Key Points
Early systemic CNS prophylaxis with high-dose methotrexate followed by dose-dense immunochemotherapy is feasible in high-risk DLBCL patients.
Most patients, including those with BCL2/MYC double-hit lymphomas, can achieve durable remissions with a low risk of CNS progression.
Abstract
Survival of patients with high-risk diffuse large B-cell lymphoma (DLBCL) is suboptimal, and the risk of central nervous system (CNS) progression is relatively high. We conducted a phase 2 trial in 139 patients aged 18 to 64 years who had primary DLBCL with an age-adjusted International Prognostic Index (aaIPI) score of 2 to 3 or site-specific risk factors for CNS recurrence. The goal was to assess whether a dose-dense immunochemotherapy with early systemic CNS prophylaxis improves the outcome and reduces the incidence of CNS events. Treatment consisted of 2 courses of high-dose methotrexate in combination with biweekly rituximab (R), cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP-14), followed by 4 courses of R-CHOP-14 with etoposide (R-CHOEP) and 1 course of high-dose cytarabine with R. In addition, liposomal cytarabine was administered intrathecally at courses 1, 3, and 5. Coprimary endpoints were failure-free survival and CNS progression rates. Thirty-six (26%) patients experienced treatment failure. Progression occurred in 23 (16%) patients, including three (2.2%) CNS events. At 5 years of median follow-up, failure-free survival, overall survival, and CNS progression rates were 74%, 83%, and 2.3%, respectively. Treatment reduced the risk of progression compared with our previous trial, in which systemic CNS prophylaxis was given after 6 courses of biweekly R-CHOEP (hazard ratio, 0.49; 95% CI, 0.31-0.77; P = .002) and overcame the adverse impact of an aaIPI score of 3 on survival. In addition, outcome of the patients with BCL2/MYC double-hit lymphomas was comparable to the patients without the rearrangements. The results are encouraging, with a low toxic death rate, low number of CNS events, and favorable survival rates. This trial was registered at www.clinicaltrials.gov as #NCT01325194.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a curable disease with combination chemotherapy. The outcome is variable but can to some extent be predicted from clinical risk factors included in the International Prognostic Index (IPI) score.1 Combination of a CD20 targeted monoclonal antibody, rituximab (R), to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP-14) or CHOP-21 regimens has substantially improved progression-free survival (PFS) and overall survival (OS) in all elderly and young low-risk DLBCL patients.2 However, dose densification of R-CHOP cycles from 21 to 14 days, or infusional dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R) has not provided further survival benefit.3-5
For young, clinically high-risk DLBCL patients, the optimal therapy has not been established. Studies comparing conventional doses of chemotherapy with high-dose therapy followed by autologous stem cell transplantation have not convincingly shown an advantage for high-dose therapy,6,7 and there is no randomized comparison of the efficacy of adding R to chemotherapy in young, high-risk patients. According to Nordic population-based studies, the addition of etoposide (E) to an R-CHOP-14 regimen improves OS of young high-risk patients.8,9 R-MegaCHOEP, in turn, is not superior to R-CHOEP-14 and is associated with significantly more toxicity.10 Likewise, rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R‐HCVAD)/rituximab, high-dose methotrexate (HD-Mtx), and cytarabine (R‐MA) did not differ from R-CHOP with respect to survival due to high treatment-related mortality.11
In addition to a high risk of systemic relapse, patients with DLBCL are at risk for progression of their lymphoma in the central nervous system (CNS). In the rituximab era, the rate and patterns of CNS involvement with DLBCL have evolved.12 The overall risk of CNS progression has been reduced to 5%; for high-risk patients with more than one extranodal site and elevated lactate dehydrogenase (LDH) levels, this risk is 10% to 15%,13-15 and particularly those with renal or adrenal involvement.16 In addition, localization of CNS relapse has shifted to the brain parenchyma in the majority of cases.12
To the best of our knowledge, no study has shown in a prospective randomized fashion that CNS prophylaxis with intrathecal or systemic Mtx prevents progression of lymphoma in the CNS. German studies of non-Hodgkin lymphoma have shown that the risk of CNS failure is reduced after addition of E or R to the CHOP regimen.13,17 Furthermore, some retrospective analyses have shown that HD-Mtx–based systemic CNS prophylaxis may reduce the risk of CNS progression.18-20
The toxicity and efficacy of the R-CHOEP-14 regimen consolidated with late systemic CNS prophylaxis in young high-risk patients was investigated in a Nordic NLG-LBC-04 (CRY-04) study.21 Three-year OS and failure-free survival (FFS) rates were 81% and 65%, respectively. Seven patients experienced CNS progression, all within 6 months of diagnosis, suggesting that the patients had a subclinical disease at diagnosis. We hypothesized that shifting of CNS prophylaxis to the beginning of the therapy could overcome the subclinical disease, and thus reduce the risk of early clinical CNS progression. To address the efficacy and toxicity of early CNS prophylaxis, we initiated the NLG-LBC-05 (CHIC) study, in which systemic CNS prophylaxis with HD-Mtx was given in the beginning of therapy, and CNS-targeted therapy further intensified by adding intrathecally administered liposomal cytarabine.
