Key Points
We report a patient with hepatosplenic T-cell lymphoma (HSTL) and compare the disease to the derived xenograft model.
Enhancer of zeste homolog 2 (EZH2) inhibitor treatment of an EZH2-mutant HSTL patient-derived xenograft model led to prolonged survival.
Introduction
Hepatosplenic T-cell lymphoma (HSTL) is a rare, poor-prognostic form of peripheral T-cell lymphoma, primarily affecting adolescents and young adults. Most patients with HSTL will succumb to this disease within a year of diagnosis.1 Development of HSTL has been associated with treatment with immunosuppressive therapies, such as azathioprine, for autoimmune disease. HSTL arises from γδ (∼80%) or αβ (∼20%) T cells, with clinical features of hepatosplenomegaly without significant lymphadenopathy and frequent bone marrow involvement. The neoplastic cells typically infiltrate the liver and spleen, as well as the bone marrow. The classically described genetic features of HSTL are isochromosome 7q and trisomy 8.2,3 Recently, whole-exome sequencing and gene-expression profiling revealed potential druggable pathways. Travert et al identified spleen tyrosine kinase as a targetable tyrosine kinase target in HSTL.2 McKinney et al defined the genomic landscape of HSTL and identified mutations in STAT5B, STAT3, and PIK3CD, as well as mutations in chromatin-modifying genes in 62% of the cases.4 SETD2 was the most frequently mutated, followed by INO80, ARID1B, and TET3. Enhancer of zeste homolog 2 (EZH2) mutations were identified in 7 of 68 patients (10%). To date, treatment of HSTL has included aggressive multiagent chemotherapy and stem cell transplantation and has not integrated genomic findings or the use of targeted therapies.
EZH2 is a histone methyltransferase that functions as the catalytic subunit of the polycomb-repressive complex 2 (PRC2). PRC2 methylates lysine 27 on histone 3 (H3K27), resulting in transcriptional silencing of target genes. Alterations in EZH2, or its partner complexes, such as the SWItch/sucrose nonfermentable (SWI/SNF) complex, have been described in a number of cancers. Specifically, recurrent gain-of-function mutations in EZH2, including hotspot mutations at tyrosine 641 (Y641), have been found in up to 30% of germinal center (GC) B-cell–like diffuse large B-cell lymphoma and 27% of follicular lymphoma.5-7 Expression of Y641-mutant EZH2 enhances the efficiency of H3K27 trimethylation (H3K27me3), leading to increased repression of EZH2 target genes.8,9 Mice expressing Y641-mutant EZH2 in GC B cells develop GC hyperplasia and accumulate high levels of H3K27me3. Several EZH2 inhibitors have been developed, including tazemetostat (EPZ-6438), and are currently being tested in clinical trials for the treatment of solid tumors and non-Hodgkin lymphoma. In fact, tazemetostat was recently approved by the US Food and Drug Administration (FDA) for the treatment of patients with epithelioid sarcoma.
There are limited models to study HSTL, especially considering the genetic heterogeneity of the disease. Here, we highlight and comprehensively describe a previously reported patient-derived xenograft (PDX) model of HSTL10 that we compare with the patient disease, and perform a targeted therapy study based on the genomic characterization of this model, a strategy that may inform treatment of future patients with this often lethal type of lymphoma.
