TO THE EDITOR:
At the end of 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as 2019-nCoV), emerged in Wuhan, China. SARS-CoV-2 infection has caused severe pneumonia, namely COVID-19, through human-to-human transmission.1 Because there have been rapidly increasing cases of COVID-19 across China and other countries, including South Korea, Iran, Japan, Italy, and the United States,2 efficient therapeutic strategies against SARS-CoV-2 are urgently needed. Although a few drugs are currently undergoing clinical trials to treat COVID-19 (eg, remdesivir [GS-5734],3 favipiravir [T-705]4 ), general and supportive therapies remain the main strategies for treating COVID-19. These include oxygen supply, interferons, glucocorticoids, human serum albumin, and antibiotics as appropriate.5 Thus, additional therapeutic options need to be explored.
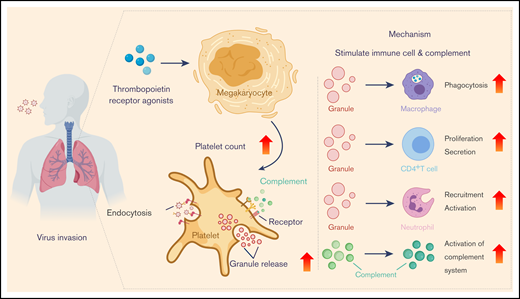
Platelets are tiny, discoid, anucleated cell fragments, with a diameter of 1 to 3 μm and a life span of 8 to 12 days. Despite their size and relatively short life span, platelets are indispensable for many important biological processes such as hemostasis, thrombosis, wound healing, angiogenesis, immunity, and inflammatory responses.6 Moreover, mounting evidence has indicated that platelets play a critical role in innate immunology in the lung, including in the defense against a variety of respiratory viruses. For example, recent findings have uncovered a role of platelets against pulmonary infection of influenza A (H1N1). Activated platelets engulf HIN1 virions and secrete antiviral molecules (eg, α-granules) to destroy virions.7 Importantly, the lung was recently shown to be a primary site for platelet biogenesis, as a large population of megakaryocytes circulate through the lung and release platelets there.8 The current review recapitulates relevant studies regarding the antiviral activities of platelets in the lung (Figure 1), aiming to exploit the platelet-dependent therapeutic options for the treatment of COVID-19.
Antiviral activity and platelet-mediated mechanisms. TPO receptor agonists increase megakaryocyte viability and proliferation, leading to an increase in platelet counts. Platelets activated by virus endocytose virions and release granules, ultimately exerting their antiviral activity by stimulating immune cells and activating the complement system.
Antiviral activity and platelet-mediated mechanisms. TPO receptor agonists increase megakaryocyte viability and proliferation, leading to an increase in platelet counts. Platelets activated by virus endocytose virions and release granules, ultimately exerting their antiviral activity by stimulating immune cells and activating the complement system.
