TO THE EDITOR:
Novel genetically engineered immunotherapies have improved the outlook for patients with advanced hematologic malignancies, for whom prognosis was once considered dismal.1,2 US, European, and other regulatory authorities have approved 5 chimeric antigen receptor (CAR) T-cell products and 1 T-cell engager (TCE), known as a bispecific T-cell engager (BiTE). More products await approval.
These breakthrough therapies are associated with short- and long-term toxicities, such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).3 The US Food and Drug Administration (FDA) requires manufacturers to report the toxicity and effectiveness of CAR T cells. Most clinical centers voluntarily report these data to the Center for International Blood and Marrow Transplant Research Cellular Immunotherapy Data Resource (CIDR).4,5
There is a significant need to collect biologic samples from patients treated with CAR T cells. Research on such samples could reveal the pathophysiology of responders vs nonresponders and of CRS and ICANS toxicities. It also would optimize selection of patients likely to benefit from these therapies. There is no consensus on the types of biomarkers to study; samples to collect; timing; standardized processing, shipping, and storing; optimal infrastructure; or analyses to perform. On 7 October 2020, the American Society of Hematology (ASH) Taskforce for Immunotherapies hosted a workshop on developing biomarkers for CAR T cell and BiTE toxicity and efficacy, which focused on 4 themes: regulation, data and samples, biomarkers, and pathophysiology.
We aim to provide a roadmap for collaboration among academic investigators, industry, funding agencies, and regulatory bodies to investigate biomarkers for current and future cellular therapies. A summary of recommendations is as follows:
- 1.
Develop a collaborative network of clinicians, academia, the pharmaceutical industry, and health authorities to share samples, models, and data in real time.
- 2.
Leverage existing biomarker pathways and guidelines from the FDA and equivalent international agencies.
- 3.
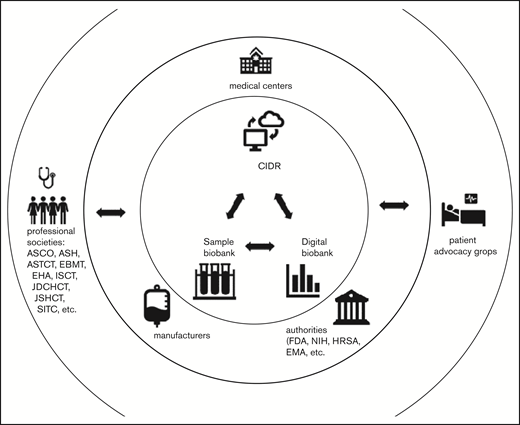
Set up centralized sample and digital biobanks6 linked to clinical data through the CIDR to facilitate collaboration among stakeholders (Figure 1).
- 4.
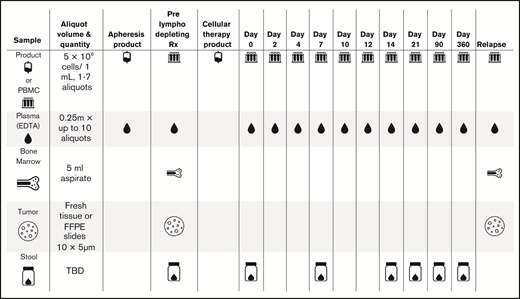
Collect samples of plasma, viable peripheral blood mononuclear cells (PBMCs), bone marrow, and tumors on a standardized schedule (Figure 2).
- 5.
Include special populations: children, older adults, and people of diverse ethnic and racial backgrounds.
- 6.
Perform harmonized and validated assays across different clinical centers, commercial products, and trials.
- 7.
Standardize the delivery of these therapeutics, with manufacturers providing dedicated samples, instructions for assays, reagents, and detailed labels for CAR T cells and TCEs.
- 8.
Investigate durability of response to treatment, immune dysfunction, CRS, and neurotoxicity.
- 9.
Collaboratively validate in vivo, in vitro, and in silico modeling approaches to enable further discovery of candidate biomarkers and preclinical testing of biomarker-based interventions.
Collaborative network to develop biomarkers. Collaborative network between clinicians, researchers, and the pharmaceutical industry. Samples and data are shared among medical centers, the CIDR, centralized sample and digital biobanks, and the pharmaceutical industry. Data are also shared with regulatory authorities. This cooperation would speed development of biomarkers that are critical to help physicians make decisions that help patients. ASCO, American Society of Clinical Oncology; ASTCT, American Society for Transplant and Cellular Therapy; EBMT, European Society for Blood and Marrow Transplantation; EHA, European Hematology Association; EMA, European Medicines Agency; HRSA, Health Resources and Services Administration; ISCT, International Society for Cell & Gene Therapy; JDCHCT, Japanese Data Center for Hematopoietic Cell Transplantation; JSHCT, Japan Society for Hematopoietic Stem Cell Transplantation; NIH, National Institutes of Health; SITC, Society for Immunotherapy of Cancer.
