Key Points
RB/RC induction followed by ASCT is well tolerated overall and achieves high rates of durable remissions.
Tracking MRD after ASCT with immunoglobulin-based next-generation sequencing is feasible and may predict disease recurrence.
Abstract
The addition of high-dose cytarabine to rituximab/bendamustine (RB) induction could improve outcomes for transplant-eligible patients with mantle cell lymphoma (MCL). We conducted a pooled analysis of 2 phase 2 trials and an off-trial cohort each testing 3 cycles of RB and 3 cycles of rituximab/high-dose cytarabine (RC) followed by autologous stem cell transplantation (ASCT) among untreated, transplant-eligible patients with MCL. Dana-Farber Cancer Institute (DFCI) and Washington University in St. Louis (WUSTL) led separate phase 2 trials testing sequential and alternating cycles of RB/RC, respectively. Patients treated at DFCI with sequential RB/RC off trial were retrospectively identified. Minimal residual disease (MRD) was assessed in the DFCI trial. A total of 88 patients (23 DFCI trial, 18 WUSTL trial, and 47 off trial) received RB/RC; 92% of patients completed induction, and 84% underwent planned consolidative ASCT. Grade 3 or 4 adverse events among trial patients included lymphopenia (88%), thrombocytopenia (85%), neutropenia (83%), and febrile neutropenia (15%). There were no treatment-related deaths during induction and 2 following ASCT. Among 87 response-evaluable patients, the end-of-induction overall and complete response rates were 97% and 90%, respectively. After a median follow-up of 33 months, 3-year progression-free survival and overall survival were 83% and 92%, respectively. Patients undergoing MRD testing experienced prolonged MRD negativity after ASCT with emergence of MRD occurring in only 1 patient who subsequently relapsed. RB/RC followed by ASCT achieves high rates of durable remissions in transplant-eligible patients with MCL. These trials were registered at www.clinicaltrials.gov as #NCT01661881 (DFCI trial) and #NCT02728531 (WUSTL trial).
Introduction
The adoption of novel induction regimens and the routine use of consolidation with autologous stem cell transplantation (ASCT) in first remission have significantly improved outcomes for patients with mantle cell lymphoma (MCL).1,2 Two randomized trials demonstrated that induction therapy with rituximab/bendamustine (RB) improves progression-free survival (PFS) compared with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).3,4 In addition, a phase 3 trial confirmed that the addition of high-dose cytarabine to RCHOP-based induction therapy significantly improves PFS among transplant-eligible patients.5 Given the benefit of cytarabine and the superiority of RB over RCHOP, the combination of RB and cytarabine may provide a superior induction regimen prior to ASCT. Indeed, the addition of low-dose cytarabine in combination with RB yielded very successful results in 2 phase 2 trials among older, transplant-ineligible patients.6,7 In 2012, Dana-Farber Cancer Institute (DFCI) launched a phase 2 trial testing 3 cycles of RB followed by 3 cycles rituximab and high-dose cytarabine (RC) in 23 transplant-eligible patients with untreated MCL. This regimen achieved a complete response rate (CRR) of 96% and a 13-month PFS of 96%.8 Based on these encouraging results, RB/RC followed by ASCT became the standard frontline regimen for transplant-eligible patients with MCL at DFCI. Simultaneously, investigators at Washington University in St. Louis (WUSTL) initiated a similar trial of alternating cycles of RB and RC in patients with untreated MCL.
With the goal of providing a more robust estimate of the efficacy of RB/RC, as well as its long-term outcomes, we conducted a pooled analysis of the DFCI trial, the WUSTL trial, and a retrospective series of transplant-eligible MCL patients who received RB/RC as off-trial, first-line therapy at DFCI. In addition, we performed minimal residual disease (MRD) testing during RB/RC induction and following ASCT in a subset of patients. Herein, we report the clinical outcomes of RB/RC induction in those 88 transplant-eligible patients.
