Key Points
SCD patients with COVID-19 display a broad range of severity, with a higher case fatality than the non-SCD population (10.9% vs 3.3%).
Older patients not treated with hydroxyurea with end organ damage who present with acute kidney injury, and elevated LDH and D-dimer level are at higher risk of death.
Abstract
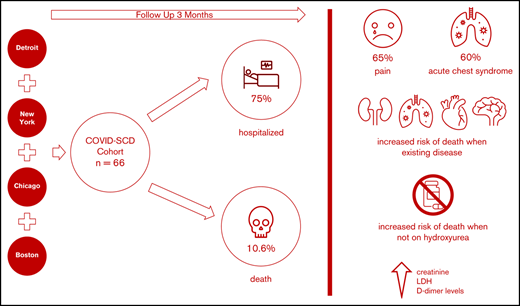
We aimed to identify predictors of outcomes and survival in patients living in 4 major metropolitan areas who had sickle cell disease (SCD) and COVID-19 to inform best approaches to prevention and care. Data were collected at baseline and during the clinical course in SCD patients diagnosed with COVID-19 in four COVID-19 epicenters. Patients were followed up posthospital discharge for up to 3 months. Of sixty-six SCD patients with COVID-19, fifty patients (75%) required hospitalization, and seven died (10.6%). Patients with preexisting kidney disease (chronic kidney disease) were more likely to be hospitalized. The most common presenting symptom was vaso-occlusive pain. Acute chest syndrome occurred in 30 (60%) of the 50 hospitalized patients and in all who died. Older age and histories of pulmonary hypertension, congestive heart failure, chronic kidney disease, and stroke were more prevalent in patients who died, as were higher creatinine, lactate dehydrogenase, and D-dimer levels. Anticoagulation use while inpatient was twice less common in patients who died. All deaths occurred in individuals not taking hydroxyurea or any other SCD-modifying therapy. Patients with SCD and COVID-19 exhibited a broad range of disease severity. We cannot definitively state that the overall mortality is higher in patients with SCD, although our case fatality rate was ∼10% compared with ∼3% in the general population, despite a median age of 34 years. Individuals with SCD aged >50 years, with preexisting cardiopulmonary, renal disease, and/or stroke not receiving hydroxyurea, who present with high serum creatinine, lactate dehydrogenase, and D-dimer levels, are at higher risk of death, irrespective of genotype or sex.
Introduction
Coronaviruses cause serious human disease; both the severe acute respiratory syndrome in 2002 and, 10 years later, the Middle East respiratory syndrome resulted in substantial mortality.1 The presence of a novel coronavirus in Wuhan, China, became apparent at the end of 2019 in a cluster of patients with pneumonia of unknown cause. Zhu et al2 identified severe acute respiratory syndrome coronavirus 2 as the seventh member of the family of coronaviruses that infects humans. The first cases described in the United States resided in Washington state.3,4 The average age of the patients reported in this initial series was 64 years, and the majority of the critically ill patients had preexisting conditions, including diabetes mellitus and chronic kidney disease (CKD). As of 19 October 2020, there are almost 8.2 million cases of COVID-19 in the United States, with ∼220 000 deaths.5
Sickle cell disease (SCD) is an inherited disorder of hemoglobin that predominantly involves individuals of African descent, affecting ∼100 000 individuals in the United States and millions in sub-Saharan Africa, the Middle East, and the Indian subcontinent.6 The disease is characterized by vaso-occlusion of blood vessels by abnormal red blood cells, white blood cells, and platelets, resulting in acute and chronic pain, as well as a broad range of complications affecting every organ system leading to end-organ damage and decreased quality of life. In addition, the survival of patients with SCD is decades less than that of the general population.7 Acute chest syndrome (ACS) and cardiopulmonary complications are common and the main causes of death.8
The SCD population may be particularly susceptible to global viral pandemics, as shown during the H1N1 pandemic when patients with SCD experienced increased vaso-occlusive events and ACS with an increased need for intensive care, including invasive ventilation and exchange transfusions.9-12 We hypothesized that patients with SCD have an increased risk for morbidity and mortality from COVID-19 infection. This increased risk would likely be multifactorial in etiology, with factors ranging from SCD-associated impairments in immunity and hypercoagulability to the fragmentation of care observed, particularly in adults with SCD and the differences in COVID-19 observed according to race potentially all playing a role in this process. At the onset of this study, we also hypothesized that individuals with SCD would have risk factors similar to those of the general population, such as presence of comorbidities, and also unique risk factors to SCD, such as high health care utilization and ACS, that would increase their probability of hospitalization or death.13,14
There have been several case series highlighting the impact of COVID-19 on patients living with SCD.15-22 A recent report from France provided data on COVID-19 severity in hospitalized SCD patients did not appear markedly worse than the general population, which may be a reflection of a younger patient population (most affected patients were aged <45 years).21 A US-based registry provided further data on morbidities and mortality risk associated with COVID-19 infection.22 The overarching aim of the current report was to add granularity to our understanding of the impact of COVID-19 infection on clinical outcomes in patients with SCD to inform best approaches to prevention and care. Specifically, the objectives included identifying risk factors for hospitalization as a marker of disease severity in this population and the impact that specific SCD and/or COVID-19–related therapies had on clinical outcomes and mortality.
Methods
Study population
The study was conducted at 5 academic centers in the United States, located in New York City (2 centers), Boston, Chicago, and Detroit, serving ∼3500 pediatric and adult patients living with SCD. Each respective institutional review board approved data collection as minimal risk research and waived the requirement for informed consent. Patients with SCD and COVID-19 infection confirmed by results of polymerase chain reaction testing of a nasopharyngeal sample between 16 February 2020 and 8 May 2020 were included. A high false-negative rate (38%) of testing has been observed in other cohorts; thus, in the patients who were hospitalized, we included those who were initially negative but for whom subsequent test results during the same hospitalization were found to be positive.23 Patients were followed up clinically after diagnosis for up to 3 months. No patients were excluded from analysis, and there were no patients who were lost to follow-up. Of note, 37 adult patients from New York and 11 adult patients from Boston in this cohort were included in the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Overview (SECURE-SCD) registry and were likely included in a previously published case series.22
Data collection and clinical definitions
Clinical data were prospectively collected in patients with SCD infected with COVID-19 by using a standardized form from each institution’s electronic medical record. Demographic information, medical history and medication usage, vital signs at presentation and during hospitalization, laboratory tests with maximum and minimum values observed for each patient during the hospitalization, inpatient medications and treatments (including noninvasive or invasive ventilation and hemodialysis), and outcomes (hospital length of stay, need for intensive care, and mortality) were collected from all patients. For patients who were hospitalized more than once during the study period, data from their first hospitalization are presented. All data were checked for accuracy by two physicians (A.U.Z. and C.P.M.). Four patients in this cohort were included in a previous publication.15
Mean arterial pressure (MAP) was calculated as follows: CKD was defined according to local guidelines at each institution. Pulmonary hypertension was diagnosed with a right heart catheterization (defined as a mean pulmonary artery pressure >20 mm Hg), if available, or a combination of tricuspid regurgitation velocity ≥2.9 m/s and an elevated N-terminal pro–B-type natriuretic peptide level at baseline (normal values were determined by local institutions). Congestive heart failure, stroke, and ACS were abstracted from diagnoses reported in the electronic medical records. Venous thromboembolism included both deep venous thrombosis and pulmonary embolism. A neutrophil-to-lymphocyte ratio (NLR) was computed at presentation and during hospitalization.
