Key Points
Checkpoint inhibition before haplo-SCT seems to improve PFS in patients receiving haplo-SCT with PTCy as GVHD prophylaxis.
Programmed death 1 blockade as bridge to haplo-SCT with PTCy as GVHD prophylaxis does not increase toxicities and NRM.
Abstract
We report on 59 Hodgkin lymphoma patients undergoing haploidentical stem cell transplantation (SCT; haplo-SCT) with posttransplant cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis, comparing outcomes based on pretransplant exposure to checkpoint inhibitors (CPIs). Considering pretransplant characteristics, the 2 cohorts (CPI = 29 patients vs no-CPI = 30 patients) were similar, except for the number of prior lines of therapy (6 vs 4; P < .001). With a median follow-up of 26 months (range, 7.5-55 months), by univariate analysis, the 100-day cumulative incidence of grade 2-4 acute GVHD was 41% in the CPI group vs 33% in the no-CPI group (P = .456), whereas the 1-year cumulative incidence of moderate to severe chronic GVHD was 7% vs 8%, respectively (P = .673). In the CPI cohort, the 2-year cumulative incidence of relapse appeared lower compared with the no-CPI cohort (0 vs 20%; P = .054). No differences were observed in terms of overall survival (OS), progression-free survival (PFS), and nonrelapse mortality (NRM) (at 2 years, 77% vs 71% [P = .599], 78% vs 53% [P = .066], and 15% vs 21% [P = .578], respectively). By multivariable analysis, CPI before SCT was an independent protective factor for PFS (hazard ratio [HR], 0.32; P = .037). Stable disease (SD)/progressive disease (PD) was an independent negative prognostic factor for both OS and PFS (HR, 14.3; P < .001 and HR, 14.1; P < .001, respectively) . In conclusion, CPI as a bridge to haplo-SCT seems to improve PFS, with no impact on toxicity profile.
Introduction
The safety and therapeutic activity of checkpoint inhibition with monoclonal antibodies targeting the programmed death 1 (PD1) receptor in advanced classic Hodgkin lymphoma (cHL) has been demonstrated in many publications.1-5 High response rates and durable responses were observed in the majority of patients. However, with extended follow-up, progression-free survival (PFS) failed to show a plateau,3,5 thus suggesting the need for a consolidation therapy in patients responding to anti-PD1 antibodies.
Allogeneic stem cell transplantation (allo-SCT) using a reduced-intensity conditioning (RIC) regimen represents an established option in cHL patients relapsed after autologous transplantation or refractory to chemotherapy who have reached a chemosensitive disease after salvage protocols.6-9
However, some areas of uncertainty remain on checkpoint inhibition before allo-SCT because PD1 blockade might enhance not only allogeneic T-cell responses (and consequently increase the graft-versus-tumor effect), but also immunological toxicities, like graft-versus-host disease (GVHD) or graft failure. Experience in this setting is still limited, but is rapidly growing.
In patients who received checkpoint inhibitors (CPIs) before allo-SCT, a higher than expected incidence of GVHD and nonrelapse mortality (NRM) has been reported.3,10-12 In addition, a steroid-requiring febrile syndrome, without any identified infectious agent, was reported and some patients developed veno-occlusive disease (VOD), which is a very rare complication after RIC regimens.13
T-cell–replete haploidentical stem cell transplantation (SCT; haplo-SCT) with high-dose posttransplant cyclophosphamide (PTCy) as GVHD prophylaxis has widely spread in patients lacking a matched related or unrelated donor. PTCy for primary GVHD prophylaxis is associated with low rates of severe acute GVHD (aGVHD) and chronic GVHD (cGVHD), and several registry analyses have shown that the rates of GVHD are actually lower with haploidentical donors and PTCy than after allo-SCT from matched unrelated or matched related donors using conventional calcineurin inhibitor/methotrexate as GVHD prophylaxis.14,15 Furthermore, recent studies indicated that PTCy may be an effective GVHD prophylaxis for patients receiving PD1 blockade therapy.16,17
Here, we analyzed the effect of CPIs before haplo-SCT with PTCy in cHL patients, with the aim of comparing outcomes of patients who did or did not receive CPIs before haplo-SCT.
