d) | 5; 18; 50; 21; 48 | ||
| T-cell–independent vaccinations | |||||||||||
| Pneumococcal polysaccharide (PCV-23) | |||||||||||
| Albert et al, 200865 | Cohort | USA | RTX | SLE | 14 | 43 | 93 | 7 mo | 29 | ||
| Bar-Or et al, 202062 | Phase 3 | USA and Canada | OCR | Relapsing MS; Disease controls: relapsing MS on IFN or no treatment | 68; 34 | 40 | 66 | 14 wk | 37% to ≥12 serotypes; 97% to ≥12serotypes | ||
| Berglund et al, 201430 | Cohort | Sweden | RTX | Lymphoma on treatment; Disease controls with cancer on active treatment other than RTX | 13; 83 | 63† | 59† | All patients on active treatment | 25; 68 | 0; 42 | |
| Bingham et al, 201066 | Phase 3 | USA | RTX | Active RA; Disease controls: active RA on MTX alone | 68; 32 | 50 | 78 | 22-26 wk | 19% to ≥6 serotypes; 61% to ≥6 serotypes | ||
| Horwitz et al, 200468 | Cohort | USA | RTX | R/R BCL post-ASCT and consolidation RTX | 22 | 51† | 34† | 6-9 mo | 41 | ||
| Mustafa et al, 202069 | Cohort | USA | RTX | B-cell NHL | 15 | 71 | 48 | Median, 9 mo (range, 1-24 mo; IQR, 9-13 mo) | 33% to ≥7 serotypes | 20 | |
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP ITP treated with placebo | 17; 7 | 40 | 71 | 6 mo | 21; 67 |
1D, response at the end of 1 dose of a 1-dose series; 2D, response at the end of 2 doses of a 2-dose series; AIRD, autoimmune rheumatic disease; ASCT, autologous stem cell transplant; CBT, cord blood transplant; CR, complete response; D1, dose 1 of a 2-dose series; D2, dose 2 of a 2-dose series; DM, diabetes mellitus; DMARD, disease-modifying anti-rheumatic drug; F, female; HBV, hepatitis B virus; IBD, inflammatory bowel disease; IFN, interferon; IQR, interquartile range; ITP, immune thrombocytopenia; MMF, mycophenolate mofetil; MS, multiple sclerosis; MTX, methotrexate; NHL, non-Hodgkin lymphoma; NMOSD, neuromyelitis optica spectrum disorder; NR, not reported; OCR, ocrelizumab; OFA, ofatumumab; RA, rheumatoid arthritis; R/R BCL, relapsed/refractory B-cell lymphoma; RTX, rituximab; SLE, systemic lupus erythematosus; SC, seroconversion; SP, seroprotection; SR, seroresponse; T1DM, type 1 diabetes mellitus; UK, United Kingdom; USA, United States of America.
Median age; where not reported, mean age reported.
Rituximab-specific age and percentage female not reported, thus age and percentage female of entire study population reported.
Literature summary: cellular responses
| Study, year . | Design . | Location . | Anti-CD20 drug . | Population . | N . | Median age, y* . | Sex, % F . | Interval between anti-CD20 and vaccination . | Vaccine type . | Measurement of T-cell response and outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|
| Arad et al, 201139 | Cohort | Israel | RTX | RA | 29 | 62 | 79 | 16 patients: <5 mo; 13 patients: >5 mo | Seasonal influenza | Measurement: Flow cytometry for activated CD4 T cells; influenza-specific cells were defined as the percentage of CD69+/IFN-γ–producing cells within the total CD4+ T-cell population in the antigen-stimulated cultures subtracted by those in the negative control cultures Outcome: No difference in percentage increase in influenza-specific CD4+ subsets in patients treated with anti-CD20 therapy compared with DMARD-treated patients (median, 0.1% to 0.3% and 0.1% to 0.2%, respectively); healthy controls had higher baseline influenza-specific CD4+ T cells, but their CD4+ subsets decreased by half postvaccination (0.6% to 0.3%) |
| de Lavallade et al, 201140 | Cohort | United Kingdom | RTX | B-cell malignancies | 12 | 57† | 40† | 19 mo (range, 2-83 mo) | Pandemic influenza | Measurement: Effector function of antigen-specific CD8+ and CD4+ T cells assessed by intracellular-cytokine staining for IFN-γ and TNF-α; response considered positive if combined percentage of H1N1-specific TNF-α plus IFN-γ–producing CD4+ or CD8+ T cells was twofold or higher compared with background level (nonstimulated PBMCs) and if there was a minimum of 0.05% H1N1-specific TNF-α plus IFN-γ–producing CD4+ or CD8+ T cells (after subtracting background) Outcome: Cellular data only available for all B-cell malignancy patients together (not RTX specific); 7% pre- and 36% postvaccine had H1N1-specific T-cell response; no effect of prior chemotherapy; healthy controls had 44% pre and 48% post; no effect of prior chemotherapy on induction of H1N1-specific T cells after 2 doses of vaccine (data NR) |
| Eisenberg et al, 201342 | Cohort | United States | RTX | Rheumatologic disease | 17 | 49 | 94 | 7-9 mo | Seasonal influenza | Measurement: T-cell ELISPOTs were performed using a standard γ-IFN T-cell ELISPOT assay Outcome: T-cell responses similar in anti-CD20–treated patients at baseline compared with healthy controls, but no significant increases after vaccination, no increase in proliferation (data NR); no changes in the T-cell repertoire as detected by spectratyping over time (data NR) |