Methods
Patients
Eligible patients were 18 to 64 years old with previously untreated, histologically confirmed CD20+ DLBCL or follicular lymphoma grade 3B based on the World Health Organization 2008 Lymphoma Classification.22 The following subgroups and variants were allowed: ALK-positive large B-cell lymphoma, mediastinal large B-cell lymphoma, intravascular large B-cell lymphoma, T-cell/histiocyte-rich large B-cell lymphoma, and follicular lymphomas grade 3B. Patients with posttransplantation lymphoma, discordant or transformed lymphoma, lymphomas intermediate between DLBCL and Burkitt’s lymphoma, and primary CNS lymphoma were ineligible.
Patients had to present World Health Organization performance status <4, without clinical, radiologic, or cytologic signs of CNS involvement with occult cerebrospinal fluid (CSF) involvement (flow cytometry [FCM]-positive/cytology-negative) allowed, age-adjusted IPI (aaIPI) score of 2 to 3,1 or specific risk factors for CNS recurrence defined by more than one extranodal site, testicular lymphoma, stage IIE and higher, paranasal sinus and orbital lymphoma with destruction of bone, or large cell infiltration of the bone marrow, and adequate organ function, allowing the planned treatment schedule. Additional details on inclusion and exclusion criteria, and study procedures are provided in the supplemental Methods.
The protocol was approved by the medical agencies and ethics committees in Finland, Denmark, Norway, and Sweden, and the trial was registered at ClinicalTrials.gov as #NCT01325194. All patients signed informed consent before study participation.
Molecular pathology
Patients were included in the study based on a histological diagnosis from the local pathologists. After inclusion, samples were forwarded to the National Pathology Review representatives (E.R., S.S., K.B., and M.-L.K.-L.) for the confirmation of the diagnosis and further subclassification into the 2 immunohistochemically defined subgroups of germinal center B-cell type (GCB) and non-GCB according to the Hans algorithm.23 Fluorescent in situ hybridization analysis was performed retrospectively on available tissue microarray or full paraffin-embedded 2- to 3-μm tissue sections by using the break-apart probes for c-MYC/8q24, BCL2/18q21, and BCL6/3q27 (Vysis, Abbott Molecular). The assessment of Ki67-positivity was conducted semi-quantitatively by the central pathology reviewer.
FCM immunophenotyping
CSF samples were directly collected into TransFix tubes (Immunostep SL) and shipped overnight to the central FCM laboratory in Oslo, Norway. Samples were centrifuged, supernatant discarded, and the cell pellet resuspended in phosphate-buffered saline. Immunophenotyping was performed by using multiparameter FCM as previously described.24
Treatment
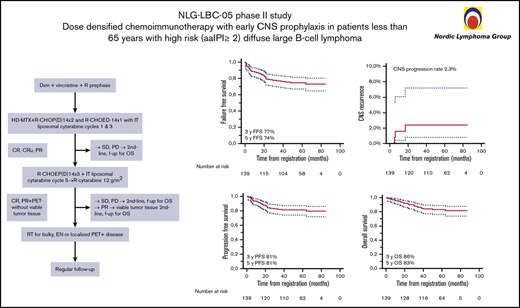
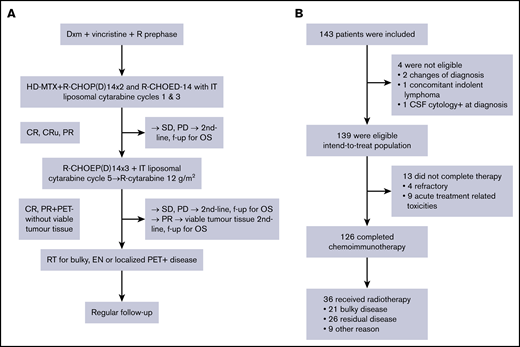
The study design is shown in Figure 1A and additional details on treatment are described in the supplemental Methods.
Study schema. (A) Trial profile. (B) Patient disposition. CR, complete response; CRu,complete response, unconfirmed; CSF, cerebrospinal fluid; EN, extranodal; f-up, follow-up; IT, intrathecal; OS, overall survival; PD, progressive disease; PET, positron emission tomography; PR, partial response; RT, radiotherapy; SD, stable disease.