Methods
Patients and centers
DFCI and WUSTL led independent phase 2 trials testing frontline treatment with RB/RC in transplant-eligible patients with MCL. Eligible participants were adults (aged 18-69 years in the DFCI trial and 18-65 years in the WUSTL trial) with untreated, radiographically measurable, and pathologically confirmed MCL. Participants were eligible for ASCT and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, absolute neutrophil count ≥1.0 × 109/L, platelet count ≥100 × 109/L, adequate renal function (creatinine <2.0 mg/dL [DFCI] or estimated glomerular filtration rate ≥40 mL/min [WUSTL]), and preserved liver function (transaminases ≤2.5 upper limit of normal [ULN] [DFCI] or ≤3.0 ULN [WUSTL] and total bilirubin ≤2.5 ULN [DFCI] or ≤2.0 ULN [WUSTL]).
Patients with MCL who received frontline treatment with RB/RC outside of a clinical trial were retrospectively identified using DFCI pharmacy and transplant databases. Patients were included if they were ≥18 years old, had a diagnosis of MCL (confirmed by DFCI pathology review), initiated RB/RC therapy after 1 January 2014, and had an end-of-induction (EOI) positron emission tomography/computed tomography (PET/CT) available for review. Patients evaluated at DFCI were included if they received their treatment at DFCI or at a community center.
Both clinical trials and the retrospective review were approved by relevant institutional review boards and were conducted in accordance with principles defined by the Declaration of Helsinki. All trial patients provided informed consent, and a waiver of informed consent was granted for retrospective analysis of nontrial patients.
Treatment
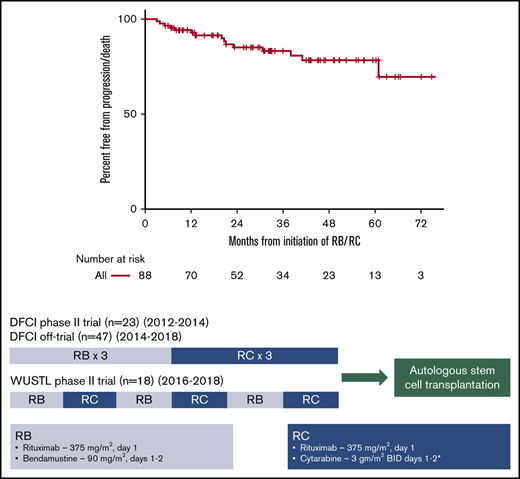
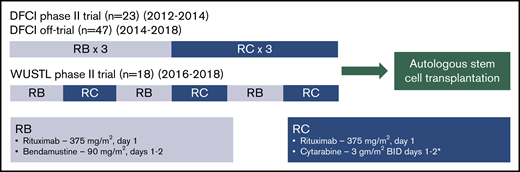
Treatment of each cohort consisted of 3 cycles of outpatient RB (rituximab 375 mg/m2 [day 1] and bendamustine 90 mg/m2 [days 1 and 2] every 4 weeks) and 3 cycles of inpatient RC (rituximab 375 mg/m2 [day 1] and cytarabine every 12 hours for 4 doses [days 1 and 2] every 3 weeks). The starting dose of cytarabine was 3 g/m2 in the DFCI and WUSTL trials, with dose reductions for age, renal dysfunction, and/or neurotoxicity (Figure 1). After enrollment of 14 patients in the WUSTL trial, the WUSTL protocol was amended to lower the starting cytarabine dose to 2 g/m2 for subsequent patients due to frequent prolonged thrombocytopenia following ASCT. In patients treated off trial, cytarabine dose was chosen by the treating physicians.