Collaborative network to develop biomarkers. Collaborative network between clinicians, researchers, and the pharmaceutical industry. Samples and data are shared among medical centers, the CIDR, centralized sample and digital biobanks, and the pharmaceutical industry. Data are also shared with regulatory authorities. This cooperation would speed development of biomarkers that are critical to help physicians make decisions that help patients. ASCO, American Society of Clinical Oncology; ASTCT, American Society for Transplant and Cellular Therapy; EBMT, European Society for Blood and Marrow Transplantation; EHA, European Hematology Association; EMA, European Medicines Agency; HRSA, Health Resources and Services Administration; ISCT, International Society for Cell & Gene Therapy; JDCHCT, Japanese Data Center for Hematopoietic Cell Transplantation; JSHCT, Japan Society for Hematopoietic Stem Cell Transplantation; NIH, National Institutes of Health; SITC, Society for Immunotherapy of Cancer.
Proposed sample collection (eg, for CD19 CAR T cells). EDTA, ethylenediaminetetraacetic acid; FFPE, formalin fixed, paraffin embedded; TBD, to be determined.
Proposed sample collection (eg, for CD19 CAR T cells). EDTA, ethylenediaminetetraacetic acid; FFPE, formalin fixed, paraffin embedded; TBD, to be determined.
We recommend using biomarker definitions from the “BEST Resource: Harmonizing Biomarker Terminology,”7,8 developed by the FDA, and using FDA biomarker qualification pathways to integrate biomarkers into drug development.9-11
Next, we advocate coordinating efforts by multiple stakeholders, including academia, the pharmaceutical industry, and health authorities. These stakeholders should standardize assays. They should use the CIDR and also establish new research networks to discover and validate novel biomarkers. Together, they should use real-world data sets to validate early signals. Finally, researchers should design prospective, randomized studies of these biomarkers.
Clinical data are currently collected in a standardized manner through the CIDR about patients who receive commercial CAR T cells. Likewise, industry and academia should coordinate mechanisms and schedules for collecting samples. They should set up centralized biorepositories for sample collection and processing, including central laboratories that provide more complex assessments. These laboratories should be able to accept samples from all practitioners. Laboratory data should be linked with CIDR clinical data or additional data for a cross-sectional assessment of clinical phenotype.
Next, clinicians should collect samples of product, PBMCs, plasma, bone marrow, tumor, and stool for microbiome (Figure 2). The first 5 types of samples are the highest priority, because bone marrow and tumor may be harder to collect. Given the varying onset of toxicities in the first 28 days, schedules for sample collection can be adapted to different diseases and/or products.
To support these efforts, the collaborative network (clinicians, academia, pharmaceutical industry, and health authorities) must identify funding mechanisms and define the role of data sharing and big-data bioinformatics.
Results from samples analyzed in real time should be centralized in a digital biobank.6 To achieve this, we first must standardize product characterization and immune monitoring, such as peak levels of CAR T cells, plus point-of-care cytokines, such as interleukin-6 levels. All centers and clinical trials should run the same validated assays using standardized reagents (or, if local laboratories do not have the required expertise, send samples to central laboratories) to produce comparable data on response and toxicity. Central qualified laboratories may help (eg, Cancer Immune Monitoring and Analysis Centers).
Treating physicians and the pharmaceutical industry must share data on key biomarkers in real time, such as quantification and pharmacokinetics of infused CAR T cells. These should be developed as hospital tests compliant with US Clinical Laboratory Improvement Amendments (CLIA).
To this end, industry should provide 4 items: (1) dedicated samples of commercial products, sent with the product, that can be used by treating centers to perform baseline assays; (2) standard operating procedures for analytic assays for detection of CAR T cells; (3) reagents to detect specific CAR T cells by flow cytometry and polymerase chain reaction; and (4) a detailed product specification label and analysis certificate.
Treating centers should provide both clinical and laboratory data to the CIDR and digital biobank, respectively, to allow a path for development of CLIA-approved assays and to assist in clinical decision making.