Study schema. (A) Trial profile. (B) Patient disposition. CR, complete response; CRu,complete response, unconfirmed; CSF, cerebrospinal fluid; EN, extranodal; f-up, follow-up; IT, intrathecal; OS, overall survival; PD, progressive disease; PET, positron emission tomography; PR, partial response; RT, radiotherapy; SD, stable disease.
Statistical analyses
Coprimary end points were to estimate the proportion of patients who were failure- and CNS progression-free at 3 and 1.5 years, respectively. FFS was defined as the interval between the registration date and the date of documented progression or lack of response, first relapse, death for any reason, or discontinuation/change of therapy because of toxicity, whichever occurred first. Otherwise, patients were censored at the last date they were known to be alive. For patients not responding at any time point on study treatment, FFS is defined as day 1. CNS recurrence was defined as the interval between registration date and the date of documented CNS progression. Of the secondary end points, OS was defined as the interval between the registration date and death from any cause, and PFS as the period between the registration date and lymphoma progression or death from any cause. Other secondary end points were response rate, toxicity, biological risk factors, and prognostic role of CSF (cytology-negative/FCM-positive) for CNS recurrence.
Survival rates were estimated by using the Kaplan-Meier method. Clinical and tumor-related factors were analyzed by using χ2 tests or nonparametric trend tests for response rates, and log-rank tests and the Cox proportional hazards multivariate analysis for survival. Statistical analyses were performed with SPSS version 25.0 (IBM SPSS Statistics, IBM Corporation). Probability values <0.05 were considered statistically significant. All comparisons and all comparative tests were 2-tailed.
Additional details, including power calculations, are provided in the supplemental Methods.
Results
Patient demographic characteristics
Between March 2011 and December 2014, a total of 143 previously untreated patients, 18 to 64 years of age, were recruited. At central pathology review, 2 cases were excluded as non-DLBCL/non-grade 3B follicular lymphoma. One patient was excluded due to a concomitant CNS lymphoma and one due to concomitant cutaneous follicular lymphoma, leaving 139 evaluable patients (intention-to-treat population) (Figure 1B). The majority of the patients had DLBCL (96%); the other subtypes are specified in Table 1. The patient characteristics were typical for high-risk DLBCL with a median age of 56 years (range, 20-64 years), advanced clinical stage, elevated LDH level, more than one extranodal site, and B symptoms. A bulky lesion (>10 cm) was present in 37% of the patients, 45% had a high CNS-IPI score, and 11 CSF samples (8%) were FCM-positive.
Patient characteristics (N = 139)
| Characteristic . | Value, n . | % . |
|---|---|---|
| Age, median (range), y | 56 (20-64) | NA |
| Sex | ||
| Male | 88 | 63 |
| Female | 51 | 37 |
| DLBCL NOS | 113 | 81 |
| GCB | 56 | 49 |
| Non-GCB | 47 | 42 |
| Not determined | 10 | 9 |
| DHL/examined (missing) | 9/77 (36) | 12 |
| TCRB | 5 | 3.6 |
| PMBCL | 8 | 5.8 |
| Intravascular | 1 | 0.7 |
| Follicular lymphoma grade 3B | 5 | 4.3 |
| Not reviewed | 7 | 5.0 |
| ECOG PS >1 | 43 | 31 |
| Stage | ||
| I-II | 11 | 8 |
| III | 26 | 19 |
| IV | 102 | 73 |
| B symptoms | 88 | 63 |
| LDH increase | 127 | 91 |
| aaIPI | ||
| 0-1* | 10 | 7.2 |
| 2 | 83 | 60 |
| 3 | 46 | 33 |
| Site-specific risk factor for CNS recurrence among aaIPI score of 0-1 | ||
| >1 extranodal site | 5 | 3.6 |
| Testicular lymphoma stage IIE or higher | 2 | 1.4 |
| Paranasal sinus and orbital lymphoma with destruction of bone | 3 | 2.2 |
| CNS IPI | ||
| Low (0-1 factor) | 4 | 2.9 |
| Intermediate (2-3 factors) | 72 | 52 |
| High (≥4 factors) | 63 | 45 |
| Bulky disease | 52 | 37 |
| >1 extranodal sites | 81 | 67 |
| CSF FCM-positive | 11 | 8 |
| Characteristic . | Value, n . | % . |