Treatment schema: a pooled analysis of 2 phase 2 clinical trials and a retrospectively identified nontrial cohort. In each cohort, patients received 3 cycles of RB and 3 cycles of RC. In the DFCI trial, the starting dose of cytarabine was reduced from 3 g/m2 to 2 g/m2 for patients >60 years with a further dose reduction to 1.5 g/m2 for patients >60 years with either renal dysfunction (creatinine, 1.3-2.0) or preexisting neurotoxicity or 1.0 g/m2 for patients >60 years with both renal dysfunction and preexisting neurotoxicity. In the WUSTL trial, the starting dose of cytarabine was reduced to 2 g/m2 for patients >60 years and/or with renal dysfunction (estimated glomerular filtration rate, 40-59). *The starting cytarabine dose was chosen by the treating physician for patients treated in the nontrial cohort. BID, twice a day.
Treatment schema: a pooled analysis of 2 phase 2 clinical trials and a retrospectively identified nontrial cohort. In each cohort, patients received 3 cycles of RB and 3 cycles of RC. In the DFCI trial, the starting dose of cytarabine was reduced from 3 g/m2 to 2 g/m2 for patients >60 years with a further dose reduction to 1.5 g/m2 for patients >60 years with either renal dysfunction (creatinine, 1.3-2.0) or preexisting neurotoxicity or 1.0 g/m2 for patients >60 years with both renal dysfunction and preexisting neurotoxicity. In the WUSTL trial, the starting dose of cytarabine was reduced to 2 g/m2 for patients >60 years and/or with renal dysfunction (estimated glomerular filtration rate, 40-59). *The starting cytarabine dose was chosen by the treating physician for patients treated in the nontrial cohort. BID, twice a day.
The DFCI trial and off-trial cohorts used sequential cycles of RB (cycles 1-3) followed by RC (cycles 4-6), whereas the WUSTL trial tested alternating cycles of RB (cycles 1, 3, and 5) and RC (cycles 2, 4, and 6) (Figure 1). Growth factor support and prophylactic fluoroquinolone treatment were used during RC cycles in the DFCI and WUSTL trials. In the retrospective cohort, growth factor support and antibiotic prophylaxis were determined by the treating physician.
Response assessment
Patients in all cohorts underwent imaging assessments at baseline and at the end of induction. The DFCI trial, which was designed before guideline recommendation for fluorodeoxyglucose (FDG) PET/CT use in MCL, used CT, and response assessments were based on the International Working Group Criteria.9 The WUSTL and off-trial cohorts used FDG-PET/CT, and responses were assessed using the 2014 Lugano classification.10 FDG-PET/CT scans for all off-trial patients were reviewed by an expert DFCI nuclear medicine radiologist. Both trials mandated a baseline bone marrow aspirate and biopsy, and if positive, a repeat bone marrow aspirate and biopsy after induction therapy to confirm response. Flow cytometry was not required for response reassessment.
Autologous transplantation
Stem cell mobilization and leukapheresis were performed based on institutional standards (filgrastim with plerixafor used as needed for poor mobilizers) and were not a part of either phase 2 trial. ASCT conditioning consisted of either CBV (cyclophosphamide, carmustine, and etoposide) in 13 patients transplanted at DFCI before 15 April 2014 or BEAM (carmustine, etoposide, cytarabine, and melphalan) in all other patients. Radiographic assessments after ASCT were at the discretion of treating physicians.
MRD assessment
When feasible, peripheral blood mononuclear cells (PBMCs) and plasma were collected from patients enrolled on the DFCI trial at baseline, after 3 cycles of induction, after 6 cycles of induction, and every 3 to 6 months thereafter. Samples were assessed for MRD using immunoglobulin-based next-generation sequencing (IgNGS) with the ClonoSeq platform (Adaptive Biotechnologies, Seattle, WA), as previously described.11,12 The analytical sensitivity of the ClonoSeq platform is estimated at detection of 1 clonal molecule in a background of 1 × 106 nonclonal molecules. As the average input into the IgNGS reaction for PBMCs was 1.79 × 106 genomes, the nominal sensitivity for this assay is 1 in 1 × 106. For detection of plasma circulating tumor DNA (ctDNA), the average input into the IgNGS reaction was 1.96 × 104 genomes; therefore, the nominal sensitivity of the assay for plasma samples is 1 in 1.96 × 104. MRD testing was considered positive for any nonzero result in either plasma or PBMCs.