Case description
A 15-year-old female patient with a history of Crohn disease, which had been treated with mercaptopurine and methotrexate, developed cytopenias, fevers, fatigue, and abdominal distention over 6 months leading to the hospital presentation. As part of her workup at her referring institution, she had 2 bone marrow evaluations 2 months apart that were negative for malignancy. The second biopsy showed evidence of hemophagocytic lymphohistiocytosis and she was started on corticosteroids and IV immunoglobulin with modest improvement. Her disease progressed with symptoms of hypoxia, liver failure with symptoms of hypoglycemia, metabolic acidosis, and hyperammonemia, as well as renal failure. At the time of her presentation to our institution, her physical examination showed significant hepatosplenomegaly, which was confirmed on an abdominal computed tomography scan (Figure 1A), and lung infiltrates concerning for disease or infection. A bone marrow evaluation at this time showed an abnormal infiltrative process, with a population of T cells positive for CD2, CD3, and CD8 and negative for CD4 and CD5 (Figure 1B). Bone marrow analysis showed 35% abnormal T cells, with cytogenetics showing 45, X, −X, i(7)(q10)[8]/46,XX[12]. A diagnosis of stage IV αβ HSTL was made. The patient started treatment with a dose-modified cycle of ifosfamide, cytarabine, and etoposide chemotherapy, as well as multiple antimicrobial agents. Her clinical course and selection of treatment regimen were complicated by severe metabolic acidosis, coagulopathy, and renal failure of unknown etiology prior to chemotherapy and continuing throughout her course of treatment. She had a modest response to the first chemotherapy cycle with improvement of her liver, renal, and respiratory function. Unfortunately, she had nearly immediate recurrence of significant life-threatening metabolic alterations and infectious complications. The patient expired 2 months after initial presentation to our institution. Autopsy demonstrated marked HSTL throughout the solid organs with leukemic evolution and fungal infiltrates in the lungs.
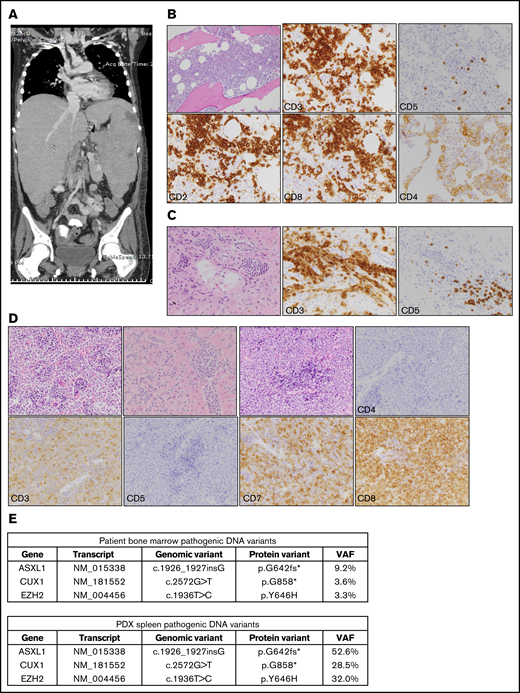
PDX model of HSTL closely recapitulated features of patient’s disease. (A) Coronal image of computed tomography scan of the chest, abdomen, and pelvis of the patient described in the case scenario. (B) Histopathology images of patient’s bone marrow. Top row, left to right: hematoxylin and eosin (H&E), CD3, CD5; original magnification ×200. Bottom row, left to right: CD2, CD8, and CD4; original magnification ×400. (C) Histopathology images of patient’s liver at the time of autopsy. Images from left to right: H&E, CD3, and CD5; original magnification ×400. (D) Histopathology images of the PDX HSTL model; original magnification ×400 for all images. Top row, left to right: H&E, bone marrow; H&E, liver; H&E, spleen; CD4, spleen. Bottom row, left to right: CD3, spleen; CD5, spleen; CD7, spleen; CD8, spleen. (E) Table showing pathogenic variants detected using targeted panel sequencing in both patient bone marrow and PDX spleen. An asterisk (*) identifies a translation termination codon. VAF, variant allele frequency.
PDX model of HSTL closely recapitulated features of patient’s disease. (A) Coronal image of computed tomography scan of the chest, abdomen, and pelvis of the patient described in the case scenario. (B) Histopathology images of patient’s bone marrow. Top row, left to right: hematoxylin and eosin (H&E), CD3, CD5; original magnification ×200. Bottom row, left to right: CD2, CD8, and CD4; original magnification ×400. (C) Histopathology images of patient’s liver at the time of autopsy. Images from left to right: H&E, CD3, and CD5; original magnification ×400. (D) Histopathology images of the PDX HSTL model; original magnification ×400 for all images. Top row, left to right: H&E, bone marrow; H&E, liver; H&E, spleen; CD4, spleen. Bottom row, left to right: CD3, spleen; CD5, spleen; CD7, spleen; CD8, spleen. (E) Table showing pathogenic variants detected using targeted panel sequencing in both patient bone marrow and PDX spleen. An asterisk (*) identifies a translation termination codon. VAF, variant allele frequency.