Steady-state values are called “baseline”: they were obtained at least 3 weeks after any acute vaso-occlusive event and were obtained up to 12 months before presentation with COVID-19.
Statistical analysis
Data are reported by using median (interquartile range [IQR]) and frequency tables. Variables were compared between those patients who were hospitalized and those who were not, using a Student t test (or Mann-Whitney U test if required) and Fisher’s exact test. To test the trend of vital signs during the hospitalization, a paired Student t test (or Wilcoxon signed rank test) was used. We compared clinical and laboratory variables between patients who survived and those who died by using the Mann-Whitney U test or Fisher’s exact test. For variables with P < .1 in this analysis, an exact logistic regression was used to calculate the age-adjusted odds ratio (OR) (P value) on mortality. For variables with P < .1 in this analysis, a penalized Firth logistic regression was used to calculate the age-adjusted OR (P value) on mortality. Age was selected as the only possible confounder due to sample size and the importance of age as a prognostic factor in COVID-19 patients without SCD. All analyses were performed in Stata 16.2 (StataCorp, College Station, TX). Missing data were not imputed, and complete case analyses were performed to test the hypotheses.
Results
Demographic and clinical characteristics of the patients
Sixty-six SCD patients with COVID-19 were included in this study; 27 patients (41%) in the cohort received treatment outside of New York City. The age range was 8 months to 69 years; the median age was 34 years (IQR, 24-40 years). There were 9 pediatric patients (range, 8 months to 21 years; median age, 18 years; mean age, 16.2 years); 36 (55%) were female. Fifty-eight patients (88%) self-identified as Black, and 10 (15%) self-identified as Hispanic. Forty-seven (71%) patients had HbSS or HbSβ0 thalassemia genotypes, reflecting the typical distribution observed in North America (Table 1). A history of tobacco use (cigarette smoking) was present in 15 patients (23%), and 7 (11%) used home oxygen. A history of ACS was the most common preexisting comorbidity, observed in 41 patients (62%). CKD was present in 12 patients (33%). Four (6%) adult patients suffered from end stage renal disease (ESRD) prior to the COVID-19 diagnosis, and were already receiving hemodialysis, which was continued during hospitalization.
Preinfection characteristics in the entire cohort
| Characteristic . | All patients . | Not hospitalized . | Hospitalized . | P . | |||
|---|---|---|---|---|---|---|---|
| n . | Results . | n . | Results . | n . | Results . | ||
| Demographic | |||||||
| Age, median (IQR), y | 66 | 34 (24-40) | 16 | 36 (26-40) | 50 | 33 (24-40) | .72 |
| Female sex | 66 | 36 (55) | 16 | 12 (75) | 50 | 24 (48) | .084 |
| Hispanic | 66 | 10 (15) | 16 | 3 (19) | 50 | 7 (14) | .70 |
| Genotype | 66 | 16 | 50 | .79 | |||
| SS/Sβ0 | 47 (71) | 13 (81) | 34 (68) | ||||
| SC | 14 (21) | 2 (13) | 12 (24) | ||||
| Sβ+ | 5 (8) | 1(6) | 4 (8) | ||||
| BMI, median (IQR), kg/m2 | 65 | 24.3 (20.2-27.8) | 16 | 24.4 (19.2-27.2) | 49 | 23.9 (20.2-27.8) | .93 |
| Medical history | |||||||
| Tobacco use (cigarette) | 66 | 15 (23) | 16 | 3 (19) | 50 | 12 (24) | >.9 |
| Venous thromboembolism* | 66 | 19 (29) | 16 | 6 (38) | 50 | 13 (26) | .53 |
| Pulmonary hypertension | 66 | 14 (21) | 16 | 1 (6) | 50 | 13 (26) | .16 |
| ACS | 66 | 41 (62) | 16 | 10 (63) | 50 | 31 (62) | >.9 |
| Stroke | 66 | 12 (18) | 16 | 2 (13) | 50 | 10 (20) | .72 |
| CKD† | 66 | 23 (35) | 16 | 1 (6) | 50 | 22 (44) | .006 |
| Diabetes | 66 | 6 (9) | 16 | 1 (6) | 50 | 5 (10) | >.9 |
| Splenectomy‡ | 66 | 12 (18) | 16 | 3 (19) | 50 | 9 (18) | >.9 |
| Congestive heart failure | 66 | 10 (15) | 16 | 1 (6) | 50 | 9 (18) | .43 |
| Treatment (chronic) | |||||||
| HU | 66 | 28 (42) | 16 | 10 (63) | 50 | 18 (36) | .08 |
| Chronic transfusion | 66 | 5 (8) | 16 | 1 (6) | 50 | 4 (8) | >.9 |
| l-glutamine | 66 | 9 (14) | 16 | 3 (19) | 50 | 6 (12) | .68 |
| Crizanlizumab | 66 | 1 (2) | 16 | 0 | 50 | 1 (2) | >.9 |
| Voxelotor | 66 | 3 (5) | 16 | 1 (6) | 50 | 2 (4) | >.9 |
| Any disease modifier | 66 | 35 (53) | 16 | 11 (69) | 50 | 24 (48) | .17 |
| Characteristic . | All patients . | Not hospitalized . | Hospitalized . | P . | |||
|---|---|---|---|---|---|---|---|
| n . | Results . | n . | Results . | n . | Results . | ||
| Demographic | |||||||
| Age, median (IQR), y | 66 | 34 (24-40) | 16 | 36 (26-40) | 50 | 33 (24-40) | .72 |
| Female sex | 66 | 36 (55) | 16 | 12 (75) | 50 | 24 (48) | .084 |
| Hispanic | 66 | 10 (15) | 16 | 3 (19) | 50 | 7 (14) | .70 |
| Genotype | 66 | 16 | 50 | .79 | |||
| SS/Sβ0 | 47 (71) | 13 (81) | 34 (68) | ||||
| SC | 14 (21) | 2 (13) | 12 (24) | ||||
| Sβ+ | 5 (8) | 1(6) | 4 (8) | ||||