The lung is a crucial organ in triggering the body’s natural immune defenses against pathogen invasion, especially after a respiratory viral infection. Once respiratory viruses invade, both innate and adaptive immune responses in the lung are rapidly activated to enable viral clearance.7 As part of innate immunity, the direct interactions between viral particles and platelets in the lung enable the endocytosis of virions by these platelets. The recognition of viral ligands by platelet receptors is indispensable for endocytosis. Respiratory syncytial virus (RSV) is a common respiratory virus that causes bronchiolitis and pneumonia in children and the elderly. Toll-like receptors (TLRs) expressed on the surface of activated platelets allow for RSV recognition to prevent RSV dissemination outside of the respiratory tract and further disease progression.6 FcγRIIA is the only Fcγ receptor expressed in activated platelets. FcγRIIA is also involved in the endocytosis of viral pathogens such as H1N1 and occurs through the direct binding to immunoglobulin G (IgG)–H1N1 complexes.9 IgG-H1N1–containing endosomes are subsequently formed by plasma membrane invagination in these platelets. Afterward, these endosomes are fused with granules that contain microbicidal and antiviral components, leading to virion destruction.10
Newly formed platelets in pulmonary circulation exert their function in fighting against respiratory viruses through granule production and release. Platelets contain 3 types of granules: α-granules, dense granules, and lysosomes. The α-granules range from 200 to 500 nm in size and represent the most abundant granule type found in platelets. The α-granules are composed of various chemokines, cytokines, membrane proteins, and proteases, as well as proinflammatory and anti-inflammatory mediators.6 In the lung, the interplay between platelets and respiratory viruses stimulates platelet activation and degranulation. Granules in activated platelets diffuse into the extracellular matrix by fusing with the open canalicular system, which is associated with the invaginations of the plasma membrane.11 This process is essential for the human respiratory defense in that a variety of chemokines, cytokines, kinocidins, and microbicidal protein are released from the degranulation of platelets. Interleukin-18 (IL-18) is a multifunctional and proinflammatory cytokine belonging to the IL-1 family, and is a component of α-granules. Pro–IL-18 is cleaved by caspase-1 into a mature and biologically active 18 kDa protein. Mature IL-18 is then released from activated platelets and promotes the development and proliferation of T helper 1 (Th1)–type CD4+ T cells. Subsequently, it enhances the secretion of interferon-γ from both natural killer (NK) and T cells to boost overall antiviral and antimicrobial activities.12
Upon activation, platelets release chemokine (CXC motif) ligand 4 (CXCL4), which is also known as platelet factor 4 (PF4). PF4 contributes to the recruitment of neutrophils and also facilitates neutrophil exocytosis to release myeloperoxidase and lysozyme. However, the ability to effectively clear influenza viruses such as H1N1 from the lung is impaired when PF4 is deficient.13 These PF4-stimulating antigen-presenting cells (APCs) induce substantial lymphoproliferation and NK cytotoxicity after respiratory virus invasion, suggesting that platelets have a critical role in protecting the host from respiratory infection. Regarding immunoregulation, PF4 in conjunction with IL-4 contributes to monocyte differentiation into specialized APCs. The mechanisms behind these processes involve upregulation of several activation markers, including CD86 and major histocompatibility complex class II, as well as the induction of dramatic morphologic changes. Studies have defined a role of PF4 in T cell–mediated immunoregulation. PF4 modulates the T-cell proliferation, among which the proliferation of CD4+CD25+ T regulatory cells is promoted while the proliferation of CD4+CD25− T cells is suppressed.14 PF4 also modulates the transcription factors T‐bet and GATA‐3, thus further modulating Th1/Th2 polarization.15 In addition, PF4 induces intracellular calcium release in T lymphocytes through CXCR3 coupled with pertussis toxin–sensitive G proteins, resulting in their chemotactic migration.16
Platelet-derived chemokine (C-C motif) ligand 5 (CCL5) has been shown to be a major factor in host defense against HIV-1, hepatitis C virus (HCV), and influenza A viral infections.17 NF‐κB and IRF‐3/7 synergistically promote CCL5 expression on platelets in response to viral infection. CCL5 then interacts with several G protein–coupled receptors (CCR1, CCR3, and CCR5) on monocytes, NK cells, and both CD4+ and CD8+ lymphocytes, leading to the migration of these cells toward the site of infection.18 CCL5 may not only modulate cytotoxic lymphocytes but also aid in increasing and activating Th1 cells. Phosphatidylinositol 3-kinase/AKT and MEK/extracellular signal–regulated kinase signaling pathways can also be activated by CCL5 during viral infection. This action is crucial for macrophage involvement in the clearance of both virus-infected and apoptotic cells and, ultimately, accelerates recovery from the infection.17 Immune defense and inflammatory responses in the lung after a respiratory viral infection determine the outcome of pulmonary pathogenesis. Platelet-secreted molecules not only promote the antiviral functions of various immune cells in the lung but also attach to the virus particles directly to exert their antiviral effects (Figure 1).