|---|---|---|
| Age, median (range), y | 56 (20-64) | NA |
| Sex | ||
| Male | 88 | 63 |
| Female | 51 | 37 |
| DLBCL NOS | 113 | 81 |
| GCB | 56 | 49 |
| Non-GCB | 47 | 42 |
| Not determined | 10 | 9 |
| DHL/examined (missing) | 9/77 (36) | 12 |
| TCRB | 5 | 3.6 |
| PMBCL | 8 | 5.8 |
| Intravascular | 1 | 0.7 |
| Follicular lymphoma grade 3B | 5 | 4.3 |
| Not reviewed | 7 | 5.0 |
| ECOG PS >1 | 43 | 31 |
| Stage | ||
| I-II | 11 | 8 |
| III | 26 | 19 |
| IV | 102 | 73 |
| B symptoms | 88 | 63 |
| LDH increase | 127 | 91 |
| aaIPI | ||
| 0-1* | 10 | 7.2 |
| 2 | 83 | 60 |
| 3 | 46 | 33 |
| Site-specific risk factor for CNS recurrence among aaIPI score of 0-1 | ||
| >1 extranodal site | 5 | 3.6 |
| Testicular lymphoma stage IIE or higher | 2 | 1.4 |
| Paranasal sinus and orbital lymphoma with destruction of bone | 3 | 2.2 |
| CNS IPI | ||
| Low (0-1 factor) | 4 | 2.9 |
| Intermediate (2-3 factors) | 72 | 52 |
| High (≥4 factors) | 63 | 45 |
| Bulky disease | 52 | 37 |
| >1 extranodal sites | 81 | 67 |
| CSF FCM-positive | 11 | 8 |
ECOG, Eastern Cooperative Oncology Group; NA, not applicable; NOS, not otherwise specified; PMBCL, primary mediastinal B-cell lymphoma; PS, performance status; TCRB, T-cell rich B-cell lymphoma.
With site-specific risk factors for CNS recurrence defined by >1 EN site, testicular lymphoma stage IIE and higher, paranasal sinus and orbital lymphoma with destruction of bone, and large cell infiltration of the bone marrow.
Most patients (n = 127; 96%) received the full treatment schedule. The second cycle was given 19 days (median) after the first cycle, which means a median delay of 2 days, while the third cycle was given after 16 days (median) from the second cycle. The second HD-Mtx dose was reduced or not given in 34% of the patients mostly due to renal toxicity; reductions for the second doxorubicin and cyclophosphamide doses were performed in 6% and 6.5% of patients, respectively. The second and the third cycles were delayed by >1 week in 8.3% and 5.3% of the patients.
Intrathecal liposomal cytarabine was given to 81 (61%) patients and omitted from the rest of the patients due to a transient production halt. Local radiotherapy was administered to 39 (30%) patients; 25 patients received it due to a bulky lesion at diagnosis, and 17 patients due to lesions positive according to positron emission tomography (PET) at the end of immunochemotherapy.
Biomarker analysis
CD10, BCL6, MUM/IRF4, BCL2, and CD5 positivity was observed in 38 (30%), 104 (83%), 45 (38%), 96 (82%), and 10 (9%) samples, respectively. On the basis of the Hans algorithm, 56 (54%) of the patients were classified as GCB DLBCLs, and 47 (46%) were classified as non-GCB DLBCLs. Double positivity for BCL2 and MYC (double protein expressor) was found in 15 (40%) of the examined 38 samples. Among the samples displaying interpretable fluorescent in situ hybridization signals, BCL2/18q21, BCL6/3q27, and c-MYC/8q24 gene rearrangements were found in 23 (27%), 16 (19%), and 11 (14%) of the cases. BCL2/18q21 and c-MYC/8q24 rearrangements were strongly associated with the GCB subgroup according to the Hans classifier (P = .001 and P = .007). BCL6/3q27 rearrangement was not correlated to either category. Double-hit lymphomas (DHLs) were found in 9 (12%) of the examined 77 samples, all within the GCB subgroup. All DHL cases had advanced stage, eight (85%) had elevated LDH levels, and three (33%) had high CNS-IPI scores.
Toxicity and treatment failures
The fraction of patients with reported grade 3 to 4 toxic effects, treatment failures due to acute and late toxicities, and toxic deaths is shown in Table 2 and supplemental Table 1. Thirty-six patients (26%) experienced treatment failure. Of these, 9 were due to acute treatment-related toxicity and 20 due to primary refractory or progressive lymphoma. Four patients developed acute myeloid leukemia/myelodysplastic syndrome, one died of lung cancer, and one from unknown reasons. Five patients died of treatment-related toxicity.