| BMI, median (IQR), kg/m2 | 65 | 24.3 (20.2-27.8) | 16 | 24.4 (19.2-27.2) | 49 | 23.9 (20.2-27.8) | .93 |
| Medical history | |||||||
| Tobacco use (cigarette) | 66 | 15 (23) | 16 | 3 (19) | 50 | 12 (24) | >.9 |
| Venous thromboembolism* | 66 | 19 (29) | 16 | 6 (38) | 50 | 13 (26) | .53 |
| Pulmonary hypertension | 66 | 14 (21) | 16 | 1 (6) | 50 | 13 (26) | .16 |
| ACS | 66 | 41 (62) | 16 | 10 (63) | 50 | 31 (62) | >.9 |
| Stroke | 66 | 12 (18) | 16 | 2 (13) | 50 | 10 (20) | .72 |
| CKD† | 66 | 23 (35) | 16 | 1 (6) | 50 | 22 (44) | .006 |
| Diabetes | 66 | 6 (9) | 16 | 1 (6) | 50 | 5 (10) | >.9 |
| Splenectomy‡ | 66 | 12 (18) | 16 | 3 (19) | 50 | 9 (18) | >.9 |
| Congestive heart failure | 66 | 10 (15) | 16 | 1 (6) | 50 | 9 (18) | .43 |
| Treatment (chronic) | |||||||
| HU | 66 | 28 (42) | 16 | 10 (63) | 50 | 18 (36) | .08 |
| Chronic transfusion | 66 | 5 (8) | 16 | 1 (6) | 50 | 4 (8) | >.9 |
| l-glutamine | 66 | 9 (14) | 16 | 3 (19) | 50 | 6 (12) | .68 |
| Crizanlizumab | 66 | 1 (2) | 16 | 0 | 50 | 1 (2) | >.9 |
| Voxelotor | 66 | 3 (5) | 16 | 1 (6) | 50 | 2 (4) | >.9 |
| Any disease modifier | 66 | 35 (53) | 16 | 11 (69) | 50 | 24 (48) | .17 |
Data are reported by using median (IQR) and frequency tables. Variables were compared between those patients who were hospitalized and those who were not. Results are presented as n (%) unless otherwise indicated. P values compare hospitalized with nonhospitalized patients. Bold P values indicate statistically significant results (P < .05).
BMI, body mass index.
Includes pulmonary embolism.
†Defined as a serum creatinine level >1.0 in HbSS/Sβ0 patients and >1.5 in HbSC/Sβ+ patients, and/or proteinuria.
Indicates surgical splenectomy.
Prior venous thromboembolism (19 of 66 [29%]), pulmonary hypertension (14 of 66 [21%]), stroke (12 of 66 [18%]), and surgical splenectomy (12 of 66 [18%]) were also common in the cohort. Thirty-four (53%) patients were treated with at least one SCD-specific therapy. This was primarily hydroxyurea (HU), in 28 individuals (42%); new therapies such as l-glutamine, voxelotor, and/or crizanlizumab were used in 13 patients (20%).
Clinical characteristics of hospitalized patients compared with nonhospitalized patients
We first compared the baseline characteristics of those who required hospitalization vs those who did not (Tables 1-3). Overall, 50 (76%) of 66 patients required hospitalization. There was no difference in SCD genotype or age in those who were hospitalized. CKD was more prevalent in the hospitalized patients than in nonhospitalized patients (10 of 50 [20%] vs 2 of 16 [13%]; P = .013). Patients who were hospitalized had a higher white blood cell count (P = .019). The use of SCD-modifying therapy was more common in nonhospitalized patients (23 of 50 hospitalized patients [46%] compared with 11 of 16 nonhospitalized patients [69%]; P = .08). In an age-adjusted logistic regression, CKD was associated with hospitalization (OR, 9.1; 95% confidence interval [CI], 1.7-94.5; P = .02) and treatment with HU was more common in patients who were not hospitalized (OR, 0.35; P = .08). Using a stepwise variable selection, male sex (OR, 4.5; 95% CI, 1.2-17.3; P = .03) and CKD (OR, 15.5; 95% CI, 1.8-129.2; P = .012) predicted high risk of hospital admission.
Clinical features during infection
| Presenting condition . | All patients . | Not hospitalized . | Hospitalized . | P . | |||
|---|---|---|---|---|---|---|---|
| n . | Results . | n . | Results . | n . | Results . | . | |
| Pain | 66 | 43 (65) | 16 | 9 (56) | 50 | 34 (68) | .55 |
| ACS | 62 | 31 (50) | 13 | 1 (8) | 49 | 30 (61) | .001 |
| Temperature ≥38.0°C | 62 | 5 (8) | 14 | 1 (7) | 48 | 4 (8) | >.9 |
| Heart rate >100 beats per minute | 60 | 23 (38) | 12 | 7 (58) | 48 | 16 (33) | .18 |
| Oxygen saturation <92% on room air | 60 | 8 (13) | 12 | 1 (8) | 48 | 7 (15) | >.9 |
| MAP, median (IQR), mm Hg | 55 | 86 (79-94) | 10 | 81 (73-88) | 45 | 86 (81-96) | .07 |
| Presenting condition . | All patients . | Not hospitalized . | Hospitalized . | P . | |||
|---|---|---|---|---|---|---|---|
| n . | Results . | n . | Results . | n . | Results . | . | |
| Pain | 66 | 43 (65) | 16 | 9 (56) | 50 | 34 (68) | .55 |
| ACS | 62 | 31 (50) | 13 | 1 (8) | 49 | 30 (61) | .001 |
| Temperature ≥38.0°C | 62 | 5 (8) | 14 | 1 (7) | 48 | 4 (8) | >.9 |
| Heart rate >100 beats per minute | 60 | 23 (38) | 12 | 7 (58) | 48 | 16 (33) | .18 |
| Oxygen saturation <92% on room air | 60 | 8 (13) | 12 | 1 (8) | 48 | 7 (15) | >.9 |
| MAP, median (IQR), mm Hg | 55 | 86 (79-94) | 10 | 81 (73-88) | 45 | 86 (81-96) | .07 |
Data are reported using median (IQR) and frequency tables. Variables were compared between those patients who were hospitalized and those who were not. Results are presented as n (%) unless otherwise indicated. Bold P values indicate statistically significant results (P < .05).