Activation of the complement system plays a distinct role in host defense against respiratory infection.19 The participation of platelets in the activation of the complement system gives rise to enhanced inflammatory cell activation, the lysis of infected cells, and the removal of apoptotic cells and cell debris from the lung.20 Respiratory viral infections and host inflammatory responses provoke platelet activation, accompanied by the release of pro-activating platelet mediators. Chondroitin sulfate A is released from α-granules during platelet activation and is a potent mediator of crosstalk between platelets and the complement system.19 It directly activates the complement reaction in plasma, resulting in the release of anaphylatoxins that mediate leukocyte activation. During viral infection, the respiratory virus (eg, influenza) is internalized and degraded by platelets, after which single-stranded RNA is released.7 TLR7 is an intracellular, pattern recognition receptor that is expressed in platelets and specifically recognizes viral single-stranded RNA. TLR7 activation induces rapid phosphorylation of both Akt and p38-MAPK, which then enhances the release of complement C3 from platelets.21 Platelet-derived complement C3 further induces neutrophil-DNA release and neutrophil aggregation in the lung, triggering the response to influenza pathogens. Neutrophils produce a large number of lysosomes to allow for virion lysis, thereby preventing the further spread of the influenza virus into deeper regions of the lung parenchyma.19 In addition to clearing pulmonary virion particles after neutrophil recruitment, platelet-derived complement C3 participates in activating the complement cascade and inducing the complement system that regulates both humoral and adaptive immunity.20 Complement system activation then implicates several plasmatic proteins with immunologic and inflammatory properties (eg, cC1q, gC1q, C3a, C5a) that are recognized by complement receptors expressed on the platelet’s surface. Therefore, activated platelets (eg, through thrombin activation pathway) activate the complement cascade, leading to a series of antiviral events in the lung (Figure 1).
Taken together, platelets are involved in several endocytic pathways to the respiratory virus directly. Moreover, platelets participate in an antiviral inflammatory response through the activation of both granules and the complement system in the lung.
The acute respiratory distress syndrome caused by both H1N1 and SARS-CoV-2 is characterized by a significant increase in neutrophils in the lung that accelerate the elimination of invading pathogens. Platelet interaction is required for neutrophil recruitment and activation in the lung, which allows for modulation of the innate immune response.22 Platelets adhere to neutrophils through P-selectin/P-selectin glycoprotein ligand-1–mediated binding. This adherence is accompanied by binding of integrin αMβ2 (Mac-1) located on neutrophils to the glycoprotein Ibα located on platelets.23 This direct binding activates neutrophils and causes the generation of neutrophil-derived extracellular vesicles. Ultimately, this action allows for the shuttling of arachidonic acid into the platelets.24 Arachidonic acid is a substrate that functions to enhance the production of thromboxane A2 in platelets. Platelet-derived thromboxane A2 plays a crucial role in pathogen clearance and neutrophil recruitment. Notably, platelets also modulate immune cells in other ways, including through the binding of CD40L to its receptor CD40, which is located on the surface of macrophages and neutrophils as well as B and T cells.25 CD40L is a type II transmembrane protein belonging to the tumor necrosis factor superfamily. Platelet-derived CD40L activates macrophages by binding to CD40, which induces the release of both nitric oxide and reactive oxygen species and subsequent pathogen destruction in the lung. Activated platelets secrete serotonin and release granule-stored inflammatory chemokines, which includes IL-1β and RANTES. The released serotonin then promotes direct activation of recruited neutrophils.11 These interactions further amplify the activation of both platelets and neutrophils, giving rise to the formation of neutrophil extracellular traps (NETs).26 NETs can be released from these activated neutrophils and contain numerous nuclear (eg, histones) and granular proteins. Because respiratory viruses infect and rapidly replicate in bronchial and alveolar epithelium, they are likely to spread into deeper regions of the lung parenchyma. These lattice-like structures serve as a defense mechanism against various respiratory viruses by trapping and killing these viruses, thereby limiting their further spread.27