Statistical analysis
The primary end point of the DFCI trial was ORR, as previously reported.8 The primary end point of the WUSTL trial was stem cell mobilization success rate (defined as a yield >2 × 106 CD34+ stem cells/kg). PFS and overall survival (OS) were estimated using the Kaplan-Meier method, and differences in survival between groups were assessed using log-rank tests. PFS was defined as the time from initiation of RB/RC to death from any cause, relapse, or progression, with patients censored at the last time seen alive and progression-free. OS was defined as the time from initiation of RB/RC to death from any cause, with patient censored at the last time seen alive. Between groups, nominal, continuous, and ordinal variables were compared by Fisher’s exact test, Kruskal-Wallis rank-sum, or Kruskal-Wallis trend test, respectively. Univariate Cox proportional hazards regression were used to evaluate associations between prognostic factors and PFS, and Wald P values were reported for covariates. All analyses were performed using R version 3.5.0 (2018-04-23, R Core Team).
Results
Patients and treatment
The DFCI trial enrolled 23 patients from August 2012 to March 2014. The WUSTL trial enrolled 18 patients from August 2016 to September 2018. Forty-seven patients initiated RB/RC therapy outside of a clinical trial between July 2014 and August 2018 and were included in the off-trial cohort. The baseline characteristics of all 88 patients are summarized in Table 1. The median age was 58 years (range, 30-72). Seventeen patients (19%) had a high MIPI score, 21 (24%) had a Ki67 >30%, and 11 (12%) had blastoid or pleomorphic histology. WUSTL trial patients had more frequent high-risk features (ECOG performance status of 2, high MIPI, blastoid/pleomorphic histology) compared with the other cohorts.
Baseline characteristics
| . | Total (N = 88) . | Patient cohort, n (%) . | P . | ||
|---|---|---|---|---|---|
| DFCI off-trial (n = 47 [53%]) . | DFCI trial (n = 23 [26%]) . | WUSTL trial (n = 18 [20%]) . | |||
| Age, y | |||||
| Median (range) | 58 (30-72) | 58 (30-72) | 57 (42-69) | 60 (38-65) | .97* |
| >60 | 36 (41) | 20 (43) | 8 (35) | 8 (44) | .84† |
| Male sex | 64 (73) | 32 (68) | 15 (65) | 17 (94) | .056† |
| Stage at diagnosis | |||||
| 1 | 1 (1) | 0 (0) | 0 (0) | 1 (6) | .80‡ |
| 2 | 1 (1) | 1 (2) | 0 (0) | 0 (0) | |
| 3 | 11 (12) | 7 (15) | 3 (13) | 1 (6) | |
| 4 | 75 (85) | 39 (83) | 20 (87) | 16 (89) | |
| ECOG PS | |||||
| 0-1 | 84 (95) | 47 (100) | 22 (96) | 15 (83) | .013† |
| 2 | 4 (5) | 0 (0) | 1 (4) | 3 (17) | |
| Days from diagnosis to treatment, median (range) | 32 (0-1539) | 31 (3-1388) | 40 (12-245) | 21 (0-1539) | .031* |
| MIPI score | |||||
| Low | 47 (53) | 22 (47) | 16 (70) | 9 (50) | .13‡ |