Patients and methods
Patients’ eligibility
This is a retrospective study including 59 consecutive cHL patients who received a haplo-SCT at 3 different institutions (Humanitas Cancer Center [Rozzano, Italy], Institut Paoli Calmettes [Marseille, France], and Hospital Sant-Antoine [Paris, France]) between February 2014 and December 2018. This time frame was selected because treatment with PD1 inhibitors has been available, in clinical trials or in extended access program, in these Centers since 2014.
Written informed consent for treatment was obtained from all patients. This retrospective study was approved by an institutional review board (ONC-OSS-15-2019) and conducted in the respect of the Helsinki declaration. All patients had a biopsy-proven cHL diagnosis.
Eligibility criteria for transplant included availability of a haploidentical related donor in the absence of a related or unrelated HLA-compatible donor. Additional transplant eligibility criteria included absence of active infection, Karnofsky performance status >60, lack of major organ dysfunctions, including a left ventricular ejection fraction <40%, diffusing capacity for carbon monoxide <50%, or creatinine clearance <50 mL/min, which would preclude administration of the cytoreductive therapy.
Conditioning regimens
The nonmyeloablative-conditioning regimen (n = 45) consisted of fludarabine 30 mg/m2 from days −6 to −2, cyclophosphamide 14.5 mg/kg at days −6 and −5, and total-body irradiation 200 cGy at day −1. The reduced toxicity regimen (n = 14) comprised thiotepa 5 to 10 mg/kg on day −6, cyclophosphamide 30 to 60 mg/kg on day −5, fludarabine 120 mg/m2 on day −5 to day −2, and low-dose total-body irradiation (2 Gy) on day −1.
GVHD prophylaxis and supportive care
For all patients, GVHD prophylaxis consisted of posttransplant cyclophosphamide 50 mg/kg at days +3 and +4, cyclosporine A (CsA), and mycophenolate mofetil. CsA was started at day +5 at the dose of 3 mg/kg as a continuous infusion until discharge and was converted to an oral formulation thereafter. CsA dosage was adjusted based on range of activity (between 100 and 200 ng/mL). CsA was tapered by days +100 to 180. Mycophenolate mofetil was administered at 15 mg/kg postoperatively 3 times per day from day +5 until day +35. Granulocyte colony-stimulating factor was started on day +5 in all patients. Antimicrobial prophylaxis and monitoring infections were applied as per center guidelines.
Stem cell sources and donors
Potential family members were typed at the HLA-A, HLA-B, and HLA-DRB1 loci at a high level of resolution. Selected donors were also typed at the HLA-C locus at a high-resolution level.
Some donors underwent bone marrow (BM) harvest under general anesthesia for a target dose of 3 × 108 to 4 × 108 total nuclear cells per kilogram of recipient weight. Other donors were mobilized by the subcutaneous administration of granulocyte colony-stimulating factor for 5 to 6 days at 10 μg/kg per day. The target was a minimum of 4 × 106 CD34+ per kilogram. Regarding the stem source choice, initially we used BM as reported by Luznik et al.18 Subsequently, it was gradually replaced by peripheral blood stem cells (PBSCs) for logistical reasons. Furthermore, the choice to donate PBSCs or BM depended on the donor decision and the availability of a specific prospective protocol. Unmanipulated BM and PBSCs were used for stem cell support on day 0.
Definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/L after transplantation. Platelet engraftment was defined as a platelet count of 20 × 109/L, with no transfusions during the preceding 7 days. aGVHD was graded according to the Keystone criteria19 and cGVHD was retrospectively graded following the National Institutes of Health (NIH) criteria.20 Hyperacute GVHD was defined as that occurring within 14 days after transplantation.21 The Hematopoietic Stem Cell Transplant–Comorbidity Index (HCT-CI) was calculated according to previously published methods.22 Cheson criteria were used to assess response to therapy.23 The LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) were used to define response after checkpoint inhibition.24 Standard definitions were used for overall survival (OS), progression-free survival (PFS), NRM, and relapse incidence.25
Statistical analysis
Data were summarized as frequencies and proportions or as median and range. Differences between groups were evaluated by using the Student t test for continuous distributions and the χ2 or the Fisher's exact test for categorical distributions. Survival analysis end points were defined according to the European Society for Blood and Marrow Transplantation (EBMT) criteria.25 PFS, relapse incidence, OS, and NRM were estimated starting from allogeneic transplantation. Survival curves were generated by using the Kaplan-Meier method and differences between groups were compared by the log-rank test. Cumulative incidence was used to estimate aGVHD, cGVHD, relapse incidence, and NRM, and the Gray method was applied to test differences between groups. The Cox proportional hazard model was used to estimate hazard ratio (HR) using a 95% confidence interval. A multivariable model was built to adjust the effect of checkpoint inhibition. Only prognostic factors statistically significant in the univariate model were included in the multivariable model. Statistical significance was set at <0.05. All analyses were performed using SAS v. 9.4 and R 3.4.1 software.