Methods
Compound
EPZ-6438 was purchased from Active Biochem.
Targeted panel sequencing
We used the Rapid heme panel, an amplicon-based 95-gene next-generation sequencing panel targeted for hematological malignancies.11 This was used for both the patient bone marrow sample and for sequencing the spleen PDX tissue.
In vivo study
Mononuclear cells from the marrow cells obtained at the time of diagnosis were isolated using Ficoll gradient and injected into irradiated NOD/SCID IL2Rγnull (NSG) mice; disease was established in the mouse (Passage 0 [P0]). The isolated spleen cells were injected into irradiated NSG mice to establish P1 disease. Spleen cells were isolated, banked, and used for the subsequent in vivo study. This PDX model is available from the PRoXe repository (www.proxe.org, under number CBTL-81777). For the in vivo PDX study, NSG mice were injected with 2 × 106 leukemic blasts via tail-vein injection and bled weekly to determine the percentage of circulating human CD45+ (hCD45) cells in the peripheral blood. Once hCD45 was detectable above 1% average in the peripheral blood of all animals, mice were assigned to receive vehicle or tazemetostat (350 mg/kg by oral gavage twice a day) and were treated for 35 days. Mice were monitored and euthanized when determined to be moribund. All animal studies were conducted under the auspices of protocols approved by the Dana-Farber Cancer Institute Animal Care and Use Committee. Samples for pathology and immunoblotting were collected in a subset of mice after 7 days of drug treatment.
Flow cytometry analysis
Peripheral blood was obtained at 1 week after injection and red blood cells were lysed. Cells were then stained with anti-human CD45 antibody. hCD45 was followed weekly until engraftment.
Immunoblotting
A segment of the mouse spleen was disaggregated in phosphate-buffered saline and red blood cells were lysed. Cells were washed twice with ice-cold phosphate-buffered saline and then histones were extracted by overnight acid extraction using an Abcam protocol (http://www.abcam.com/protocols/histone-extraction-protocol-for-western-blot). Immunoblots were run as previously described.12 Blots were incubated in primary antibody to H3K27me3 (Millipore) and total H3 (Millipore), followed by the secondary antibodies anti-rabbit conjugated to horseradish peroxidase (Amersham) or anti-mouse conjugated to horseradish peroxidase (Amersham).
Statistical analysis and consent
Statistical significance was determined by the 2-tailed Student t test for pairwise comparison of groups and by the log-rank test for survival curves. Consent was obtained for participation in a Dana-Farber Cancer Institute Institutional Review Board–approved research study, per the Declaration of Helsinki.
Results and discussion
The patient’s bone marrow mononuclear cells were injected into NSG mice. The mice developed hepatosplenomegaly and leukemia, reminiscent of the patient’s disease. Pathological evaluation showed the mouse disease to be positive for CD3, CD7, CD8, and negative for CD4 and CD5 (Figure 1D), thus meeting criteria for HSTL. Targeted panel sequencing of the patient’s bone marrow cells and mouse spleen cells revealed an identical mutation pattern. Both demonstrated the activating EZH2 mutation p.Y646H (same as Y641H using RefSeq transcript NM_001203247) in addition to mutations in ASXL1 p.G642fs* and CUX1 p.G858* in similar percentages relative to one another (Figure 1E). Given the same relative variant allele frequency (VAF) within each sample, the higher EZH2 VAF in the spleen samples likely represents more significant disease involvement of the organ, compared with the 35% bone marrow involvement, rather than enrichment of a clone upon engraftment in NSG mice.
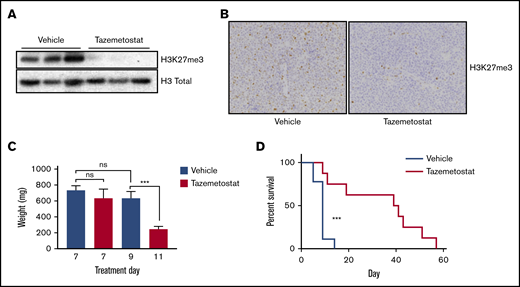
EZH2 inhibitors, such as tazemetostat, have been tested in vitro and in mouse models of B-cell lymphoma and are currently in clinical trials with early promising results.13-17 We thus conducted a mouse preclinical study with tazemetostat. NSG mice were injected with spleen cells from the PDX model and monitored for disease establishment. After 2 weeks, peripheral blood flow cytometry was positive for hCD45. The mice were then randomized to receive vehicle vs tazemetostat. Pharmacodynamic analysis at 7 days of treatment showed a decrease in H3K27me3 by western analysis (Figure 2A), as well as by immunohistochemistry (Figure 2B), in mice treated with tazemetostat, a marker of on-target activity. There was no difference in spleen size at day 7. However, by day 11, mice treated with tazemetostat had a significant decrease in spleen size (Figure 2C). Treatment with tazemetostat resulted in significantly prolonged survival in this very aggressive PDX model of HSTL (Figure 2D).