Feasibility and toxicity
| Variable . | n . | % . |
|---|---|---|
| Adverse event (grades >2) | ||
| Grade 4 infection | 16 | 12 |
| Grade 3-4 mucositis | 28 | 20 |
| Grade 3 arachnoiditis | 2 | 1.4 |
| Grade 3-4 gastrointestinal toxicity | 28 | 20 |
| AML/MDS | 4 | 3.1 |
| PML | 1 | 0.7 |
| Treatment failure due to acute toxicity | 9 | 6.5 |
| Gastrointestinal hemorrhage | 1 | |
| Multiorgan failure | 1 | |
| Septicemia | 2 | |
| Unspecified toxicity | 4 | |
| Subdural hematoma | 1 | |
| Treatment-related death | 5 | 3.6 |
| Gastrointestinal hemorrhage | 1 | |
| Multiorgan failure | 1 | |
| PML* | 1 | |
| Endocarditis† | 1 | |
| Toxicity unspecified | 1 |
| Variable . | n . | % . |
|---|---|---|
| Adverse event (grades >2) | ||
| Grade 4 infection | 16 | 12 |
| Grade 3-4 mucositis | 28 | 20 |
| Grade 3 arachnoiditis | 2 | 1.4 |
| Grade 3-4 gastrointestinal toxicity | 28 | 20 |
| AML/MDS | 4 | 3.1 |
| PML | 1 | 0.7 |
| Treatment failure due to acute toxicity | 9 | 6.5 |
| Gastrointestinal hemorrhage | 1 | |
| Multiorgan failure | 1 | |
| Septicemia | 2 | |
| Unspecified toxicity | 4 | |
| Subdural hematoma | 1 | |
| Treatment-related death | 5 | 3.6 |
| Gastrointestinal hemorrhage | 1 | |
| Multiorgan failure | 1 | |
| PML* | 1 | |
| Endocarditis† | 1 | |
| Toxicity unspecified | 1 |
AML/MDS, acute myeloid leukemia/myelodysplastic syndrome; PML, promyelocytic leukemia.
First CR.
After relapse.
Responses and survival
Response to therapy is summarized in supplemental Tables 2 and 3. Eight patients were not evaluable for response due to toxicity. Of the 119 patients who underwent PET/computed tomography (CT) imaging at the end of immunochemotherapy, 91 (77%) achieved a metabolic complete remission (CR), and 19 of 35 patients (21%) with CT scan–based complete response unconfirmed/partial remission were in metabolic CR according to PET/CT imaging. Of note, only 1 (9%) of 11 biopsy samples from the PET-positive lesions contained viable lymphoma.
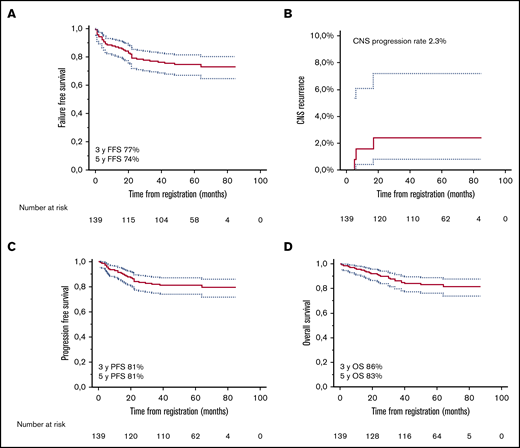
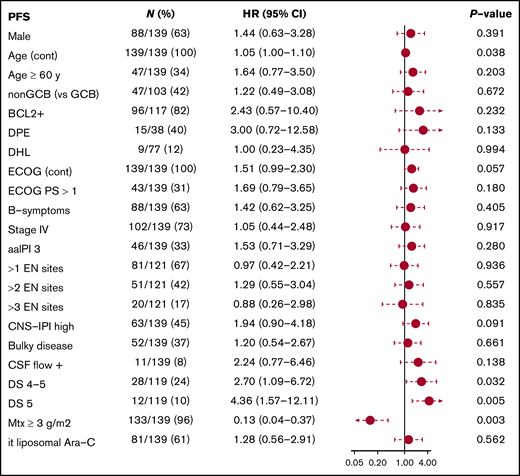
After a median follow-up of 60 months, 23 patients had relapsed, three in the CNS (1 with intermediate and 2 with high CNS-IPI scores), of whom only 1 had pretherapeutic FCM-positive CSF. Twenty-three had died, 16 due to lymphoma (supplemental Table 2). Three-year FFS, CNS progression, PFS, and OS rates were 77%, 2.3%, 81%, and 86%, and corresponding 5-year rates were 74%, 2.3%, 81%, and 83%, respectively (Figure 2). Deauville score 5 at the end of treatment was associated with increased risk of progression and death (Figure 3; supplemental Figure 1), whereas other risk factors, such as aaIPI group (0-2 vs 3), number of extranodal sites, and pretherapeutic FCM-positive CSF were not associated with outcome. When the impact of chemotherapy on survival was tested, there was a better PFS and OS rate in patients who were treated with higher total Mtx doses (≥3 g/m2). The patients receiving lower total doses of Mtx had worse performance scores, higher aaIPI scores, and more treatment failures due to toxicity. However, favorable prognostic impact of higher total Mtx dose was sustained when adjusted by Eastern Cooperative Oncology Group performance status (PFS hazard ratio [HR], 0.16; 95% confidence interval [CI], 0.05-0.49; P = .003), aaIPI (PFS HR, 0.13; 95% CI, 0.04-0.37; P < .001), or toxicity (PFS HR, 0.17; 95% CI, 0.05-0.57; P = .004). Conversely, PFS and the number of CNS events were not affected by intrathecal liposomal cytarabine. When the association of biological markers with outcome was examined, none of them correlated significantly with survival.