| Intermediate | 16 (18) | 11 (23) | 5 (22) | 0 (0) | |
| High | 17 (19) | 6 (13) | 2 (9) | 9 (50) | |
| Missing | 8 (9) | 8 (17) | 0 (0) | 0 (0) | |
| LDH > ULN | |||||
| No | 54 (61) | 27 (57) | 18 (78) | 9 (50) | .16† |
| Yes | 27 (31) | 13 (28) | 5 (22) | 9 (50) | |
| Missing | 7 (8) | 7 (15) | 0 (0) | 0 (0) | |
| Ki67 | |||||
| ≤30% | 49 (56) | 27 (57) | 12 (52) | 10 (56) | >.99† |
| >30% | 21 (24) | 12 (26) | 5 (22) | 4 (22) | |
| Missing | 18 (20) | 8 (17) | 6 (26) | 4 (22) | |
| MCL subtype | |||||
| Other | 76 (86) | 40 (85) | 23 (100) | 13 (72) | .037† |
| Blastoid/pleomorphic | 11 (12) | 7 (15) | 0 (0) | 4 (22) | |
| Missing | 1 (1) | 0 (0) | 0 (0) | 1 (6) | |
| Treating center | |||||
| Community | 8 (9) | 8 (17) | 0 (0) | 0 (0) | .025† |
| Academic | 80 (91) | 39 (83) | 23 (100) | 18 (100) | |
| . | Total (N = 88) . | Patient cohort, n (%) . | P . | ||
|---|---|---|---|---|---|
| DFCI off-trial (n = 47 [53%]) . | DFCI trial (n = 23 [26%]) . | WUSTL trial (n = 18 [20%]) . | |||
| Age, y | |||||
| Median (range) | 58 (30-72) | 58 (30-72) | 57 (42-69) | 60 (38-65) | .97* |
| >60 | 36 (41) | 20 (43) | 8 (35) | 8 (44) | .84† |
| Male sex | 64 (73) | 32 (68) | 15 (65) | 17 (94) | .056† |
| Stage at diagnosis | |||||
| 1 | 1 (1) | 0 (0) | 0 (0) | 1 (6) | .80‡ |
| 2 | 1 (1) | 1 (2) | 0 (0) | 0 (0) | |
| 3 | 11 (12) | 7 (15) | 3 (13) | 1 (6) | |
| 4 | 75 (85) | 39 (83) | 20 (87) | 16 (89) | |
| ECOG PS | |||||
| 0-1 | 84 (95) | 47 (100) | 22 (96) | 15 (83) | .013† |
| 2 | 4 (5) | 0 (0) | 1 (4) | 3 (17) | |
| Days from diagnosis to treatment, median (range) | 32 (0-1539) | 31 (3-1388) | 40 (12-245) | 21 (0-1539) | .031* |
| MIPI score | |||||
| Low | 47 (53) | 22 (47) | 16 (70) | 9 (50) | .13‡ |
| Intermediate | 16 (18) | 11 (23) | 5 (22) | 0 (0) | |
| High | 17 (19) | 6 (13) | 2 (9) | 9 (50) | |
| Missing | 8 (9) | 8 (17) | 0 (0) | 0 (0) | |
| LDH > ULN | |||||
| No | 54 (61) | 27 (57) | 18 (78) | 9 (50) | .16† |
| Yes | 27 (31) | 13 (28) | 5 (22) | 9 (50) | |
| Missing | 7 (8) | 7 (15) | 0 (0) | 0 (0) | |
| Ki67 | |||||
| ≤30% | 49 (56) | 27 (57) | 12 (52) | 10 (56) | >.99† |
| >30% | 21 (24) | 12 (26) | 5 (22) | 4 (22) | |
| Missing | 18 (20) | 8 (17) | 6 (26) | 4 (22) | |
| MCL subtype | |||||
| Other | 76 (86) | 40 (85) | 23 (100) | 13 (72) | .037† |
| Blastoid/pleomorphic | 11 (12) | 7 (15) | 0 (0) | 4 (22) | |
| Missing | 1 (1) | 0 (0) | 0 (0) | 1 (6) | |
| Treating center | |||||
| Community | 8 (9) | 8 (17) | 0 (0) | 0 (0) | .025† |
| Academic | 80 (91) | 39 (83) | 23 (100) | 18 (100) | |
Significant P values (ie, P < .05) are bolded.
LDH, lactate dehydrogenase; MIPI, MCL International Prognostic Index; PS, performance status.
Kruskal-Wallis rank-sum test.
Fisher’s exact test.
Kruskal-Wallis trend test (Monte Carlo simulation with 10 000 replicates).