Tazemetostat was effective in the treatment of the HSTL PDX model. (A) Immunoblotting showing on-target activity of tazemetostat, with a decrease in H3K27me3. Total histones were extracted from mouse spleen samples after 7 days of treatment. (B) Immunohistochemistry for H3K27Me3 in mouse spleen samples; original magnification ×400. (C) Bar graph showing spleen weights from mice in the PD cohort (treated for 7 days, n = 3 per group), and mice who were moribund at day 9 (n = 6) and day 11 (n = 2). (D) Kaplan-Meier curves showing overall survival of mice treated with vehicle (n = 9) or tazemetostat (n = 8). ***P = .0007 calculated using the log-rank test. ns, not significant.
Tazemetostat was effective in the treatment of the HSTL PDX model. (A) Immunoblotting showing on-target activity of tazemetostat, with a decrease in H3K27me3. Total histones were extracted from mouse spleen samples after 7 days of treatment. (B) Immunohistochemistry for H3K27Me3 in mouse spleen samples; original magnification ×400. (C) Bar graph showing spleen weights from mice in the PD cohort (treated for 7 days, n = 3 per group), and mice who were moribund at day 9 (n = 6) and day 11 (n = 2). (D) Kaplan-Meier curves showing overall survival of mice treated with vehicle (n = 9) or tazemetostat (n = 8). ***P = .0007 calculated using the log-rank test. ns, not significant.
We report a clinical case of a patient with HSTL, a rare lymphoma with a dismal prognosis, and compare it to the established PDX model. This disease and model had activating mutations in EZH2, as well as mutations in CUX1 and ASXL1. Treatment with tazemetostat, an EZH2 inhibitor, showed in vivo on-target activity, a decrease in disease burden, and significantly prolonged survival in this aggressive mouse model. The development of additional models of HSTL and the testing of targeted therapies, such as EZH2 inhibitors, will be essential to further progress in the treatment of this disease. Given the significant morbidity and mortality associated with HSTL and its treatment, genomic characterization of this disease, in real time, may inform therapy for future patients.
Acknowledgments
This work was supported by grants from the Wong Family Fund for Translational Research (Y.P.), National Institutes of Health, National Cancer Institute grants R35 CA210030 (K.S.) and K08 CA222684 (Y.P.), and grants from the St. Baldrick’s Foundation (K.S. and Y.P.).
Authorship
Contribution: Y.P., L.B.S., D.M.W., and K.S. were responsible for study concept, design, and analysis; Y.P., A.S.C., A.L.R., and S.K. conducted experiments; A.J.C. and M.H.H. performed patient and mouse pathology; K.S., A.L.K., and A.L.B. participated in the care of the patient; and all authors participated in manuscript preparation, editing, and review.
Conflict-of-interest disclosure: D.M.W. is a founder and Scientific Advisory Board (SAB) member of Ajax Therapeutics and Travera; an SAB member of Bantam Therapeutics; a consultant for Myeloid Therapeutics, Ossium Health, Magnetar, and ASELL; and receives research funding from Daiichi-Sankyo and Verastem. K.S. has previously consulted for Rigel Pharmaceuticals and Novartis Pharmaceuticals and receives grant funding through the Novartis/Dana-Farber Cancer Institute Drug Discovery Program. The remaining authors declare no competing financial interests.
Correspondence: Kimberly Stegmaier, Dana-Farber Cancer Institute, 360 Longwood Ave, Boston, MA 02215; e-mail: kimberly_stegmaier@dfci.harvard.edu.