Survival analyses. Kaplan-Meier survival estimates for FFS (A), risk of CNS relapse (B), PFS (C), and OS (D).
Survival analyses. Kaplan-Meier survival estimates for FFS (A), risk of CNS relapse (B), PFS (C), and OS (D).
Subgroup analyses. Forest plot showing subgroup analysis of PFS. Ara-C, cytarabine; DPE, double-protein expressor; EN, extranodal; PS, performance status.
Subgroup analyses. Forest plot showing subgroup analysis of PFS. Ara-C, cytarabine; DPE, double-protein expressor; EN, extranodal; PS, performance status.
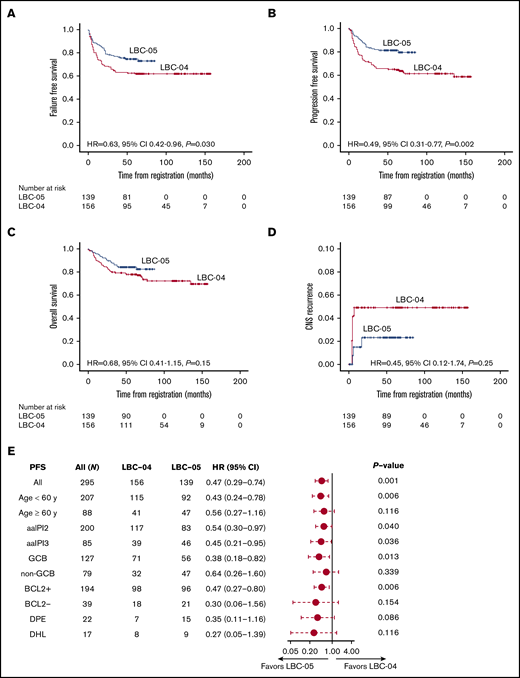
Outcome for patients treated in the NLG-LBC-05 trial compared with the previous NLG-LBC-04 trial
In the NLG-LBC-05 trial, patients more often had >1 extranodal site than patients in the NLG-LBC-04 study,21 which served as a preplanned historical control. Otherwise, patient demographic characteristics and response rates were comparable between the 2 trial cohorts (supplemental Table 4). However, the LBC-05 regimen improved outcome over LBC-04 in terms of better 5-year FFS and PFS (Figure 4A,C). The differences in 5-year OS and cumulative incidence rates of CNS recurrence did not reach statistical significance (Figure 4B,D). A favorable impact of the LBC-05 regimen on PFS was particularly evident among the patients aged <60 years or with an aaIPI score of 3 (Figure 4E; supplemental Figure 2). In multivariate analysis, which included age, aaIPI score, molecular subtype, and regimen, the aaIPI score and regimen remained independent prognostic factors for progression (supplemental Table 5).
Comparison of survival rates between LBC-04 and LBC-05 trials. Kaplan-Meier survival estimates for FFS (A), PFS (B), OS (C), and risk of CNS relapse (D) according to the LBC-04 and LBC-05 trials. (E) Forest plot showing subgroup analysis of PFS in the LBC-04 vs LBC-05 trials. In panel E, PFS in case of all patients is adjusted for aaIPI score.
Comparison of survival rates between LBC-04 and LBC-05 trials. Kaplan-Meier survival estimates for FFS (A), PFS (B), OS (C), and risk of CNS relapse (D) according to the LBC-04 and LBC-05 trials. (E) Forest plot showing subgroup analysis of PFS in the LBC-04 vs LBC-05 trials. In panel E, PFS in case of all patients is adjusted for aaIPI score.
To investigate the impact of early HD-Mtx on the outcome within the biological subgroups, the patients were divided according to their biological subgroup and study cohort. In the entire study population, double-protein expressor of BCL2 and c-MYC was the only marker to be significantly correlated with a worse outcome (5-year PFS 77% vs 50%; HR, 2.30; 95% CI, 1.087-4.851; P = .029). No single immunohistochemical marker or the GCB/non-GCB subtype or DHL status significantly affected outcome. However, when treatment interaction in the 2 Nordic studies was tested, DHL status was associated with a worse PFS and OS after the LBC-04 regimen (P = .011 and P = .001), as previously reported25 and now updated at the 75-month median follow-up. DHL had no adverse prognostic impact among the patients who received the LBC-05 regimen (P = .99) (supplemental Figure 2).