Presenting laboratory values during infection
| Variable . | Not hospitalized . | Hospitalized . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline . | Presentation . | Baseline . | Hospitalization . | |||||||
| n . | Results . | n . | Results . | P . | n . | Results . | n . | Results . | P . | |
| Hemoglobin, g/dL | 15 | 9.1 (7.3-10.3) | 12 | 8.1 (7.0-10.0) | .30 | 38 | 8.8 (7.7-9.7) | 46 | 8.2 (7.3-10.3) | .006 |
| White blood cell, ×109/L | 15 | 8.1 (7.4-10.6) | 12 | 9.8 (4.7-13.3) | .88 | 38 | 10.8 (8.0-13.3) | 46 | 11.4 (7.1-16.1) | .10 |
| Platelet, ×109/L | 12 | 337 (287-419) | 12 | 342 (244-420) | .07 | 26 | 317 (247-521) | 46 | 314 (207-375) | .24 |
| Absolute lymphocyte count, ×109/L | 11 | 2.6 (2.0-4.0) | 11 | 2.0 (1.1-2.9) | .06 | 48 | 2.1 (1.4-3.4) | 38 | 2.2 (1.5-3.1) | .14 |
| Absolute neutrophil count, ×109/L | 12 | 4.8 (4.1-5.8) | 12 | 5.9 (2.6-10.4) | .41 | 26 | 5.0 (4.0-9.3) | 46 | 7.1 (3.8-10.6) | .19 |
| NLR* | 11 | 2.3 (1.0-2.5) | 11 | 2.2 (1.4-4.5) | .14 | 28 | 2.2 (1.3-3.0) | 48 | 3.2 (1.9-5.1) | .025 |
| Lactate dehydrogenase, U/L | 12 | 418 (298-503) | 9 | 336 (305-564) | .17 | 30 | 384 (255-585) | 39 | 438 (319-631) | .26 |
| Reticulocyte count, k/mm3 | 14 | 293 (150-500) | 8 | 172 (101-281) | .050 | 37 | 220 (120-350) | 38 | 207 (95-320) | .67 |
| Creatinine, mg/dL | 15 | 0.6 (0.5-0.8) | 12 | 0.7 (0.6-1.3) | .07 | 38 | 0.7 (0.6-0.9) | 41 | 0.8 (0.6-1.0) | .019 |
| Total bilirubin, mg/dL | 12 | 2.0 (1.4-2.6) | 11 | 1.8 (1.2-3.1) | .58 | 31 | 1.9 (1.1-4.1) | 41 | 2.1 (1.3-3.3) | .22 |
| Direct bilirubin, mg/dL | 12 | 0.4 (0.3-0.5) | 10 | 0.5 (0.2-0.6) | .50 | 30 | 0.4 (0.3-0.6) | 39 | 0.6 (0.3-0.8) | .025 |
| C-reactive protein, mg/L | 4 | 0.8 (0.4-1.9) | 4 | 3.3 (1.4-7.6) | NA | 11 | 1.8 (0.6-3.9) | 28 | 14.6 (5.1-27.5) | .046 |
| ALT, IU/L | 12 | 20 (17-25) | 11 | 22 (19-25) | .003 | 31 | 19 (14-28) | 41 | 26 (17-42) | .012 |
| Ferritin, ng/mL | 12 | 610 (64-3374) | 3 | 611 (237-2249) | .18 | 31 | 571 (236-1835) | 23 | 649 (196-2785) | .053 |
| Variable . | Not hospitalized . | Hospitalized . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline . | Presentation . | Baseline . | Hospitalization . | |||||||
| n . | Results . | n . | Results . | P . | n . | Results . | n . | Results . | P . | |
| Hemoglobin, g/dL | 15 | 9.1 (7.3-10.3) | 12 | 8.1 (7.0-10.0) | .30 | 38 | 8.8 (7.7-9.7) | 46 | 8.2 (7.3-10.3) | .006 |
| White blood cell, ×109/L | 15 | 8.1 (7.4-10.6) | 12 | 9.8 (4.7-13.3) | .88 | 38 | 10.8 (8.0-13.3) | 46 | 11.4 (7.1-16.1) | .10 |
| Platelet, ×109/L | 12 | 337 (287-419) | 12 | 342 (244-420) | .07 | 26 | 317 (247-521) | 46 | 314 (207-375) | .24 |
| Absolute lymphocyte count, ×109/L | 11 | 2.6 (2.0-4.0) | 11 | 2.0 (1.1-2.9) | .06 | 48 | 2.1 (1.4-3.4) | 38 | 2.2 (1.5-3.1) | .14 |
| Absolute neutrophil count, ×109/L | 12 | 4.8 (4.1-5.8) | 12 | 5.9 (2.6-10.4) | .41 | 26 | 5.0 (4.0-9.3) | 46 | 7.1 (3.8-10.6) | .19 |
| NLR* | 11 | 2.3 (1.0-2.5) | 11 | 2.2 (1.4-4.5) | .14 | 28 | 2.2 (1.3-3.0) | 48 | 3.2 (1.9-5.1) | .025 |
| Lactate dehydrogenase, U/L | 12 | 418 (298-503) | 9 | 336 (305-564) | .17 | 30 | 384 (255-585) | 39 | 438 (319-631) | .26 |
| Reticulocyte count, k/mm3 | 14 | 293 (150-500) | 8 | 172 (101-281) | .050 | 37 | 220 (120-350) | 38 | 207 (95-320) | .67 |
| Creatinine, mg/dL | 15 | 0.6 (0.5-0.8) | 12 | 0.7 (0.6-1.3) | .07 | 38 | 0.7 (0.6-0.9) | 41 | 0.8 (0.6-1.0) | .019 |
| Total bilirubin, mg/dL | 12 | 2.0 (1.4-2.6) | 11 | 1.8 (1.2-3.1) | .58 | 31 | 1.9 (1.1-4.1) | 41 | 2.1 (1.3-3.3) | .22 |
| Direct bilirubin, mg/dL | 12 | 0.4 (0.3-0.5) | 10 | 0.5 (0.2-0.6) | .50 | 30 | 0.4 (0.3-0.6) | 39 | 0.6 (0.3-0.8) | .025 |
| C-reactive protein, mg/L | 4 | 0.8 (0.4-1.9) | 4 | 3.3 (1.4-7.6) | NA | 11 | 1.8 (0.6-3.9) | 28 | 14.6 (5.1-27.5) | .046 |
| ALT, IU/L | 12 | 20 (17-25) | 11 | 22 (19-25) | .003 | 31 | 19 (14-28) | 41 | 26 (17-42) | .012 |
| Ferritin, ng/mL | 12 | 610 (64-3374) | 3 | 611 (237-2249) | .18 | 31 | 571 (236-1835) | 23 | 649 (196-2785) | .053 |
Results are presented as median (IQR). P values compare each group, hospitalized and nonhospitalized, vs their own baseline. Bold P values indicate statistically significant results (P < .05).
ALT, alanine transaminase; NA, not available.
Calculated as follows: NLR = (absolute neutrophil count)/(absolute lymphocyte count).