Platelets are pivotal mediators in cellular communication during virus-induced pulmonary inflammatory responses. The interactions between platelets and dendritic cells (DCs) direct DCs to the sites of viral infection, such as the bronchial epithelium and/or alveolar epithelium.28 In return, the activated DCs release inflammatory chemokines that enhance the antigen-presenting capacity of DCs.29 Accumulating evidence has shown that platelets present pathogen-derived antigens to promote T-cell responses in vivo, defining a novel antigen-presentation role of platelets.26 Further experimental data have shown the role of platelets in directly promoting T-cell responses, in which platelets express costimulatory molecules and present antigens to these T cells along with major histocompatibility complex class I molecules.30 Because there are more platelets than either leukocytes or APCs in the lung, the antigen presentation function of platelets may provide an early surveillance mechanism in acquired immunity. Furthermore, pathogen-activated platelets could release exosomes with a diameter <100 nm. These platelet-originated exosomes would act to activate CD4+ T cells, thereby increasing transforming growth factor β1 production and shifting the differentiation of CD4+ T cells toward functional regulatory T cells (Tregs). This action would suggest an important immune-modulatory role of platelet-derived ectosomes.31
Taken together, these findings reveal that platelets present pathogen antigens directly and promote T-cell responses indirectly, highlighting the overall important role of platelets in immunoreactions. Considering the large amounts of platelets in the lung, the antigen-presenting capability of platelets undoubtedly helps exert their antiviral activities in the lung.
All the aforementioned findings reveal the important role of platelets in combating viral infection, which has been tested through a variety of approaches thus far. For example, chemokine PF4, one of the most abundant proteins in platelet-secreted α-granules, is used as a broad-spectrum inhibitor of HIV-1 infections.32 Unlike conventional mechanisms, PF4 directly binds to the major viral envelope glycoprotein, gp120, and blocks the CD4-binding site to impair HIV-1 attachment and entry. These observations indicate that platelets need to be considered when treating respiratory viral infections.33 Thrombocytopenia is a common adverse outcome stemming from viral infections; it increases the risk of bleeding and prolongation of the infection, and significantly reduces the antiviral capabilities of the immune system, ultimately resulting in a higher mortality risk.34 Leukopenia, lymphopenia, and thrombocytopenia have been observed in lung-targeting SARS-CoV-2–infected patients at the prodromal phase, which is consistent with the blood symptoms of patients infected with other RNA viruses (eg, HIV-1, HCV, dengue).5,32,34,35 The occurrence of thrombocytopenia can be caused by platelet-leukocyte aggregation and resultant platelet sequestration by macrophages. Comparatively, SARS-CoV-2 directly impairs thrombopoietin (TPO) production.21 Another major cause of thrombocytopenia development is the rapid replication and spread of SARS-CoV-2, which impairs the hematopoietic infrastructure of the lung.36 Clinical observations also showed that patients with COVID-19 experienced elevated concentrations of inflammatory cytokines.5 The release of excessive cytokines damages endothelial cells and disturbs their functions, resulting in capillary leakage, vascular permeability, and shock. These adverse physiological effects and symptoms can be further exacerbated by SARS-CoV-2–induced thrombocytopenia. Thus, the timely improvement of the count and activity of effective platelets by using a TPO agonist may be beneficial in the treatment of COVID-19 at the prodromal phase (Figure 1).
It should be noted that platelets are associated with the burst of cytokine storm, which may cause lung tissue damage, airway occlusion, and the occurrence of acute respiratory distress syndrome.28 Despite this, platelets are indispensable for facilitating neutrophil influx into the lung to eliminate the viral infection. Recent studies have shown that PF4 deficiency in platelets results in diminished clearance of H1N1 virus from the lung during the early stages of the infection. PF4 deficiency in platelets also causes severe lung injuries, owing to the failure of neutrophil mobilization and subsequent impairment of viral phagocytosis during the late stages of the infection.13 Thus, a balance between platelet production and immune responses is a prerequisite in successfully combating respiratory viral infections. In this setting, it is critical to perform platelet surveillance in patients to prevent vascular permeability and the development of shock.