Discussion
To our knowledge, the current study is the largest prospective study to date addressing efficacy and toxicity of early systemic CNS prophylaxis and dose-dense immunochemotherapy in patients aged <65 years with high-risk aggressive B-cell lymphoma. The study had two coprimary end points: to determine if early administration of HD-Mtx–based CNS prophylaxis followed by dose-dense immunochemotherapy could reduce the incidence of early CNS progressions and improve FFS. Even if our study was not powered to show a significant reduction in the CNS recurrence rate, we observed fewer CNS events than in our previous LBC-04 study21 and could report a better systemic control of the disease and superior 3-year FFS (77% vs 63%) and PFS (81% vs 66%) rates. A relatively low toxic death rate of 3.6% showed that the intensive regimen is feasible for most of the patients. Overall, the LBC-05 regimen seemed to be better tolerated than other intensive treatment approaches.5,10,11 It is thus possible that the favorable outcome is partially related to lower toxicity, which does not interfere with the therapeutic efficacy.
Since the design and initiation of our trial, a specific model to estimate the risk of CNS recurrence, the CNS-IPI, has been established and validated.16 Some biological risk factors have also been described. In particular, DHL and double-protein expressor lymphomas, and occult CSF involvement (FCM-positive/cytology-negative) have been associated with increased risk of CNS relapse in retrospective series.26-28 Molecular CNS-IPI was also recently presented.29 In our study, 53% of the patients were categorized to the high-risk group according to CNS-IPI score, or biological risk factors such as DHL despite low or intermediate CNS-IPI scores (8%) with the expected CNS recurrence rate of 10% to 12%.16 However, we observed only 3 CNS events, translating to a 2.3% CNS recurrence rate in 5 years. Because neither the occult CSF involvement nor the DHL entity was associated with the risk of CNS recurrence, it is plausible to suggest that the LBC-05 regimen may overcome the adverse prognostic impact of both clinical and biological risk factors. Conversely, 47% of the patients had low or intermediate risk of CNS recurrence with the expected CNS recurrence rate <5%, and CNS prophylaxis may not be justified for this group. However, as HD-Mtx could also improve systemic control of the disease, it remains to be confirmed whether or to what extent the low number of CNS relapses observed in our study is a consequence of a better systemic efficacy of early administration of HD-Mtx.
Given the conflicting evidence base, lack of prospective randomized studies, and potential toxicity, there is no consensus whether, how, when, and to which patient groups CNS prophylaxis should be given. Retrospective analyses have shown that systemic CNS prophylaxis may reduce the risk of CNS relapses.18-20 In our previous trial,21 a CNS relapse rate of 4.5% was observed. In the current study, we aimed to reduce CNS relapse rate further without compromising systemic efficacy by combining sensitive FCM-based CSF detection analysis with earlier and more intensive systemic and intrathecally targeted CNS prophylaxis. Although shifting of HD-Mtx to the beginning of the therapy translated to significantly improved FFS, PFS, and low number of CNS events, intrathecally administered liposomal cytarabine failed to show any additional benefit. This scenario may be related to restricted penetration of intrathecal therapy to the brain parenchyma, which is the predominant location of CNS recurrence in DLBCL.12,30 We also analyzed the impact of Mtx dose and, irrespective of the impact on CNS events, found a significant quantitative association between the dose and survival. Overall, our results are promising and emphasize the importance of timing and dose of systemic HD-Mtx administration for optimal systemic control of lymphoma. Consistent with our findings, recent retrospective studies have shown that addition of HD-Mtx to a CHOP/R-CHOP regimen improves the prognosis of patients with high-risk DLBCL, irrespective of their risk for CNS relapse.31-33 Whether or to what extent the low number of CNS relapses observed in our study is a consequence of a systemic efficacy of early administration of HD-Mtx needs to be confirmed in a randomized study.
We also assessed whether PET positivity (Deauville score 4-5) at the end of immunochemotherapy could identify patients, who are unlikely to be cured with the NLG-LBC-05 regimen. As expected, we found that majority of the patients (80%) with negative fluorodeoxyglucose PET scans (Deauville score 1-3) achieved long-term metabolic remission, and 42% of the patients with Deauville score 5 relapsed. In contrast, the outcome of those with Deauville score 4 was comparable to those of PET-negative patients. Of note was also the finding that only 9% from the PET-positive lesions contained viable lymphoma. The observations emphasize the importance of histological confirmation of relapse from PET- positive lesions and a possible favorable impact of consolidating radiotherapy.