Pain was reported in 43 (65%) of 66 patients at presentation; this was the most common presenting symptom of COVID-19 in SCD patients, and it did not discriminate between hospitalized and nonhospitalized patients. Fever, defined by a temperature ≥38°C, was uncommon at initial presentation, occurring in 7 patients (11%). Tachycardia occurred in 16 (33%) of 48 patients at presentation. Hypoxia, defined by an oxygen saturation <92% on room air, was initially observed in 8 (13%) of 60 patients. An abnormal chest radiographic at presentation, consistent with ACS, was significantly more common in patients who were hospitalized, observed in 29 (60%) of 48 patients compared with 2 (14%) of 14 nonhospitalized patients (P = .001).
Clinical characteristics at steady state compared with presentation with COVID-19
We detail the laboratory data of the hospitalized and nonhospitalized patients at presentation, compared with their steady-state values, before infection with COVID-19 (Tables 2 and 3). We found that patients not hospitalized had minimal changes in their hematologic parameters except for a reduced reticulocyte count; however, patients who required hospitalization had significant alterations in their hematologic parameters, including a significant decrease in hemoglobin concentration (P = .006) and lymphocyte percentage (P = .009), and an increase in NLR (P = .025), from their steady-state values. The NLR is an established marker of systemic inflammation and a validated prognostic factor in several diseases,24,25 including COVID-19, in which a higher NLR is associated with disease severity.26
Markers of hemolysis, including reticulocyte counts, aspartate transaminase, lactate dehydrogenase, and indirect bilirubin, were not significantly different from baseline in hospitalized patients. This suggests that hemolysis did not play a significant role in patients infected with COVID-19 and that the reduction in hemoglobin observed in hospitalized patients was more likely secondary to bone marrow suppression. Hospitalized patients had a statistically significant increase in serum creatinine (P = .019), C-reactive protein (P = .046), direct bilirubin (P = .025), and alanine transaminase (P = .012) from steady state. It should be noted that data on the use of nonsteroidal anti-inflammatory drugs were not collected, and this may be a possible confounder of reduced renal function.
Disease progression and therapeutic approaches in hospitalized patients
During hospitalization, symptoms of infection became more apparent, as fever developed in 24 (53%) of 45 hospitalized patients, tachycardia occurred in 31 (69%) of 45, and MAP decreased (P < .001). Hypoxia eventually developed in 15 (33%) of 45 hospitalized patients. Overall, 13 (28%) of 46 hospitalized patients required oxygen supplementation, 8% were treated with noninvasive ventilation, and 8% required invasive ventilation. Six of the 50 hospitalized patients (12%) required an admission or transfer to an intensive care unit.
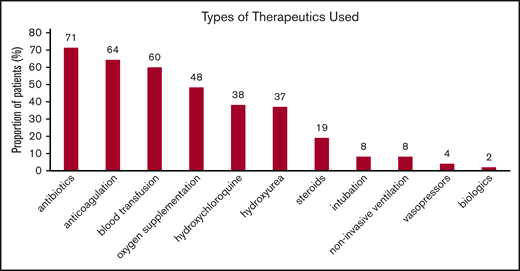
Therapeutic approaches for SCD and COVID-19 patients while hospitalized reflected the temporal standard of care at the different institutions. In Figure 1, the distribution of various therapies used during hospitalization is shown. Of those receiving anticoagulation, 8 (25%) of 32 received therapeutic doses and 24 (75%) of 32 received prophylactic doses consisting mostly of heparin, either low-molecular-weight or unfractionated. Direct oral anticoagulants alone were used in 2 patients, direct oral anticoagulants and heparin were used in 5, and warfarin was used in 1 patient. Thirty-six patients received a blood transfusion: 22 simple and 3 exchange transfusions, while 5 received both.
Types of interventions used in patients hospitalized with COVID-19. Noninvasive ventilation includes continuous positive airway pressure and bilevel pressure ventilation. Blood transfusion includes both simple and exchange transfusion. Among those receiving anticoagulation, 14% received therapeutic anticoagulation. Results are reported as percentage of 50 patients. Patients may have received >1 therapy.
Types of interventions used in patients hospitalized with COVID-19. Noninvasive ventilation includes continuous positive airway pressure and bilevel pressure ventilation. Blood transfusion includes both simple and exchange transfusion. Among those receiving anticoagulation, 14% received therapeutic anticoagulation. Results are reported as percentage of 50 patients. Patients may have received >1 therapy.
Only 2 patients received the interleukin-6 blocker tocilizumab. The first was a 64-year-old female with HbSC disease and a body mass index of 40 kg/m2, who presented with altered mental status and who developed ACS. She was treated with exchange transfusion and tocilizumab, with improvement in her clinical status. The second patient was a 39-year-old man with HbSC disease and a body mass index of 40 kg/m2, who underwent exchange transfusion for multifocal ACS and clinical deterioration, who similarly improved after receiving tocilizumab. Both patients received hydroxychloroquine, HU, and antibiotics as inpatients.
Ten (56%) of 18 patients taking HU as chronic therapy continued to receive HU during hospitalization (supplemental Table 1). There were no deaths, intensive care unit admissions, or need for invasive ventilation in these patients. Patients who were treated with anticoagulation had significantly longer mean length of stay (LOS) (8 [IQR, 4-12] days compared with 3 [IQR, 2-6] days; P = .018). Similarly, patients who received hydroxychloroquine had a significantly longer mean LOS (8 [IQR, 4-12] days compared with 4 [IQR, 2-8] days; P = .002) but also a significant increase in D-dimer levels (P = .002). It is possible that the use of anticoagulation and hydroxychloroquine identified a more severe subgroup of patients, which may also explain why the LOS in patients who were transfused was longer (P = .002). The use of steroids was limited to 19% of patients and had no discernible effect on outcomes (data not shown).
Mortality
There have been 7 deaths in our cohort up to 15 June 2020 (Table 4), with an overall mortality rate of 10.6%. Four deaths occurred during the initial COVID-19 infection presentation (3 were hospitalized and 1 died precipitously in the emergency department). Three deaths occurred 3 to 8 weeks after discharge from the initial hospitalization. The first patient was a 59-year-old man with HbSS, ESRD on hemodialysis, history of pulmonary emboli on anticoagulation, severe iron overload, and pulmonary hypertension. He died within 24 hours of presentation to the emergency department from the home nursing facility where he resided, with altered mental status and severe hypoxia. During his first admission, he presented with altered mental status, severe anemia, and had a 15-day LOS. The second patient was a 46-year-old man with HbSS disease, ESRD, chronic hepatitis B, obstructive sleep apnea, severe iron overload, and arrhythmias; he became noncompliant with hemodialysis because of fear of COVID-19 re-exposure and died suddenly at home 4 weeks after initial discharge. During his first admission, the patient presented with severe anemia and shortness of breath without ACS. He had been hospitalized for 8 days in that initial admission. The third patient was a 68-year-old woman with HbSC disease, who died in a subsequent hospitalization 6 weeks after her initial hospital discharge with multiorgan failure and toxic megacolon in the setting of Clostridium difficile infection. During her initial presentation, she had severe COVID-19 with acute on chronic renal failure that did not require dialysis but did not completely recover before discharge. She required oxygen supplementation and had ACS. The patient was discharged on oral bicarbonate supplementation and sevelamer as a phosphate binder. We have elected to include these patients in the mortality analysis, even though it cannot be elucidated clearly whether this mortality stems directly from COVID-19. We believe this is the best way to report all deaths during the time frame of the study in our analysis.