| Muller et al, 201343 | Cohort | Switzerland | RTX | Autoimmune rheumatic diseases | 16 | 45 | 88 | Median, 6 mo (range, 1-36 mo) | Pandemic influenza | Measurement: Presence of IFN-γ–producing 2009 H1N1 influenza virus–specific CD4+ and CD8+ T cells Outcomes: After first vaccination, virus-specific CD4+ and CD8+ T-cell responses were significantly lower in patients with low B cells than those with normal B cells; booster vaccination stimulated the antiviral T-cell response only in patients with low B cells |
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP | 17 | 40 | 71 | 6 mo | Tetanus toxoid | Measurement: IFN-γ ELISPOT assay to measure frequency of tetanus toxoid–specific T cells Outcomes: Mean number of IFN-γ–producing T cells was reduced in patients who had received rituximab compared with placebo at 1 wk (38 cells vs 93 cells per 5 × 105 total cells) and 1 mo (14 cells vs 43 cells per 5 × 105 total cells) |
| Parrino et al, 201741 | Phase 1 | Worldwide | RTX | B-cell lymphoma | 80 | 61 | 54 | On active treatment | Inactivated VZV | Measurement: IFN-γ ELISPOT assay was used to detect the presence of IFN-γ–secreting VZV-specific cells from PBMCs before and after immunization Outcome: GMFR met primary outcome at 4.34 (95% CI, 3.01-6.24) |
| Study, year . | Design . | Location . | Anti-CD20 drug . | Population . | N . | Median age, y* . | Sex, % F . | Interval between anti-CD20 and vaccination . | Vaccine type . | Measurement of T-cell response and outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|
| Arad et al, 201139 | Cohort | Israel | RTX | RA | 29 | 62 | 79 | 16 patients: <5 mo; 13 patients: >5 mo | Seasonal influenza | Measurement: Flow cytometry for activated CD4 T cells; influenza-specific cells were defined as the percentage of CD69+/IFN-γ–producing cells within the total CD4+ T-cell population in the antigen-stimulated cultures subtracted by those in the negative control cultures Outcome: No difference in percentage increase in influenza-specific CD4+ subsets in patients treated with anti-CD20 therapy compared with DMARD-treated patients (median, 0.1% to 0.3% and 0.1% to 0.2%, respectively); healthy controls had higher baseline influenza-specific CD4+ T cells, but their CD4+ subsets decreased by half postvaccination (0.6% to 0.3%) |
| de Lavallade et al, 201140 | Cohort | United Kingdom | RTX | B-cell malignancies | 12 | 57† | 40† | 19 mo (range, 2-83 mo) | Pandemic influenza | Measurement: Effector function of antigen-specific CD8+ and CD4+ T cells assessed by intracellular-cytokine staining for IFN-γ and TNF-α; response considered positive if combined percentage of H1N1-specific TNF-α plus IFN-γ–producing CD4+ or CD8+ T cells was twofold or higher compared with background level (nonstimulated PBMCs) and if there was a minimum of 0.05% H1N1-specific TNF-α plus IFN-γ–producing CD4+ or CD8+ T cells (after subtracting background) Outcome: Cellular data only available for all B-cell malignancy patients together (not RTX specific); 7% pre- and 36% postvaccine had H1N1-specific T-cell response; no effect of prior chemotherapy; healthy controls had 44% pre and 48% post; no effect of prior chemotherapy on induction of H1N1-specific T cells after 2 doses of vaccine (data NR) |
| Eisenberg et al, 201342 | Cohort | United States | RTX | Rheumatologic disease | 17 | 49 | 94 | 7-9 mo | Seasonal influenza | Measurement: T-cell ELISPOTs were performed using a standard γ-IFN T-cell ELISPOT assay Outcome: T-cell responses similar in anti-CD20–treated patients at baseline compared with healthy controls, but no significant increases after vaccination, no increase in proliferation (data NR); no changes in the T-cell repertoire as detected by spectratyping over time (data NR) |
| Muller et al, 201343 | Cohort | Switzerland | RTX | Autoimmune rheumatic diseases | 16 | 45 | 88 | Median, 6 mo (range, 1-36 mo) | Pandemic influenza | Measurement: Presence of IFN-γ–producing 2009 H1N1 influenza virus–specific CD4+ and CD8+ T cells Outcomes: After first vaccination, virus-specific CD4+ and CD8+ T-cell responses were significantly lower in patients with low B cells than those with normal B cells; booster vaccination stimulated the antiviral T-cell response only in patients with low B cells |
| Nazi et al, 201338 | Phase 3 substudy | Canada | RTX | ITP | 17 | 40 | 71 | 6 mo | Tetanus toxoid | Measurement: IFN-γ ELISPOT assay to measure frequency of tetanus toxoid–specific T cells Outcomes: Mean number of IFN-γ–producing T cells was reduced in patients who had received rituximab compared with placebo at 1 wk (38 cells vs 93 cells per 5 × 105 total cells) and 1 mo (14 cells vs 43 cells per 5 × 105 total cells) |
| Parrino et al, 201741 | Phase 1 | Worldwide | RTX | B-cell lymphoma | 80 | 61 | 54 | On active treatment | Inactivated VZV | Measurement: IFN-γ ELISPOT assay was used to detect the presence of IFN-γ–secreting VZV-specific cells from PBMCs before and after immunization Outcome: GMFR met primary outcome at 4.34 (95% CI, 3.01-6.24) |
DMARD, disease-modifying antirheumatic drug; ELISPOT, enzyme-linked immune absorbent spot; GMFR, geometric mean fold rise; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor; VZV, varicella zoster virus. See Table 1 for expansion of other abbreviations.