Romiplostim is a potent peptide agonist of the TPO receptor. Critically, it has been approved for clinical use by the US Food and Drug Administration to improve platelet counts in patients with thrombocytopenia.37 Romiplostim contains 14 amino acids that can bind to the distal cytokine homology region of the TPO receptor, inducing activation of downstream JAK-, STAT-, and MAP-related pathways as well as antiapoptotic signal pathways. Ultimately, these pathways increase megakaryocyte viability and proliferation. After romiplostim administration, platelet count can be increased by 1 to 2 million.38 Thus, romiplostim may be useful in treating patients with thrombocytopenia resulting from a diverse array of respiratory infections.
Eltrombopag is another TPO receptor agonist and is also clinically used to treat immune thrombocytopenia. Unlike romiplostim, eltrombopag interacts with the transmembrane domain of the TPO receptor, resulting in an additive effect in the presence of TPO.39 Eltrombopag stimulates hematopoietic stem cell differentiation and promotes hematopoietic recovery. Furthermore, eltrombopag modulates Tregs and restores the balance of Fcγ receptors in phagocytes.40 Eltrombopag treatment in patients with immune thrombocytopenia enhances transforming growth factor β release and reduces the production of IL-2 by CD4+ T cells. This scenario leads to an increased immunomodulation function of Tregs.41 Eltrombopag also reduces the content of intracellular irons by chelating iron to inhibit viral replication.42 Eltrombopag enables the regulation of both the chemotaxis and phagocytosis of neutrophils and macrophages; it also increases the antiviral activity of other immune cells.41 Thus far, eltrombopag has been approved by the US Food and Drug Administration for the treatment of HCV, human cytomegalovirus, HIV-1,41,43,44 and refractory aplastic anemia.45 These approvals were all based on the drug’s ability to increase platelet count. Notably, eltrombopag improves the therapeutic outcome of ganciclovir, which is a recommended drug in the guidelines for antiviral therapy (eg, human cytomegalovirus–infected cases43 ). Collectively, these findings and previous approvals indicate that it is promising to use eltrombopag in treating more respiratory viral infections.
Platelet involvement in regulating the homeostasis of both innate and adaptive immune responses gives platelets remarkable potential for use in the fight against respiratory viral infections. Of note, thrombocytopenia and leukopenia have been identified as complications in patients with COVID-19, accompanied by the dysregulated synthesis of diverse cytokines as well as leukocytic infiltration in the lung.5,46 Therefore, it is rational that targeting thrombocytopenia would serve as a useful, adjuvant therapeutic strategy to treating COVID-19 infection. Both romiplostim and eltrombopag are effective, clinically approved antiviral medications that work by increasing platelet production. Importantly, both drugs are also well tolerated in patients. Platelet-based therapeutic agents therefore warrant further exploration. Potential side effects should also be considered when using therapies to elevate platelet counts, including increased inflammation. Thus, customized strategies are recommended to balance the antiviral and pro-inflammatory effects of platelets.
Contribution: J.Q. wrote the manuscript and prepared the figure; J.M. and S.Z. designed, wrote, and revised the manuscript; and J.H. and S.L. reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shuping Zhang, The First Affiliated Hospital of Shandong First Medical University, 16766 Jingshi Rd, Lixia District, Jinan, China; e-mail: spzhang@sdfmu.edu.cn; or Juan Ma, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 18 Shuangqing Rd, Haidian District, Beijing 100085 China; e-mail: juanm@rcees.ac.cn.
Data-sharing requests should be submitted via e-mail to the corresponding authors, Shuping Zhang (spzhang@sdfmu.edu.cn) and Juan Ma (juanm@rcees.ac.cn).
Acknowledgments:
This work was supported by the National Natural Science Foundation of China (grant number 21707161), the “Outstanding University Driven by Talents” program of the Shandong First Medical University, and Academic Promotion Programme of Shandong First Medical University (grant number 2019LJ001).