Demographic and clinical variables of hospitalized patients who died
| Variable . | Alive* . | Death . | P† . | ||
|---|---|---|---|---|---|
| n . | Results . | n . | Results . | ||
| Age, median (IQR), y | 44 | 31 (23-38) | 7 | 53 (39-68) | <.001 |
| Age ≥21 y | 36 (82) | 7 (100) | .58 | ||
| Female sex | 44 | 23 (52) | 7 | 4 (57) | >.9 |
| Hispanic | 44 | 6 (14) | 7 | 1 (14) | >.9 |
| Genotype | 44 | 7 | .66 | ||
| SS/Sβ0 | 30 (68) | 4 (50) | |||
| SC | 11 (25) | 2 (29) | |||
| Sβ+ | 3 (7) | 1 (14) | |||
| Smoking history | 44 | 10 (23) | 7 | 3 (43) | .35 |
| Body mass index, median (IQR), kg/m2 | 43 | 23.9 (20.4-28.0) | 7 | 25.3 (19.8-26.8) | .81 |
| History | |||||
| Venous thromboembolism‡ | 44 | 10 (23) | 7 | 4 (57) | .08 |
| Pulmonary hypertension | 44 | 8 (18) | 7 | 6 (86) | .001 |
| ACS | 44 | 25 (57) | 7 | 6 (86) | .22 |
| Stroke | 44 | 6 (14) | 7 | 4 (57) | .021 |
| CKD§ | 44 | 16 (36) | 7 | 6 (86) | .034 |
| Diabetes | 44 | 3 (7) | 7 | 2 (29) | .13 |
| Splenectomyǁ | 44 | 7 (16) | 7 | 2 (29) | .59 |
| Congestive heart failure | 44 | 5 (12) | 7 | 4 (57) | .013 |
| Presenting symptoms | |||||
| Pain | 44 | 33 (75) | 7 | 2 (29) | .025 |
| Abnormal chest radiograph at presentation | 43 | 24 (56) | 7 | 7 (100) | .035 |
| Fever | 42 | 3 (7) | 7 | 1 (14) | .47 |
| Hypoxia¶ | 42 | 7 (17) | 7 | 0 | .57 |
| Presenting laboratory tests, median (IQR) | |||||
| Hemoglobin, g/dL | 42 | 8.4 (7.5-10.4) | 7 | 5.8 (4.6-9.7) | .07 |
| White blood cells, ×109/L | 42 | 11.3 (6.5-16.1) | 7 | 11.3 (10.6-21.3) | .30 |
| Lymphocyte count, ×109/L | 42 | 2.1 (1.2-3.6) | 7 | 2.2 (1.6-3.0) | .81 |
| NLR | 42 | 3.0 (1.8-5.2) | 7 | 3.5 (2.3-8.0) | .34 |
| Platelet, ×109/L | 42 | 321 (207-376) | 7 | 277 (201-344) | .63 |
| Creatinine, mg/dL | 37 | 0.8 (0.6-1.0) | 7 | 4.1 (2.0-8.5) | <.001 |
| CRP, mg/dL | 26 | 11.9 (4.8-24) | 2 | 29.8 | .20 |
| LDH, U/L | 35 | 431 (313-583) | 7 | 631 (462-1293) | .012 |
| D-dimer, μg/mL | 20 | 1.9 (0.9-3.4) | 5 | 9.2 (2.1-12.7) | .011# |
| Treatment during hospitalization | |||||
| HU | 44 | 16 (36) | 7 | 2 (29) | >.9 |
| Blood transfusion | 44 | 25 (57) | 7 | 5 (71) | .69 |
| Anticoagulation | 44 | 30 (68) | 7 | 2 (29) | .09 |
| Mechanical ventilation | 44 | 2 (5) | 7 | 3 (43) | .015 |
| ICU admission | 44 | 5 (11) | 7 | 1 (14) | >.9 |
| LOS | 44 | 5 (3-10) | 7 | 8 (1-11) | .75 |
| Treatment (chronic) | |||||
| HU | 44 | 18 (41) | 7 | 0 | .042 |
| Chronic transfusion | 44 | 4 (9) | 7 | 0 | >.9 |
| l-glutamine | 44 | 6 (13) | 7 | 0 | .58 |
| Crizanlizumab | 44 | 1 (2) | 7 | 0 | >.9 |
| Voxelotor | 44 | 2 (5) | 7 | 0 | >.9 |
| Any disease modifier | 44 | 24 (55) | 7 | 0 | .011 |
| Variable . | Alive* . | Death . | P† . | ||
|---|---|---|---|---|---|
| n . | Results . | n . | Results . | ||
| Age, median (IQR), y | 44 | 31 (23-38) | 7 | 53 (39-68) | <.001 |
| Age ≥21 y | 36 (82) | 7 (100) | .58 | ||
| Female sex | 44 | 23 (52) | 7 | 4 (57) | >.9 |
| Hispanic | 44 | 6 (14) | 7 | 1 (14) | >.9 |
| Genotype | 44 | 7 | .66 | ||
| SS/Sβ0 | 30 (68) | 4 (50) | |||
| SC | 11 (25) | 2 (29) | |||
| Sβ+ | 3 (7) | 1 (14) | |||
| Smoking history | 44 | 10 (23) | 7 | 3 (43) | .35 |
| Body mass index, median (IQR), kg/m2 | 43 | 23.9 (20.4-28.0) | 7 | 25.3 (19.8-26.8) | .81 |
| History | |||||
| Venous thromboembolism‡ | 44 | 10 (23) | 7 | 4 (57) | .08 |
| Pulmonary hypertension | 44 | 8 (18) | 7 | 6 (86) | .001 |
| ACS | 44 | 25 (57) | 7 | 6 (86) | .22 |
| Stroke | 44 | 6 (14) | 7 | 4 (57) | .021 |
| CKD§ | 44 | 16 (36) | 7 | 6 (86) | .034 |
| Diabetes | 44 | 3 (7) | 7 | 2 (29) | .13 |
| Splenectomyǁ | 44 | 7 (16) | 7 | 2 (29) | .59 |
| Congestive heart failure | 44 | 5 (12) | 7 | 4 (57) | .013 |
| Presenting symptoms | |||||
| Pain | 44 | 33 (75) | 7 | 2 (29) | .025 |
| Abnormal chest radiograph at presentation | 43 | 24 (56) | 7 | 7 (100) | .035 |
| Fever | 42 | 3 (7) | 7 | 1 (14) | .47 |
| Hypoxia¶ | 42 | 7 (17) | 7 | 0 | .57 |
| Presenting laboratory tests, median (IQR) | |||||
| Hemoglobin, g/dL | 42 | 8.4 (7.5-10.4) | 7 | 5.8 (4.6-9.7) | .07 |
| White blood cells, ×109/L | 42 | 11.3 (6.5-16.1) | 7 | 11.3 (10.6-21.3) | .30 |
| Lymphocyte count, ×109/L | 42 | 2.1 (1.2-3.6) | 7 | 2.2 (1.6-3.0) | .81 |
| NLR | 42 | 3.0 (1.8-5.2) | 7 | 3.5 (2.3-8.0) | .34 |
| Platelet, ×109/L | 42 | 321 (207-376) | 7 | 277 (201-344) | .63 |