Median age; where not reported, mean age reported.
Rituximab-specific age and percentage female not reported, thus age and percentage female of entire study population reported.
Risk of bias within studies
Supplemental Table 5 lists the results of the risk-of-bias assessment for individual studies. Twenty-eight studies had a low risk of bias.
Results of individual studies
Humoral responses to pandemic influenza, seasonal influenza, tetanus, diphtheria, pertussis, Haemophilus influenzae B, hepatitis B, hepatitis A, polio, and pneumococcal vaccinations.
Table 3 provides a high-level summary of all of the reports of humoral immune responses in patients receiving rituximab. Details of individual studies are available in Tables 1 and 2. As outlined in both tables, SP, SC, and/or SR rates were lower in anti-CD20–treated patients than in healthy controls or disease controls, regardless of the time that had elapsed from treatment. In patients on active anti-CD20 therapy, response to vaccination was poor regardless of the vaccine studied, with SC rates of 0% to 25%.30-37 Response rates appeared to improve as more time elapsed after anti-CD20 therapy, with the best responses in patients >12 months from therapy, though this did not always translate to an equivalent response to controls.
Summarized data from all studies listed in Table 1, by average duration of time since last anti-CD20 therapy
| Duration of time since anti-CD20 therapy . | Summarized SP rate, % . | Summarized SC rate, % . | Summarized SR rate, % . |
|---|---|---|---|
| Pandemic influenza (N = 13 studies, 222 patients treated with anti-CD20 therapy)* | |||
| Active treatment, <3 mo | 0-3330-34,40,61 | 0-2530-34,40 | |
| 3-6 mo | 1960 | 3860 | 38-4443 |
| 6-12 mo | 5040 | 5040 | |
| >12 mo | 66-10040,44 | 66-10040,44 | |
| Seasonal influenza (N = 12 studies, 252 patients treated with anti-CD20 therapy)† | |||
| Active treatment, <3 mo | 8-2530,64 | 030,35 | |
| 3-6 mo | 56-8062 | 10-6062 | |
| 6-12 mo | 69-7763 | 2535 | 1742 |
| >12 mo | 23-7944,52 | 3-4344,52 | |
| Tetanus, diphtheria, and pertussis (12 studies, 309 patients treated with anti-CD20 therapy)‡ | |||
| Active treatment, <3 mo | 10062 | 24-2536,62 | |
| 3-6 mo | 64-7367 | 9-3966,67 | |
| 6-12 mo | 66-9350,69 | 7-2069 | 31-6950,65,68,70 |
| >12 mo | 0-947,53 | 9-7546,53 | 047 |
| Haemophilus influenzae B (3 studies, 52 patients treated with anti-CD20 therapy) | |||
| Active treatment, <3 mo | |||
| 3-6 mo | |||
| 6-12 mo | 2938 | 73-77 after 1-2 doses68 | |
| >12 mo | 8546 | ||
| Hepatitis B (3 studies, 61 patients treated with anti-CD20 therapy)§ | |||
| Active treatment, <3 mo | |||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | 56-6454,71 | 5654 | |
| Hepatitis A (2 studies, 57 patients treated with anti-CD20 therapy) | |||
| Active therapy, <3 mo | 047 | ||
| 3-6 mo | |||
| 6-12 mo | 47 (slightly lower than disease controls of 67)70 | ||
| >12 mo | |||
| Polio virus (3 studies, 49 patients treated with anti-CD20 therapy)ǁ | |||
| Active treatment, <3 mo | 2036 | ||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | 62, slightly lower than in disease controls (80)46 | ||
| Pneumococcal conjugate (PCV-7) (1 study, 55 patients treated with anti-CD20 therapy) | |||
| Active treatment, <3 mo | 5, compared with 21-50 in disease controls73 | ||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | |||
| Pneumococcal polysaccharide (PCV-23) (7 studies, 217 patients treated with anti-CD20 therapy)¶ | |||
| Active treatment, <3 mo | 2530 | 030 | |
| 3-6 mo | 19 to ≥6 serotypes66 | ||
| 6-12 mo | 33 (to ≥7 serotypes) to 4168 | 20-2138,69 | 2965 |
| >12 mo |
| Duration of time since anti-CD20 therapy . | Summarized SP rate, % . | Summarized SC rate, % . | Summarized SR rate, % . |
|---|---|---|---|
| Pandemic influenza (N = 13 studies, 222 patients treated with anti-CD20 therapy)* | |||
| Active treatment, <3 mo | 0-3330-34,40,61 | 0-2530-34,40 | |
| 3-6 mo | 1960 | 3860 | 38-4443 |
| 6-12 mo | 5040 | 5040 | |
| >12 mo | 66-10040,44 | 66-10040,44 | |
| Seasonal influenza (N = 12 studies, 252 patients treated with anti-CD20 therapy)† | |||
| Active treatment, <3 mo | 8-2530,64 | 030,35 | |
| 3-6 mo | 56-8062 | 10-6062 | |
| 6-12 mo | 69-7763 | 2535 | 1742 |
| >12 mo | 23-7944,52 | 3-4344,52 | |