Results
Patients
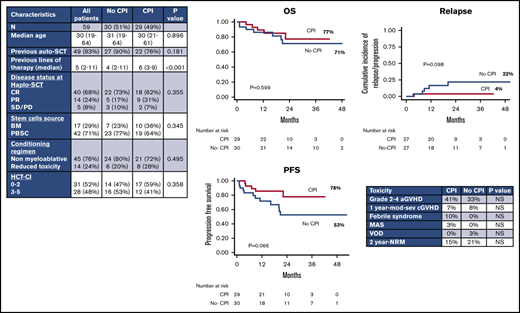
From February 2014 to December 2018, 59 consecutive cHL patients received a haplo-SCT with PTCy either with CPIs (n = 29) or without CPIs (no-CPI; n = 30) before SCT. Patient characteristics are summarized in Table 1. The 2 cohorts were similar in terms of age at SCT, previous autologous transplant, pretransplant disease status, CMV serostatus, stem cell source, intensity of conditioning regimen, and HCT-CI. The only difference was represented by the median number of previous lines of therapy, which was higher in the CPI group (6 vs 4; P < .001). Within this cohort, 28 patients (96%) received nivolumab and 1 patient was treated with pembrolizumab. The median number of CPI cycles before transplant was 12 (range, 4-55) and the median time from the last PD1 inhibitor infusion to haplo-SCT was 60 days (range, 27-372 days). Nine patients (31%) received chemotherapy after CPI in order to improve quality of response at transplant.
Characteristics of patients with cHL undergoing haplo-SCT
| Characteristics . | All patients . | No-CPI . | CPI . | P . |
|---|---|---|---|---|
| n (%) | 59 (100) | 30 (51) | 29 (49) | |
| Median age (range), y | 30 (19-64) | 31 (19-64) | 30 (21-61) | .896 |
| Previous auto-SCT, n (%) | 49 (83) | 27 (90) | 22 (76) | .181 |
| Previous lines of therapy (range) | 5 (2-11) | 4 (2-11) | 6 (3-9) | <.001 |
| Disease status at haplo, n (%) | ||||
| CR | 40 (68) | 22 (73) | 18 (62) | .355 |
| PR | 14 (24) | 5 (17) | 9 (31) | |
| SD/PD | 5 (8) | 3 (10) | 2 (7) | |
| CMV serostatus, n (%) | ||||
| Recipient − | 20 (34) | 10 (33) | 10 (36) | .849 |
| Recipient + | 39 (66) | 20 (67) | 19 (64) | |
| Stem cell source, n (%) | ||||
| BM | 17 (29) | 7 (23) | 10 (36) | .345 |
| PBSC | 42 (71) | 23 (77) | 19 (64) | |
| Conditioning regimen, n (%) | ||||
| Nonmyeloablative | 45 (76) | 24 (80) | 21 (72) | .495 |
| Reduced toxicity | 14 (24) | 6 (20) | 8 (28) | |
| HCT-CI, n (%) | ||||
| 0-2 | 31 (52) | 14 (47) | 17 (59) | .358 |
| 3-5 | 28 (48) | 16 (53) | 12 (41) |
| Characteristics . | All patients . | No-CPI . | CPI . | P . |
|---|---|---|---|---|
| n (%) | 59 (100) | 30 (51) | 29 (49) | |
| Median age (range), y | 30 (19-64) | 31 (19-64) | 30 (21-61) | .896 |
| Previous auto-SCT, n (%) | 49 (83) | 27 (90) | 22 (76) | .181 |
| Previous lines of therapy (range) | 5 (2-11) | 4 (2-11) | 6 (3-9) | <.001 |
| Disease status at haplo, n (%) | ||||
| CR | 40 (68) | 22 (73) | 18 (62) | .355 |
| PR | 14 (24) | 5 (17) | 9 (31) | |
| SD/PD | 5 (8) | 3 (10) | 2 (7) | |
| CMV serostatus, n (%) | ||||
| Recipient − | 20 (34) | 10 (33) | 10 (36) | .849 |
| Recipient + | 39 (66) | 20 (67) | 19 (64) | |
| Stem cell source, n (%) | ||||
| BM | 17 (29) | 7 (23) | 10 (36) | .345 |
| PBSC | 42 (71) | 23 (77) | 19 (64) | |
| Conditioning regimen, n (%) | ||||
| Nonmyeloablative | 45 (76) | 24 (80) | 21 (72) | .495 |
| Reduced toxicity | 14 (24) | 6 (20) | 8 (28) | |
| HCT-CI, n (%) | ||||
| 0-2 | 31 (52) | 14 (47) | 17 (59) | .358 |
| 3-5 | 28 (48) | 16 (53) | 12 (41) |
The P value shown in bold represents <.05.