Modeling in cell-based immunotherapy has 3 major goals: mechanistic understanding of treatment failure and toxicity to define favorable conditions and modifiable factors, development of novel biomarkers for clinical risk stratification, and preclinical modeling of new therapies to predict risks before clinical translation.
Animal models need to recapitulate the complex interactions of the immune system with the tumor microenvironment and specific organs, such as the brain. Although these interactions are limited in mouse xenograft models, advances in humanized mouse models have led to better understanding of the interaction of CAR T cells with the host immune system. Immunocompetent mouse and large animal models provide naturalistic immune environments but do not allow testing of clinical CAR constructs. Thus, no single model can do it all, and it is crucial to coordinate and build upon existing efforts: in vivo, in vitro, organoid/organ chip, and in silico.
To increase the success of modeling, we need to define relevant readouts of preclinical investigations for efficacy and toxicity. Novel biomarkers can be discovered via unbiased screening approaches or candidate-based validation, but both will require high-quality annotated clinical data.
It is essential for clinicians, researchers, and industry scientists to share samples and data in real time. This will accelerate discoveries that help clinicians prevent or manage toxicities in patients.
We have outlined a consensus on the timing and type of samples, sharing of methodologies for analysis, and digital and sample biobanking.6 This approach may be extended to clinical trials that integrate children, older adults, and people of diverse racial and ethnic backgrounds.12 These efforts should extend to other international registries, such as the European Society for Blood and Marrow Transplantation.
Many issues remain to be resolved, including but not limited to formalizing the structure that would oversee this work and ensuring academic independence and academic credit to investigators while balancing this with protection of the proprietary information of industry partners, as well as the priorities of other stakeholders, such as regulators and health authorities. Work is currently ongoing in collaboration with ASH and the CIDR to address these issues, and future workshops and meetings are being planned.
CAR T-cell and TCE therapies are transformative; however, they are complex to administer and generate uniquely challenging adverse events. Validated biomarkers can significantly improve the outcomes for patients who receive these therapies.
Acknowledgments
The authors thank the ASH Task Force on Immunotherapies for organizing this workshop (see collaborators in the supplemental Appendix). The authors also thank Jennifer Motl, freelance medical writer (Milwaukee, WI), for editorial support, funded by ASH (Washington, DC), according to Good Publication Practice.
The CIDR at the Center for International Blood and Marrow Transplant Research is supported by National Cancer Institute, National Institutes of Health, grant U24CA233032.
Contribution: All authors contributed to the workshop and wrote the manuscript.
Conflict-of-interest disclosure: A.A. is employed by and has equity ownership in Bristol Myers Squibb. C.B. is on the scientific advisory board of Myst Therapeutics (just acquired by Turnstone Biologics), has consulted for and received research funding from Iovance Biotherapeuticsto develop tumor-infiltrating lymphocyte therapy in several solid tumor indications. J.G. has consulted for Johnson & Johnson. L.K. has received research funding from Bluebird Bio, Bristol Myers Squibb, Magenta, Novartis, and Regeneron; consulted for EMD Serono, Equilium, FortySeven, Gilead, Interrius, Kymab, Magenta, and Sana; and served on a board for HiFi Bio. S.Z.P. has received royalties for the patent application on “Methods of detection of graft-versus-host disease,” licensed to Viracor-IBT Laboratories. M.C.P., as part of employment at Center for International Blood and Marrow Transplant Research (CIBMTR), has consulted for Amgen, served on an advisory board for Celgene, and received research funding from Bristol Myers Squibb, Celgene, Kite Pharma, and Novartis. M.-A.P. reports honoraria from AbbVie, Astellas, Celgene, Bristol Myers Squibb, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, and Takeda; data and safety monitoring board membership for Cidara Therapeutics, Servier, and Medigene and scientific advisory board membership for NexImmune; research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis; and volunteer membership on Be The Match board of directors (National Marrow Donor Program) and CIBMTR CIDR executive committee. S.S. is employed by the National Marrow Donor Program. V.S. has received research funding from the Department of Defense Congressionally Directed Medical Research Program Neurofibromatosis Research Program, National Cancer Institute K08 and U01 grants, and the Sontag Distinguished Scientist Award; served on a board for the Gilbert Family Foundation; and has worked on patents and royalties at Johns Hopkins University. S.P. declares no competing financial interests.
A complete list of the ASH Task Force on Immunotherapies workshop collaborators appears in the supplemental appendix.
Correspondence: Miguel-Angel Perales, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, Box 59, New York, NY 10021; e-mail: peralesm@mskcc.org.
References
Author notes
The full-text version of this article contains a data supplement.