| Tetanus, diphtheria, and pertussis (12 studies, 309 patients treated with anti-CD20 therapy)‡ | |||
| Active treatment, <3 mo | 10062 | 24-2536,62 | |
| 3-6 mo | 64-7367 | 9-3966,67 | |
| 6-12 mo | 66-9350,69 | 7-2069 | 31-6950,65,68,70 |
| >12 mo | 0-947,53 | 9-7546,53 | 047 |
| Haemophilus influenzae B (3 studies, 52 patients treated with anti-CD20 therapy) | |||
| Active treatment, <3 mo | |||
| 3-6 mo | |||
| 6-12 mo | 2938 | 73-77 after 1-2 doses68 | |
| >12 mo | 8546 | ||
| Hepatitis B (3 studies, 61 patients treated with anti-CD20 therapy)§ | |||
| Active treatment, <3 mo | |||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | 56-6454,71 | 5654 | |
| Hepatitis A (2 studies, 57 patients treated with anti-CD20 therapy) | |||
| Active therapy, <3 mo | 047 | ||
| 3-6 mo | |||
| 6-12 mo | 47 (slightly lower than disease controls of 67)70 | ||
| >12 mo | |||
| Polio virus (3 studies, 49 patients treated with anti-CD20 therapy)ǁ | |||
| Active treatment, <3 mo | 2036 | ||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | 62, slightly lower than in disease controls (80)46 | ||
| Pneumococcal conjugate (PCV-7) (1 study, 55 patients treated with anti-CD20 therapy) | |||
| Active treatment, <3 mo | 5, compared with 21-50 in disease controls73 | ||
| 3-6 mo | |||
| 6-12 mo | |||
| >12 mo | |||
| Pneumococcal polysaccharide (PCV-23) (7 studies, 217 patients treated with anti-CD20 therapy)¶ | |||
| Active treatment, <3 mo | 2530 | 030 | |
| 3-6 mo | 19 to ≥6 serotypes66 | ||
| 6-12 mo | 33 (to ≥7 serotypes) to 4168 | 20-2138,69 | 2965 |
| >12 mo |
See Tables 4 and 5 for meta-analyzed data. In all studies, SP, SC, and/or SR rates were similar or lower in anti-CD20–treated patients than healthy controls or disease controls. One study did not report the average duration since last dose of anti-CD20 therapy but had SP and SR rates of 0% and 0% to 9%, respectively.45 One study indicated that patients had received anti-CD20 therapy <12 months ago but did not specify the average time interval, and reported a SP rate of 9%.59
In all studies, SP, SC, and/or SR rates were lower in anti-CD20–treated patients than in healthy controls or disease controls.
In all studies, SP, SC, and/or SR rates were similar or lower in anti-CD20–treated patients than healthy controls or disease controls. Other than in 2 studies47,53 (a study of patients post–autologous stem cell transplant plus rituximab for non-Hodgkin lymphoma, and a study of pediatric patients treated with rituximab for relapsing/steroid-dependent idiopathic nephrotic syndrome) where the SP rates were only 0% to 9%, SP rates were moderate to high regardless of the duration that had elapsed from anti-CD20 therapy.
One study did not report the interval after anti-CD20 therapy during which patients were vaccinated.72 Overall values for anti-CD20–treated patients were similar to healthy controls ≥55 years and disease controls, but worse than healthy controls ≤35 years.
One study did not report the interval after anti-CD20 therapy during which patients received vaccination but had a SR rate of 91%.54
All studies showed that SP, SC, and/or SR rates were lower in anti-CD20–treated patients compared with disease controls.
Based on the descriptive nature of the data, ascertaining whether patients had better responses to T-cell–dependent vaccinations than T-cell–independent vaccinations is difficult.
Meta-analysis of humoral response to pandemic influenza vaccine.
Thirteen studies (222 anti-CD20 treated patients and 993 control patients) reported on response rates to the pandemic influenza vaccine. Ten of the 13 studies included patients with hematologic malignancies (rather than autoimmune disease) treated with anti-CD20 therapy. The pooled estimates of SC for patients treated with anti-CD20 therapy are listed in Table 4. Patients on active (<3 months since last dose) anti-CD20 therapy had poor response to vaccination, with a pooled estimate of SC after 1 vaccine dose of 3% (95% CI, 0% to 9%; 6 studies, 75 patients). This improved incrementally over time with pooled estimates of 21% (95% CI, 0% to 65%), 50% (95% CI, 4% to 96%), and 41% (95% CI, 19% to 65%) within 3 to 6 months, 6 to 12 months, or >12 months from last dose of anti-CD20 therapy, respectively, though the sample size was limited (see Table 4).