auto-SCT, autologous stem cell transplant; CMV, cytomegalovirus; CR, complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
Engraftment
All patients were evaluable for ANC engraftment, whereas only 57 patients (97%) were evaluable for platelets engraftment, as 2 died on day +33 and +35, before platelet engraftment. Median time for ANC recovery and transfusion-independent platelet count was 20 days (range, 13-28 days) and 26 days (range, 11-139 days), respectively. For 3 patients, platelets remained >20 × 109/L for all of the posttransplant period. The cumulative incidence of ANC engraftment at day 30 was 98% and the cumulative incidence of platelet engraftment at day 60 was 98%. No differences in engraftment were observed between patients who received CPIs before transplant and others.
GVHD and other toxicities
One hundred day cumulative incidence of grade 2-4 aGVHD was 33% for all patients without any difference between CPI (41%) and no-CPI (33%) cohorts (P = .45). Only 2 patients, 1 in the CPI group and 1 in the no-CPI group, developed grade 3-4 aGVHD. No patients developed hyperacute GVHD.
One-year cumulative incidence of moderate-severe cGVHD was 7% for the entire population and was similar between patients receiving CPI (7%) or not (8%) before SCT (P = .67). By univariate and multivariable analysis, the incidence of grade 2-4 aGVHD was not influenced by any variable (stem cell source, conditioning regimen, cytomegalovirus [CMV] serostatus, previous autologous transplantation, HCT-CI, age at transplant, disease status pre-SCT). cGVHD was only evaluated for the entire cohort because only 3 patients of the entire cohort developed moderate or severe cGVHD.
Considering other toxicities, no patients of the CPI cohort developed VOD, which was observed only in 1 patient of the no-CPI cohort. In the CPI cohort, a steroid-requiring febrile syndrome after engraftment was observed in 3 patients and a macrophage-activation syndrome (MAS) in 1 patient responding to steroids and IV immunoglobulins. No patients of the no-CPI cohort developed febrile syndrome or MAS. In the CPI cohort, no differences in terms of GVHD and other toxicities were found based on the number of previous doses of PD1 inhibitors or on the time elapsed between the last anti-PD1 infusion and haplo-SCT (data not shown).
PFS, OS, and relapse incidence
With a median follow-up of 26 months (range, 7.5-55 months), 2-year OS and PFS rates for the whole population were 74% and 65%, respectively. By univariate analysis, we did not observe any difference between CPI and no-CPI cohorts in terms of OS (2-year OS, 77% vs 71%; P = .599) (Figure 1A). PFS was higher in the CPI cohort (2-year PFS, 78% vs 53%; P = .066), even if this difference was not statistically significant (Figure 1B). Pretransplant disease status was the only variable associated with long-term outcomes by univariate analysis: 2-year OS was 81% in complete remission (CR) patients, 75% in partial remission (PR) patients, and 20% in patients in stable disease (SD)/progressive disease (PD) (P < .001) (Figure 1C); 2-year PFS was 70% vs 63% vs 20% (P < .001), respectively (Figure 1D). Furthermore, if we use the Cox proportional hazard model, SD/PD pretransplant had a negative impact on both OS and PFS (OS [HR, 11.7; 95% confidence interval, 3.07-44.82; P < .001]; PFS [HR, 7.1; 95% confidence interval, 2.19-23.54; P = .001]). However, the impact on OS and PFS of PR compared with CR at transplant was not statistically significant (OS [HR, 2.13; 95% confidence interval, 0.51-8.94; P = .302]; PFS [HR, 1.51; 95% confidence interval, 0.46-4.92; P = .492]).