| Creatinine, mg/dL | 37 | 0.8 (0.6-1.0) | 7 | 4.1 (2.0-8.5) | <.001 |
| CRP, mg/dL | 26 | 11.9 (4.8-24) | 2 | 29.8 | .20 |
| LDH, U/L | 35 | 431 (313-583) | 7 | 631 (462-1293) | .012 |
| D-dimer, μg/mL | 20 | 1.9 (0.9-3.4) | 5 | 9.2 (2.1-12.7) | .011# |
| Treatment during hospitalization | |||||
| HU | 44 | 16 (36) | 7 | 2 (29) | >.9 |
| Blood transfusion | 44 | 25 (57) | 7 | 5 (71) | .69 |
| Anticoagulation | 44 | 30 (68) | 7 | 2 (29) | .09 |
| Mechanical ventilation | 44 | 2 (5) | 7 | 3 (43) | .015 |
| ICU admission | 44 | 5 (11) | 7 | 1 (14) | >.9 |
| LOS | 44 | 5 (3-10) | 7 | 8 (1-11) | .75 |
| Treatment (chronic) | |||||
| HU | 44 | 18 (41) | 7 | 0 | .042 |
| Chronic transfusion | 44 | 4 (9) | 7 | 0 | >.9 |
| l-glutamine | 44 | 6 (13) | 7 | 0 | .58 |
| Crizanlizumab | 44 | 1 (2) | 7 | 0 | >.9 |
| Voxelotor | 44 | 2 (5) | 7 | 0 | >.9 |
| Any disease modifier | 44 | 24 (55) | 7 | 0 | .011 |
Data are presented as n (%) unless otherwise identified. Bold P values indicate statistically significant results (P < .05).
CRP, C-reactive protein; ICU, intensive care unit; LDH, lactate dehydrogenase.
Limited to hospitalized patients.
P value comparing patients who died vs those who survived.
Includes pulmonary embolism.
Defined according to local guidelines at each institution.
Indicates surgical splenectomy.
Indicated by oxygen saturation <92%.
P value from the permutation Student t test.
Of the patients who died, 4 were HbSS, 2 were HbSC, and 1 was HbSβ+; the median age was 53 years, significantly higher than the median age of the entire cohort of 31 years (P < .001), and 3 (43%) of 7 were female. In these patients, there was a statistically significant higher proportion of baseline pulmonary hypertension (P = .001), stroke (P = .021), CKD (P = .034), and CHF (P = .013). Pain was a less frequent presenting issue in those who died (P = .025), and every patient who died had evidence of ACS (P = .035). In an age-adjusted logistic regression analysis, a history of pulmonary hypertension was associated with a markedly increased mortality risk (OR, 8.1; 95% CI, 1.1-61.0; P = .042), followed by CHD (OR, 5.5; 95% CI, 0.9-33.3), stroke (OR, 3.8; 95% CI, 0.6-24.6), and CKD (OR, 2.9; 95% CI, 0.4-21.0), although these were not statistically significant. Of note, none of the patients who died was taking HU (P = .042) or any disease-modifying therapy at baseline, compared with 28 (47%) of 59 in the survival cohort (P = .011). Laboratory values at presentation revealed that those who died had higher serum lactate dehydrogenase (P = .012) and creatinine (P < .001) levels than those who survived. Although 65% of patients in our hospitalized cohort received anticoagulation, 5 of the 7 patients who died did not (71%). D-dimer levels were significantly higher in individuals who died (P = .011) (Table 4).
Discussion
Our study provides important clinical information on factors affecting the clinical course and mortality in patients with SCD and COVID-19 infection. Our cohort included consecutively identified COVID-19–infected patients with SCD at 5 major sickle cell centers who cumulatively provide care to ∼5% of the total SCD population in the United States. We observed a wide spectrum of disease severity, ranging from minimal symptoms to multisystem organ failure and death. Several case series and 2 large cohort studies, one from Europe and one from the United States (the SECURE-SCD registry),15-22,27 have reported on the clinical characteristics and mortality rate of patients with SCD and COVID-19. The US-based registry reported a 7% mortality in 178 patients, whereas the French cohort had 2 deaths in 83 patients.22 In general, younger patients had a less severe course, with only 1 pediatric death in those cohorts. The mortality rate in our cohort was 10.6%, with 4 of 7 deaths occurring during the initial presentation and the remaining 3 deaths occurring within 3 to 8 weeks of discharge from the hospital. This is a higher rate than observed in other published series to date in SCD cohorts.15-22,27 Similar to most of the existing literature, the case fatality rate in SCD could be lower, as we were only aware of patients who were symptomatic and presented to the health care system.