Pooled estimates of SC to 1 or 2 doses of the pandemic influenza vaccine in patients treated with anti-CD20 therapy
| . | . | . | Pooled estimate of SC, % (95% CI, I2 %) . | |
|---|---|---|---|---|
| Duration of time since last anti-CD20 treatment, mo . | No. of studies . | Total no. of patients . | For 1 vaccine dose . | For 2 vaccine doses . |
| Active, <3 | 6 | 75 | 3 (0-9, 0) | 12 (2-27, 37) |
| 3-6 | 2 | 21 | 21 (0-65, 71) | 4 (0-32, 0) |
| 6-12 | 1 | 12 | 50 (4-96, N/A) | 50 (4-96, N/A) |
| >12 | 2 | 26 | 41 (19-65, 0) | 75 (39-100, 41) |
| . | . | . | Pooled estimate of SC, % (95% CI, I2 %) . | |
|---|---|---|---|---|
| Duration of time since last anti-CD20 treatment, mo . | No. of studies . | Total no. of patients . | For 1 vaccine dose . | For 2 vaccine doses . |
| Active, <3 | 6 | 75 | 3 (0-9, 0) | 12 (2-27, 37) |
| 3-6 | 2 | 21 | 21 (0-65, 71) | 4 (0-32, 0) |
| 6-12 | 1 | 12 | 50 (4-96, N/A) | 50 (4-96, N/A) |
| >12 | 2 | 26 | 41 (19-65, 0) | 75 (39-100, 41) |
N/A, not applicable.
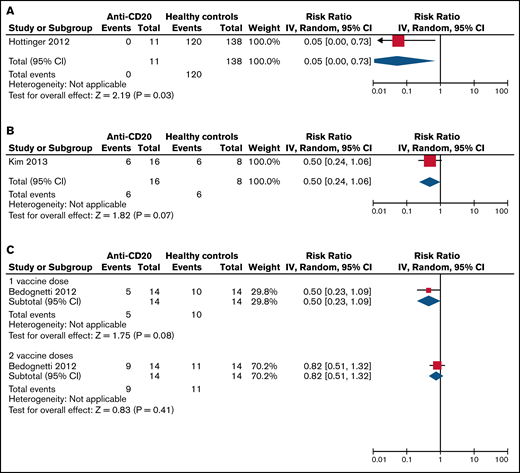
The pooled RBs for SC of patients treated with anti-CD20 therapy compared with healthy controls and compared with disease controls are listed in Table 5 and visualized in Figures 2 and 3. Patients on active anti-CD20 therapy had significantly lower SC rates after 1 dose of the pandemic influenza vaccine compared with healthy controls (RB, 0.05 [95% CI, 0-0.73]) and compared with disease controls (RB, 0.22 [95% CI, 0.09-0.56]). The results did not change significantly even when limiting only to studies of patients with hematologic malignancy (Table 5).
Risk benefit of SC to the pandemic influenza vaccine in anti-CD20–treated patients vs healthy controls. (A) On active therapy (<3 months from therapy). (B) Three to 6 months from therapy. (C) More than 12 months from therapy. Events, the number of patients with SC response to vaccination; Total, the total number of patients assessed for SC response to vaccination; Weight, the weighted contribution of each study to the meta-analysis.
Risk benefit of SC to the pandemic influenza vaccine in anti-CD20–treated patients vs healthy controls. (A) On active therapy (<3 months from therapy). (B) Three to 6 months from therapy. (C) More than 12 months from therapy. Events, the number of patients with SC response to vaccination; Total, the total number of patients assessed for SC response to vaccination; Weight, the weighted contribution of each study to the meta-analysis.
Risk ratios of SC to the pandemic influenza vaccine when treated with either 1 or 2 doses when comparing anti-CD20–treated patients to disease controls. (A) On active therapy (<3 months from therapy). (B) Three to 6 months from therapy. (C) Six to 12 months from therapy. (D) More than 12 months from therapy. Events, the number of patients with SC response to vaccination; Total, the total number of patients assessed for SC response to vaccination; Weight, the weighted contribution of each study to the meta-analysis.
Risk ratios of SC to the pandemic influenza vaccine when treated with either 1 or 2 doses when comparing anti-CD20–treated patients to disease controls. (A) On active therapy (<3 months from therapy). (B) Three to 6 months from therapy. (C) Six to 12 months from therapy. (D) More than 12 months from therapy. Events, the number of patients with SC response to vaccination; Total, the total number of patients assessed for SC response to vaccination; Weight, the weighted contribution of each study to the meta-analysis.