Kaplan-Meier curves. (A-B) OS and PFS by prior checkpoint inhibition at haplo-SCT. Two-year OS was 77% in the CPI cohort vs 71% in the no-CPI cohort (P = .599); 2-year PFS: 78% vs 53%, respectively (P = .066). (C-D) OS and PFS by disease status at time of haplo-SCT (CR, PR, SD/PD). Two-year OS was 81% in CR patients, 75% in PR patients, and 20% in patients in SD/PD (P < .001); 2-year PFS was 70% vs 63% vs 20% (P < .001), respectively.
Kaplan-Meier curves. (A-B) OS and PFS by prior checkpoint inhibition at haplo-SCT. Two-year OS was 77% in the CPI cohort vs 71% in the no-CPI cohort (P = .599); 2-year PFS: 78% vs 53%, respectively (P = .066). (C-D) OS and PFS by disease status at time of haplo-SCT (CR, PR, SD/PD). Two-year OS was 81% in CR patients, 75% in PR patients, and 20% in patients in SD/PD (P < .001); 2-year PFS was 70% vs 63% vs 20% (P < .001), respectively.
As reported in “Patients and methods,” a multivariable analysis for OS and PFS was performed, including disease status at transplant (SD/PD vs CR and PR vs CR) and CPIs before transplant as variables. CPIs before transplant confirmed a positive impact on PFS but not on OS (PFS [HR, 0.23; 95% confidence interval, 0.07-0.76; P = .015] and OS [HR, 0.52; 95% confidence interval, 0.16-1.72; P = .283]). Considering response at transplant, SD/PD was an independent negative prognostic factor for both OS and PFS (OS [HR, 14.3; 95% confidence interval, 3.46-58.95; P < .001] and PFS [HR, 14.1; 95% confidence interval, 3.49-57.18; P < .001]), whereas PR did not have any impact both on OS and PFS compared with patients in CR (OS [HR, 2.34; 95% confidence interval, 0.55-9.91; P = .283] and PFS [HR, 1.81; 95% confidence interval, 0.55-5.91; P = .327]).
Considering patients in CR and PR before haplo-SCT, 2-year cumulative incidence of disease relapse/progression was 13%. When we analyzed patients according to prior CPIs, relapse/progression rate at 2 years was higher, but not statistically different, in patients not receiving CPIs before transplant: 22% vs 4% (P = .098) (Figure 2A). Taking into account only patients in CR at transplant, 2-year cumulative incidence of relapse was 20% in the no-CPI group vs 0% in the CPI group (P = .054) (Figure 2B).
Cumulative incidence of relapse. (A) Cumulative incidence of relapse/progression by prior checkpoint inhibition at haplo-SCT. Two-year relapse/progression rate was 22% in the no-CPI cohort and 4% in the CPI cohort (P = .098). (B) Cumulative incidence of relapse by prior checkpoint inhibition at haplo-SCT. Two-year relapse cumulative incidence was 20% in the no-CPI group vs 0% in the CPI group (P = .054).
Cumulative incidence of relapse. (A) Cumulative incidence of relapse/progression by prior checkpoint inhibition at haplo-SCT. Two-year relapse/progression rate was 22% in the no-CPI cohort and 4% in the CPI cohort (P = .098). (B) Cumulative incidence of relapse by prior checkpoint inhibition at haplo-SCT. Two-year relapse cumulative incidence was 20% in the no-CPI group vs 0% in the CPI group (P = .054).
Finally, only for the CPI cohort, we analyzed whether the 9 patients who received additional chemotherapy between CPI and haplo-SCT had different outcomes compared with the 20 patients who underwent transplant early after CPI. With the limitations of the small numbers, no statistically significant differences were observed.
NRM
Two-year NRM rate was 18% for all patients with no difference between CPI and no-CPI cohorts (15% vs 21%; P = .578). In the CPI group, death was due to infection (n = 1), posttransplant lymphoproliferative disease (n = 1), and heart failure (n = 1). In the no-CPI group, causes of death were GVHD (n = 1, aGVHD; n = 1, cGVHD), infection (n = 1), VOD (n = 1), and acute myocardial infarction (n = 1).
Discussion
Our study shows that checkpoint inhibition before haplo-SCT results in enhanced PFS, without increasing immunological toxicities and NRM. Even with the limitations of the retrospective analysis, this represents the first study comparing outcomes of patients who did or did not receive CPIs before haplo-SCT. Furthermore, the median follow-up of alive patients in this analysis is 26 months, longer than in other studies on allo-SCT after PD1 blockade.