Although we cannot with certainty say that COVID-19 was a proximate cause of death in the patients who died after initial discharge, we have included these deaths because they may represent late sequelae related to COVID-19 infection that increased their subsequent mortality risk. Our findings underscore the importance of close follow up of all SCD patients with COVID-19 after initial presentation, even if the initial infection resulted in mild disease. Of all decedents regardless of SCD diagnosis reported to the Centers for Disease Control and Prevention in a circumscribed time frame, 0.1% were <18 years old, 2.8% were 18 to 44 years old, and 5.1% were 45 to 54 years old.28
In the current cohort, older age and the presence of end-organ disease, particularly pulmonary hypertension, increased the risk of death. In agreement with other cohorts, we observed that severe COVID-19 infection and death were not limited to the traditional “severe genotypes” (HbSS/HbSβ0); those with HbSC/HbSβ+ were similarly affected and represented among hospitalized and deceased individuals. Severe disease requiring hospitalization occurred more frequently in those with preexisting end-organ disease of the brain, kidneys, heart, and lungs, reflective of systemic vasculopathy. This suggests that patients with multiple end-organ diseases, especially pulmonary hypertension, CHF, and CKD, irrespective of hemoglobin genotype, should be aggressively evaluated and treated in the setting of COVID-19 infection. An abnormal chest radiograph consistent with ACS was more common in those admitted to the hospital, and in all who died. Thus, we suggest that this should be part of the diagnostic evaluation for all SCD patients presenting with COVID-19–like symptoms. Unique to this population, pain was the most common presenting feature, and this should prompt clinicians to obtain testing for COVID-19. It should be noted, however, that among the patients who died, pain was not a prominent presenting feature. Contrary to the data from patients with SCD during the H1N1 pandemic, we found that COVID-19 infection was not associated with significant hemolysis.
HU has been established as a disease-modifying therapy with a multitude of benefits in SCD in terms of both morbidity and mortality. Similarly, in this cohort, chronic HU therapy was protective of death (P = .042). Moreover, none of the deceased patients was receiving any disease-modifying therapy. The beneficial effect of HU could be secondary to the reduction of hemolysis, platelets, leukocytes, and inflammatory parameters, especially interleukin-6.29 HU is a dose-dependent donor of nitric oxide (NO),30 and modulation of intracellular levels of the NO second messenger, cyclic guanosine monophosphate, is effective in amplifying intracellular NO-dependent signaling. HU may have immediate beneficial effects on the microvasculature that are independent of the drug’s fetal hemoglobin-elevating properties and likely involve the formation of intravascular NO and inhibition of phosphodiesterase type 9.31 This finding needs to be interpreted with caution, as HU is recommended mostly for individuals with HbSS/Sβ0, and therefore HbSC and Sβ+ patients are not as likely to receive the agent. Furthermore, given that HU may be contraindicated in persons with CKD, it should be noted that there may be some confounding, which may show collinearity between HU use and the CKD findings.
Because SCD is a disease of reduced survival,7 the median age of those who died of COVID-19 infection in our cohort (53 years) was much younger than that of the US non-SCD population (median age, 78 years).28 Although not statistically significant, an interesting clinical observation that emerges from this study is that SCD patients treated with any form of anticoagulation were less likely to die of COVID-19 infection. Almost 70% of patients who survived received either prophylactic or therapeutic anticoagulation, whereas only 30% of patients who died did (P = .09). COVID-19–associated coagulopathy has been widely described in the general population.32,33 The inflammatory, hypercoagulable, and vasculopathic milieu present in patients with SCD, especially as they age, likely predisposes these patients to thrombosis if other risk factors are present. Although our results were not significant, we would favor the use of at least prophylactic anticoagulation for SCD patients hospitalized with COVID-19. The suggestion of an impaired coagulation balance has been mentioned in previous studies,33 and there has been suggestion of elevated D-dimer being associated with disease severity. Although we could not determine the association of D-dimer levels on presentation with disease severity due to missing data, we observed that patients with SCD who died were more likely to have elevated D-dimer levels during hospitalization (P = .011).
Due to limitations of a small sample, we cannot definitively state that the overall mortality is higher in patients with SCD. Based on these data, however, it seems that there is a higher case fatality rate than in the non-SCD population (10.9% vs 3.3%) (https://www.statista.com/statistics/1105431/covid-case-fatality-rates-us-by-age-group).
Finally, we remain concerned about the impact of social determinants of health and health care disparities on the observed outcomes. There is a growing body of knowledge in the United States that Black and Hispanic individuals face increased risk from COVID-19 infection, although the exact etiology of this disparity remains unclear. The limited use of biologics and novel therapeutics in our cohort (only 2 patients received tocilizumab) is striking, as is the fact that almost 50% of patients were on no SCD-modifying therapies, particularly HU, which likely increases their risk of ACS from COVID-19.
We are beginning to understand the clinical features that place those living with SCD at higher risk for hospitalization and death. Older patients regardless of hemoglobin genotype, particularly those with chronic end-organ disease of the kidneys, brain, heart, and lungs, seem to be at the highest risk for morbidity, including ACS. Patients with pulmonary hypertension of SCD may be at the highest risk for death from COVID-19. Although our findings need to be confirmed in larger cohorts, we anticipate that they will be useful for risk stratification of patients with SCD as this pandemic continues. We plan to track prospectively the patients enrolled in this cohort for further analyses.
Requests for data sharing may be submitted to the corresponding author (Caterina P. Minniti; e-mail: caterina.minniti@einsteinmed.org).
Acknowledgments
The authors greatly appreciate the efforts of their fellow health care workers and support staff at the Montefiore Medical Center and the other participating academic centers in this study for providing outstanding patient care at considerable personal risk on the front lines of this pandemic. They express their support for their patients with COVID-19 and their families.
Authorship
Contribution: C.P.M. and A.U.Z. wrote the paper; M.N. performed the statistical analysis; D.M. and G.D.C. contributed to the paper; A.S.C. and M.U.C. helped write the paper and collect data; S.C., C.J., J.H., and J.S. helped collect and analyze data; and J.G., V.R.G., and E.S.K. helped write the manuscript and analyze the data.
Conflict-of-interest disclosure: C.P.M. declares honoraria for consulting/advisory boards for GBT, Emmaus, Roche, Forma, Novartis, CSL Behring, Bluebird Bio, and research funds from GBT. A.U.Z. declares honoraria/advisory boards from Global Blood Therapeutics, Novartis, Emmaus Life Sciences, Cyclerion, Imara, and Speakers Bureau: Global Blood Therapeutics. M.U.C. declares grants and personal fees from Bayer, Biomarin, Bluebird Bio, Global Blood Therapeutics, Hema Biologics, Kedrion, Octapharma, Pfizer, Roche/Genentech, Sanofi/Bioverativ, Spark Therapeutics, and Takeda. J.G. delcares Eli Lilly research funding, and Global Blood Therapeutics consulting. E.S.K. declares research support from Bayer and Arena/United Therapeutics, consulting for Novartis, Micelle Data and Safety Monitoring Board for the Phase III trial of Omega 3 Fatty Acids for Children and Adolescents with Sickle Cell Disease, and Novartis development of a disease severity score for patients with sickle cell disease. The remaining authors declare no competing financial interests.
Correspondence: Caterina P. Minniti, Division of Hematology, Montefiore Health Systems, Albert Einstein College of Medicine, Bronx, NY 10467; e-mail: caterina.minniti@einsteinmed.org.
References
Author notes
C.P.M and A.U.Z. contributed equally to this study.
The full-text version of this article contains a data supplement.