Relative benefit ratios for SC to the pandemic influenza vaccine in healthy controls and disease controls compared with patients on anti-CD20 therapy
| Average duration of time since anti-CD20 therapy, mo . | No. of studies analyzed . | 1 dose (95% CI) . | I2 %, P . | 2 doses (95% CI) . | I2 %, P . | No. of studies analyzed . | 1 dose (95% CI) . | I2 %, P . | 2 doses (95% CI) . | I2 %, P . |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients on anti-CD20 therapy vs healthy controls | All patients on anti-CD20 therapy vs disease controls | |||||||||
| On active treatment, <3 | 1 | 0.05 (0-0.73) | N/A, 0.03 | — | — | 6 | 0.22 (0.09-0.56) | 3, .001 | 0.25 (0.12-0.55) | 0, .0006 |
| 3-6 | 1 | 0.50 (0.24-1.06) | N/A, 0.07 | — | — | 2 | 0.44 (0.23-0.84) | 0, .01 | 0.10 (0.01-1.49) | N/A, .10 |
| 6-12 | — | — | — | — | — | 1 | 0.77 (0.28-2.10) | N/A, .61 | 0.62 (0.23-1.67) | N/A, .35 |
| >12 | 1 | 0.50 (0.23-1.09) | N/A, 0.08 | 0.82 (0.51-1.32) | N/A, 0.41 | 1 | 1.03 (0.45-2.35) | N/A, .94 | 1.10 (0.74-1.63) | N/A, .65 |
| Only patients with hematologic malignancy on anti-CD20 therapy vs disease controls | ||||||||||
| On active treatment, <3 | 5 | 0.19 (0.06-0.61) | 21, .005 | 0.22 (0.09-0.52) | 0, .0007 | |||||
| 3-6 | 1 | 0.13 (0.01-1.85) | N/A, .13 | 0.10 (0.01-1.49) | N/A, .10 | |||||
| 6-12 | 1 | 0.77 (0.28-2.10) | N/A, .61 | 0.62 (0.23-1.67) | N/A, .35 | |||||
| >12 | 1 | 1.03 (0.45-2.35) | N/A, .94 | 1.10 (0.74-1.63) | N/A, .65 | |||||
| Average duration of time since anti-CD20 therapy, mo . | No. of studies analyzed . | 1 dose (95% CI) . | I2 %, P . | 2 doses (95% CI) . | I2 %, P . | No. of studies analyzed . | 1 dose (95% CI) . | I2 %, P . | 2 doses (95% CI) . | I2 %, P . |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients on anti-CD20 therapy vs healthy controls | All patients on anti-CD20 therapy vs disease controls | |||||||||
| On active treatment, <3 | 1 | 0.05 (0-0.73) | N/A, 0.03 | — | — | 6 | 0.22 (0.09-0.56) | 3, .001 | 0.25 (0.12-0.55) | 0, .0006 |
| 3-6 | 1 | 0.50 (0.24-1.06) | N/A, 0.07 | — | — | 2 | 0.44 (0.23-0.84) | 0, .01 | 0.10 (0.01-1.49) | N/A, .10 |
| 6-12 | — | — | — | — | — | 1 | 0.77 (0.28-2.10) | N/A, .61 | 0.62 (0.23-1.67) | N/A, .35 |
| >12 | 1 | 0.50 (0.23-1.09) | N/A, 0.08 | 0.82 (0.51-1.32) | N/A, 0.41 | 1 | 1.03 (0.45-2.35) | N/A, .94 | 1.10 (0.74-1.63) | N/A, .65 |
| Only patients with hematologic malignancy on anti-CD20 therapy vs disease controls | ||||||||||
| On active treatment, <3 | 5 | 0.19 (0.06-0.61) | 21, .005 | 0.22 (0.09-0.52) | 0, .0007 | |||||
| 3-6 | 1 | 0.13 (0.01-1.85) | N/A, .13 | 0.10 (0.01-1.49) | N/A, .10 | |||||
| 6-12 | 1 | 0.77 (0.28-2.10) | N/A, .61 | 0.62 (0.23-1.67) | N/A, .35 | |||||
| >12 | 1 | 1.03 (0.45-2.35) | N/A, .94 | 1.10 (0.74-1.63) | N/A, .65 | |||||
The RB is the ratio of SC rate in patients treated with anti-CD20 therapy to the SC rate in healthy or disease controls. Bold values represent statistically significant relative benefit ratios.
N/A, not applicable.
—, no available data.
Cellular responses to vaccination.
Six studies reporting cellular response data on 171 patients treated with anti-CD20 therapy were included. Descriptive data are available in Table 2. Notably, all but 1 study38 included patients who were receiving concomitant immunosuppressive therapy that may have impaired T-cell function (eg, mycophenolate, cyclosporine). Three of the included studies found no significant difference in T-cell vaccine response between anti-CD20–treated patients vs healthy or disease controls.39–41 In these studies, the average interval between anti-CD20 and vaccination ranged from being on active treatment to 19 months. The other 3 studies found that patients treated with anti-CD20 had lower T-cell responses compared with healthy or disease controls, or to patients with normal B-cell subsets.38,42,43 The average interval between anti-CD20 therapy and vaccination in these studies was 6 to 9 months.
Safety of vaccination in patients with hematologic malignancy.
Of the 19 studies reporting data on patients with hematologic malignancies, 9 reported data on adverse events related to vaccination.30,31,33,37,40,41,44–47 The majority of studies found that adverse reactions were mild, and 1 study noted that there were no differences in side effect profiles in patients compared with healthy controls.40 Only 1 study reported serious adverse events (SAEs): a phase 1 study of a novel inactivated varicella zoster vaccine schedule reported 10 SAEs related to vaccination among the 80 patients studied,41 with the most common SAEs being febrile neutropenia and pneumonia.