The 2-year PFS observed in the CPI cohort was higher relative to the no-CPI cohort (79% vs 53%) and, by multivariable analysis, PD1 inhibition before transplant was an independent protective factor for PFS (HR, 0.324). Furthermore, relapse/progression incidence was lower in patients treated with CPIs before SCT (4% vs 22% at 2 years) and, specifically, in the CPI cohort, none of the 18 CR patients relapsed. These results might be due to residual blockade of inhibitory checkpoints at the time of transplant, which may lead to a more robust donor T-cell response, and consequently to enhanced graft-versus-lymphoma activity. The low relapse rate was observed in other trials including patients treated with CPI before an allogeneic transplantation. In the CheckMate 205 trial, 44 patients received allo-SCT after nivolumab treatment and, with a median follow up of 5.5 months, the relapse rate was 7%, and overall outcomes (PFS and OS) seemed favorable.3 In a retrospective multicenter study that includes 31 cHL patients who received CPI before allo-SCT, Merryman et al reported a 1-year relapse incidence of 16%.11
A main object of discussion is whether all patients receiving anti-PD1 should receive a consolidation with allo-SCT or only those achieving a PR, which is associated with lower PFS. A recent study from LYSA centers, with a median follow up of 34.3 months, reported that patients achieving a CR after nivolumab had a significantly longer PFS than those reaching a PR (median not reached vs 9.3 months). However, consolidation with allo-SCT, both in CR and PR patients, was associated with a markedly lower relapse rate compared with patients who did not receive transplants (0% vs 62.2%), and 5 of 6 patients who received allo-SCT who were not in CR converted to CR after allo-SCT.26 In our study, PFS of patients in the CPI cohort appeared higher compared with the no-CPI group, even if a lower number of CPI patients were in CR at transplant (62% in the CPI cohort vs 73% in the no-CPI cohort; P = .35). Furthermore, a high proportion of patients who received haplo-SCT not in CR after CPIs converted to CR after transplant (9 of 11 patients).
The reason for this clinical response could be found in an imbalanced T-cell recovery after transplantation in patients previously treated with CPI. PD1 may lead to impaired differentiation of T effector cells and enhanced differentiation of T regulatory cells (Tregs).27 In a previous study, immune profiling of circulating T-cell subsets showed a decreased Treg-to-CD4Tcon ratio and a Treg-to-CD8 ratio after SCT in patients treated with anti-PD1 before transplantation compared with matched controls.11
Another factor affecting the low relapse rate observed in our study could be the use of PBSCs as graft source in the majority of patients (71%). Previous reports showed that, in haplo-SCT, a PBSC graft was associated with higher PFS and OS relative to BM cells,28,29 suggesting an enhanced graft-versus-leukemia effect potentially linked to the higher number of CD3 infused with PBSCs. However, the role of PBSCs in patients treated with PD1 inhibitors before transplant is difficult to identify and larger studies are required to clarify this topic.
The low incidence of relapse reported in our study could also be associated with the frequency of grade 2 aGVHD that was higher in the CPI cohort (41% vs 33% at 100 days). The beneficial effect of grade 2 aGVHD on outcomes was already known. In a retrospective study on 340 patients with hematologic malignancies who received a haplo-SCT with PTCy as GVHD prophylaxis, the 4-year probabilities of PFS and OS were higher in patients with grade 2 aGVHD when compared with patients who never experienced GVHD and, in multivariable models, grade 2 aGVHD was associated with improved PFS.30
Although the immunomodulatory effects of CPIs may improve outcomes of allo-SCT, PD1 inhibition may also augment alloreactive T cells and consequently increase early posttransplant side effects and NRM. For this reason, a warning and precaution label for complications after allo-SCT is included in the prescribing information for nivolumab.10 This came from data of the first 17 patients treated with CPI and allo-SCT, finding a higher than expected NRM (35%) and severe (grade 3-4) acute GVHD (29%). In addition, 1 patient developed VOD, leading to death.10 In the Merryman study, although the overall incidences of aGVHD, cGVHD, and NRM were in line with the literature,6,7 the incidence of grade 4 aGVHD appeared higher than in prior studies (23% at 1 year) and 3 patients died of grade 4 aGVHD.11 Also in the CheckMate 205 trial, a higher than expected incidence of grade 3-4 aGVHD (17% at 100 days) was reported in the 44 patients who received allo-SCT and 4 patients died of aGVHD.3 Finally, in a recent meta-analysis including 107 patients who received CPI before allo-SCT, 59% of patients developed aGVHD, and cGVHD was observed in 29%.12 Despite the retrospective nature and the small number of patients included in our analysis, our study does not confirm these observations: aGVHD and cGVHD incidences were encouraging, and no differences between the 2 cohorts were observed. In addition, only 1 patient for each cohort (CPI and no-CPI) developed grade 3-4 aGVHD and no hyperacute GVHD was reported. The 2-year NRM cumulative incidences did not differ in the 2 groups (15% in the CPI cohort vs 21% in the no-CPI cohort) and no patients treated with CPI died of GVHD.