Our exploratory analyses on clinical variables uncovered the influence of age on treatment tolerability and outcomes. Although in patients aged <60 years, the LBC-05 regimen showed clinically meaningful survival benefit over the LBC-04 regimen with manageable safety, in patients aged ≥60 years, toxicity was possibly confounding the therapeutic benefit. In addition, the benefit of the LBC-05 regimen was particularly noted in patients with an aaIPI score of 3. Based on the findings, we propose intensified therapy with early CNS prophylaxis for high-risk patients <60 years, whereas in this setting the regimen should be cautiously considered for patients aged ≥60 years.
We were also interested in the impact of the LBC-05 regimen on the outcome of patients with DHL. Several retrospective studies have shown that R-CHOP is not a sufficient therapy for patients with DHL and have proposed that more intensive Burkitt-like regimens, such as dose-adjusted EPOCH-R, R-HyperCVAD/MA, or R-CODOX-M/IVAC, should be used.26,34 Of these, the R-CODOX-M/IVAC regimen has recently been tested for the patients with high-risk DLBCL/high-grade B-cell lymphoma also in a phase 2 trial, and reported to have efficacy similar to high-risk Burkitt lymphoma.35 We found that the outcome of the patients with DHL was comparable to that of all other patients, whereas no such impact could be seen in the previous LBC-04 trial.25 It is thus plausible to suggest that early administration and/or a higher Mtx dose may overcome the adverse prognostic impact of DHL. The overall conclusion of this and previous studies is that the patients with DHL may benefit from more intensive treatment.
This study had several limitations. First, it was not powered to detect a statistically significant reduction in CNS events. Overall, the benefit of CNS prophylaxis should be ideally addressed in an adequately sized, randomized phase 3 study. The encouraging results on outcome of the few patients with DHL must also be interpreted with caution. Second, because the conclusions based on historical comparisons may be subject to differences in observed and unobserved prognostic factors, we cannot exclude the possibility that a better outcome in response to the LBC-05 regimen is related to factors other than treatment. Finally, exploratory analyses of prognostic variables were also limited by small numbers and should be interpreted with caution.
Taken together, we were able to show highly satisfactory PFS, OS, and CNS progression rates for young patients with high risk B-cell lymphoma in response to early HD-Mtx–based CNS prophylaxis followed by dose-dense immunochemotherapy. The LBC-05 regimen was also well tolerated, and it can be considered as an alternate treatment option for young patients with high-risk aggressive B-cell lymphomas. Identifying the biologically high-risk group and combining the regimen with novel agents seem to be the most logical next steps to further improve the outcome for this high-risk patient population.
Any requests for de-identified trial data and supporting material will be reviewed by the respective trial writing committee in the first instance. Requests that have a methodologically sound proposal will be considered. Proposals should be directed to the corresponding author (Sirpa Leppä; e-mail: sirpa.leppa@helsinki.fi) in the first instance; to gain access, data requestors will need to sign a data access agreement.
Acknowledgments
The authors thank all participating hospitals for patient enrollment and sample collection. Data management was provided by the Oslo University Hospital Clinical Trial Unit.
The study was supported by grants from the Finnish Cancer Foundation, Academy of Finland, Sigrid Jusélius Foundation, Nordic Cancer Union, University of Helsinki, Helsinki University Hospital, Amgen, and Mundipharma.
Authorship
Contribution: The Nordic Lymphoma Group Large B-Cell Lymphoma Working Group (S.L., S.J., M.J., M.B., J.J., Ø.F., and H.H.) designed the protocol; all authors provided study materials, or enrolled patients to the study, and collected, and assembled data; E.R., S.S., K.B., and M.-L.K.-L. performed pathology reviews; S.L., K.L., and H.H. performed the primary data analysis and interpretation; and all authors contributed to writing of the manuscript and gave final approval.
Conflict-of-interest disclosure: S.L. reports research funding from Mundipharma and Amgen during the conduct of the study; and honoraria and research funding from Celgene, Roche, Takeda, Bayer, and Janssen-Cilag outside the submitted work. J.J. reports personal fees from Roche and Gilead outside the submitted work. H.H. reports research funding from Mundipharma and Amgen during the conduct of the study; and participation in advisory boards for Novartis, Gilead, Roche, Nordic Nanovector, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Sirpa Leppä, Department of Oncology, Helsinki University Hospital Comprehensive Cancer Centre, P.O. Box 180, FI-00029 Helsinki, Finland; e-mail: sirpa.leppa@helsinki.fi.
References
Author notes
The full-text version of this article contains a data supplement.