Discussion
There are over 900 000 people living with lymphoma in the United States alone,48 and anti-CD20 therapies have been a component of first-line therapy for many lymphoproliferative disorders for over 20 years. Furthermore, anti-CD20 therapies are an important treatment modality for numerous autoimmune conditions, including multiple sclerosis and rheumatoid arthritis, where their use is widespread. Understanding vaccine responsiveness in the context of B-cell–depleting therapies, especially given the number of patients that will need vaccination against COVID-19, is imperative.
To interpret vaccine responses (SP, SC, and/or SR rates), it is important to note that there are 2 types of vaccine antigens. A primary antigen is an antigen that the patient has not been previously exposed to, which elicits an extrafollicular response that causes B-cell proliferation and differentiation into plasma cells, and generally reaches a peak value 4 weeks after immunization.49 A recall antigen is one in which an anamnestic immune response is anticipated (eg, tetanus booster vaccination), in as little as 7 days.49 For recall antigens, SP and SC rates postvaccination are expected to be high. From that perspective, we chose to focus our meta-analysis on the pandemic influenza vaccine from the 2009 H1N1 pandemic, as this vaccine was a primary antigen, analogous to the COVID-19 vaccine. As patients would have no preexisting memory B cells to this antigen, this allows the best assessment of B-cell–depleted patients’ immune response to vaccination. Additionally, the pandemic influenza vaccine has standardized definitions of SP and SC. The SC rate was chosen as the primary outcome to meta-analyze as SC demonstrates the ability to mount a response to vaccination (by either a several-fold increase in antibody titers, or conversion from negative titers to protective titers). SP rates can be adequate in patients who have had previous exposure to an antigen, even if they do not respond to the vaccination given.
Although the literature was heterogenous in terms of patient population, vaccine types, and interval since anti-CD20 therapy, we documented 4 main findings: (1) vaccination appears safe in patients on anti-CD20 therapy; (2) the response to vaccination in patients on active anti-CD20 therapy is low and approaches 0%; (3) anti-CD20 therapy lowers patients’ vaccine response beyond the impact of their disease or other treatments, given the higher response to vaccination in disease controls; and (4) response to vaccination improves incrementally over time but may not reach the level of healthy controls even 12 months after therapy. Although not the primary aim of our study, we also noted that patients with lymphoproliferative disorders not exposed to anti-CD20 therapy (on active cancer therapy, previous non-anti-CD20 treatment, or never treated) had reasonable responses to vaccination (eg, SC rates 27% to 80% to pandemic influenza vaccine, see Tables 1 and 2).
It is notable that SC rates in studies of patients on active anti-CD20 therapy were 0% to 25%,30–37 regardless of the vaccine type assessed. A small number of studies reported that vaccine responses did not vary over time.39,50,51 However, these studies may have been underpowered to detect a time trend, as our meta-analysis for pandemic influenza vaccine found that SC rates did improve as time between last anti-CD20 treatment and vaccination increased.
Whether 2 vaccine doses enhance immunogenicity in patients receiving anti-CD20 therapy remains unclear. When assessing the response to pandemic influenza vaccination, the point estimate of SC in patients on active therapy was just 3% (95% CI, 0% to 9%) after 1 dose, and 12% (95% CI, 2% to 27%) after 2 doses. Five studies assessed SC after 1 and 2 doses of pandemic influenza vaccine in patients receiving anti-CD20 vs disease controls. As illustrated in Tables 4 and 5, the point estimate of SC does not appear appreciably different in those receiving 1 or 2 doses of vaccine, nor do patients on anti-CD20 seem to improve in their SC rates compared with disease controls. This finding is unchanged when limiting the analysis only to patients with hematologic malignancies. However, due to the small sample sizes of included studies, the relative impact or lack of impact of booster vaccinations remains essentially unknown.
Our third finding was that the reduction in response to vaccination seen in patients on anti-CD20 therapy is likely above and beyond the immunocompromising effects of the disease alone and the other treatments used in the disease. The SC rates to the pandemic influenza vaccination in patients on active anti-CD20 therapy or even 3 to 6 months from therapy were significantly lower than in disease controls (RBs of 0.22 [95% CI, 0.09-0.56] and 0.44 [95% CI, 0.23-0.84], respectively). However, by an average of 6 to 12 months from anti-CD20 therapy, the difference between groups was less pronounced, and by >12 months the difference seemed trivial, though we are limited by the number of studies available for analysis.
Given the finding that anti-CD20 therapy abrogates vaccine responses, one strategy may be to delay anti-CD20 therapy (when possible) until after vaccination. However, currently we do not know how long of a gap is required between vaccination and anti-CD20 treatment of vaccination to be effective. One small study of patients with relapsed low-grade lymphoma36 assessed this strategy: 5 patients were given a primary antigen vaccine (hepatitis A) 2 weeks prior to starting on rituximab treatment. None of patients developed a humoral response. On the other hand, 5 of 10 patients immunized 2 weeks before rituximab had a response to a recall antigen (polio virus or tetanus toxoid). These results highlight the need for further research to determine the optimal gap between vaccination, particularly of a novel primary vaccine, and anti-CD20 therapy. In the absence of data, clinicians and patients need to weigh the expected benefits of initiating anti-CD20 therapy against its anticipated impact on vaccine response. In rare instances, it may be appropriate to delay anti-CD20 therapy to allow for COVID-19 vaccination.