The promising results reported in our study in terms of toxicities may be partially related to GVHD prophylaxis. All patients included in our analysis received PTCy as GVHD prophylaxis, and this could explain the surprisingly low incidence of grade 3-4 aGVHD.
Recently, Ikegawa et al evaluated the impact of PTCy on abnormal T-cell reconstitution after PD1 blockade using murine models and demonstrated that PTCy efficiently ameliorated GVHD after transplantation.17 Furthermore, the benefit of PTCy in preventing severe aGVHD after allo-SCT In patients receiving PD1 blockade therapy was also observed in a recent retrospective clinical study. The Baltimore group reported the data of 14 patients affected by hematological diseases (10 with cHL) receiving allo-SCT after CPI and PTCy as GVHD prophylaxis.16 This analysis included 10 patients who received SCT from haploidentical donors, 2 from mismatched unrelated donors, and 2 from matched unrelated donors. With a median follow-up of 1 year, no grade 3-4 aGVHD, no cGVHD, and no NRM were observed, suggesting a role of PTCy in also preventing GVHD in this setting. The reason why PTCy is effective at abrogating the CPI-induced alloreactivity is not well known. Several studies analyzed the immunological reconstitution in haploidentical transplantation with PTCy as GVHD prophylaxis,31,32 showing that the frequencies of postinfusion Tregs were preferentially high. In murine models, PTCy successfully restored T-cell homeostasis and reduced GVHD induced by PD1−/− donor T cells, rescuing PD-1−/− Tregs from apoptosis.17
Another factor that could influence the rate of GVHD is the stem cell source as previous observations suggest that using BM as graft source could reduce the incidence of aGVHD in patients treated with CPI before transplant.11,16 Despite the limitations of our analysis, our data do not seem to confirm the protective role of the BM graft on GVHD. In our study, in fact, 66% of patients in the CPI cohort received PBSCs and the rate of aGVHD and cGVHD was not different when patients were infused with BM cells or PBSCs.
In conclusion, these data suggest that using CPI as a bridge to haplo-SCT is feasible and improves the PFS in relapsed/refractory cHL, lowering the relapse rate without enhancing the toxicity. For this reason, especially for patients who obtain a response after CPI, consolidation with haploidentical transplantation and PTCy as GVHD prophylaxis should be considered. However, given the relatively small number of patients involved in our study, further studies should be performed to extend these findings.
Data-sharing requests may be e-mailed to the corresponding author, Luca Castagna, at luca.castagna@humanitas.it.
Authorship
Contribution: C.D.P. analyzed the data and wrote the article; L.G. analyzed the data and performed statistical analyses; F.L.-I., C.M.d.O., R. Duléry, R.B., A.G., R. Devillier, J.M., B.S., S.H., V.M., S.F., T.P., P.-J.W., C.L., B.C., and C.C. provided patients and/or scientific input and/or prepared the manuscript; S.B., A.S., M.M., and D.B contributed to the design of the study and revised the manuscript; and L.C. designed the research study and wrote the article.
Conflict-of-interest disclosure: A.S. served on advisory boards for Bristol-Myers Squibb (BMS), Servier, Gilead, Pfizer, Eisai, Bayer, and Merck Sharp & Dohme (MSD); provided consultancy services to ArQule and Sanofi; and participated in speaker’s bureaus for Takeda, BMS, Roche, AbbVie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, ArQule, Lilly, Sandoz, Eisai, Novartis, Bayer, and MSD. The remaining authors declare no competing financial interests.
Correspondence: Luca Castagna, Humanitas Clinical and Research Center–IRCCS, Via Manzoni 56, 20089 Rozzano, Italy; e-mail: luca.castagna@humanitas.it.