Finally, the response to vaccination does seem to improve incrementally over time as the B-cell compartment recovers following rituximab treatment. Generally, the effect of anti-CD20 therapy was least pronounced >1 year after treatment, although even these patients had impaired responses compared with healthy controls, with SC rates of 33% to 100% for pandemic infuenza,40,44 3% to 43% for seasonal influenza,44,52 9% to 75% for tetanus, diphtheria, and pertussis,46,53 85% for haemophilus influenzae type b,46 and 56% for hepatitis B.54 The finding was also noted in correlative analyses in a study by de Lavallade,40 which found that the interval between therapy and vaccination was significantly longer in patients who were seroprotected compared with those who were not (4.7 vs 17.5 months; P = .001). Whether the degree of B-cell recovery is associated with response remains unclear, as this is not generally measured in clinical practice, and thus was not a focus of this review.
Notably, we should be cautious applying immunological correlates of protection (which have only been validated in young healthy adults55) to immunocompromised patients.56 Whether an incremental rise in titers not meeting criteria for SP or SC might still be valuable in immunocompromised patients to guard against clinical infection remains unclear. Additionally, cellular responses are also important in vaccination-mediated protection,57 and although these are also affected by anti-CD20 therapy, the degree to which cellular immunity is impacted is less clear.35 Studies were small and contradictory in their findings with respect to whether T-cell immunity was preserved or diminished following anti-CD20 therapy. Some of the studies were also confounded by the inclusion of patients who were on concomitant immunosuppressive therapies that impair T-cell function (eg, mycophenolate).
There are several limitations to our work. First, the pool of studies included in this systematic review was heterogenous with respect to patient population, vaccine studied, definitions of SP and SC, and interval between anti-CD20 treatment and vaccination. Although precise estimates of interval between anti-CD20 and vaccination were available for some studies, others only provided median/mean intervals and ranges. Definitions of SP and SC varied between studies, which was expected as there are no widely used definitions for SP and SC other than for pandemic influenza vaccinations. We sought to limit the heterogeneity encountered as much as possible by categorizing studies both by vaccine studied and by average interval between anti-CD20 and vaccination. We also only meta-analyzed data related to the pandemic influenza vaccine, which has standard definitions for SP and SC. Nonetheless, heterogeneity in interval from anti-CD20 therapy does remain, and the most accurate interpretation of amalgamated data only applies to patients on active treatment as only these studies had homogenous populations. Additionally, the sample sizes of each study were small, so direct extrapolation of SP, SC, and/or SR from any 1 study should be avoided. Finally, we did not have data on baseline hypogammaglobulinemia for all studies, and whether concomitant immunoglobulin replacement therapy was used for patients, as this can provide an exogenous source of SP detected in laboratory studies. We also did not collect data on other concomitant immunomodulatory medications used for patients and the timing of their use with respect to vaccination, which may also have impacted their immune responses.
This systematic review highlights the available literature on both humoral and cellular vaccination response in patients treated with anti-CD20 therapy. We found limited and heterogenous data, suggesting the need for a robust prospective study in a large sample of patients. The COVID-19 vaccination rollout represents an opportunity to study primary vaccine responses in immunocompromised patients, including those receiving anti-CD20 therapy.
We report that SC after vaccination in patients on active anti-CD20 therapy is low and approaches 0%. The low response rate of patients on anti-CD20 therapy appears to be largely attributable to the anti-CD20 therapy rather than the disease or other treatments, and response to vaccination seems to improve incrementally over time but may not reach the level of healthy controls even 12 months after therapy. During the current pandemic, this argues for the need for rapid, real-world studies to guide clinical practice. We need to quickly determine the SC rate following COVID-19 vaccination, whether it can be improved upon with booster shots, and/or influenced via the timing of vaccination relative to anti-CD20 therapy. Additionally, these data serve as a reminder to continue stringent evidence-based infection-prevention strategies such as infection control measures, physical distancing, and appropriate shielding for patients on anti-CD20 therapy.58 Additionally, in an effort to decrease the household risk of exposure, advocating for vaccination of the caregivers and household contacts of patients receiving anti-CD20 therapy may be appropriate. Finally, while we await future prospective data addressing this issue, we do recommend COVID-19 vaccination for patients receiving anti-CD20 therapy as even a muted response to vaccination is likely better than no response.
Acknowledgment
The authors thank the authors and patients of all studies included in this review.
Authorship
Contribution: A.V. performed literature search, article selection, analysis, and manuscript writing; I.G. performed article selection and manuscript review and revision; S.D.B. and M.C. reviewed and revised the manuscript; and L.K.H. conceived of the study, assisted with analysis, and reviewed and revised the manuscript.
Conflict-of-interest disclosure: L.K.H. was one of the principal investigators on a study partially funded by Gilead Sciences. The remaining authors declare no competing financial interests.
ORCID profiles: A.V., 0000-0001-5369-1501; I.G., 0000-0001-9306-2945.
Correspondence: Lisa K. Hicks, St. Michael’s Hospital, Room 2-084, Donnelly Wing, 30 Bond St, Toronto, ON M5B 1W8, Canada: e-mail lisak.hicks@unityhealth.to.
References
Author notes
All data are reported in the paper in tables or figures. Data tables are available from the corresponding author at lisak.hicks@unityhealth.to.
The full-text version of this article contains a data supplement.