All trial patients were treated at an academic center, whereas 8 out of 47 off-trial patients (17%) received all 6 cycles of RB/RC at a community center. Among off-trial patients, where the starting dose of cytarabine was determined by the treating physician, a cytarabine dose of <3 g/m2 was used more frequently compared with patients treated on the DFCI or WUSTL trials (83% vs 39% vs 44%, P < .001). In total, 31 out of 47 off-trial patients (66%) received a lower starting dose of cytarabine than intended per the DFCI trial protocol (median starting cytarabine dose: off-trial cohort, 2 g/m2; DFCI trial, 3 g/m2; WUSTL trial, 3 g/m2). Among all cohorts, 24 patients (27%) had either a dose reduction in cytarabine or received <3 cycles of RC (17 patients with dose reduction only, 5 patients with ≥1 missed RC cycles, and 2 patients with both dose reductions and missed RC cycles). Rates of cytarabine modification were higher in the off-trial cohort (14/47 [30%]) and WUSTL trial (9/18 [50%]) compared with the DFCI trial (1/23 [4%]) (P < .01). The median cumulative dose of cytarabine was 24 g/m2 among all patients (24 g/m2 in the off-trial cohort, 36 g/m2 in the DFCI trial cohort, and 24 g/m2 in the WUSTL trial cohort).
Efficacy
One patient (WUSTL trial) was removed from the trial prior to an EOI assessment due to a type III hypersensitivity reaction to rituximab. Among 87 response-evaluable patients, the EOI overall response rate (ORR) was 97% (90% complete response [CR]; 7% partial response [PR]), 96% (96% CR; 0% PR) in the DFCI trial, 100% (CR 94% CR, 6% PR) in the off-trial cohort, and 88% (CR 71%; PR 18%) in the WUSTL trial (Table 2). High ORRs were observed for poor-risk patient subgroups (Table 2). Response rates were similar among patients receiving a starting cytarabine dose of 3 g/m2 (ORR, 100%; CRR, 89%) and <3 g/m2 (ORR, 95%; CRR, 89%) (P = .55 for ORR, P = 1.00 for CRR). Similarly, among patients completing 3 cycles of RC, cumulative cytarabine dose was not a significant predictor of response (Table 2).
EOI response rates among response-evaluable patients
| . | ORR, n/N (%) (95% CI) . | CR, n/N (%) (95% CI) . | PR, n/N (%) (95% CI) . |
|---|---|---|---|
| Overall | 84/87 (97) (90-99) | 78/87 (90) (81-95) | 6/87 (7) (3-14) |
| Cohort | |||
| DFCI off trial | 47/47 (100) (92-100) | 44/47 (94) (82-99) | 3/47 (6) (1-18) |
| DFCI trial* | 22/23 (96) (78-100) | 22/23 (96) (78-100) | 0/23 (0) (0-15) |
| WUSTL trial | 15/17 (88) (64-99) | 12/17 (71) (44-90) | 3/17 (18) (4-43) |
| MIPI | |||
| Low | 47/47 (100) (92-100) | 43/47 (91) (80-98) | 4/47 (9) (2-20) |
| Intermediate | 15/16 (94) (70-100) | 15/16 (94) (70-100) | 0/16 (0) (0-21) |
| High | 14/16 (88) (62-98) | 12/16 (75) (48-93) | 2/16 (12) (2-38) |
| Ki67 | |||
| ≤30% | 48/49 (98) (89-100) | 45/49 (92) (80-98) | 3/49 (6) (1-17) |
| >30% | 19/20 (95) (75-100) | 17/20 (85) (62-97) | 2/20 (10) (1-32) |
| Histologic subtype | |||
| Other | 73/75 (97) (91-100) | 69/75 (92) (83-97) | 4/75 (5) (1-13) |
| Blastoid/pleomorphic | 10/11 (91) (59-100) | 9/11 (82) (4-98) | 1/11 (9) (0-41) |
| Starting cytarabine dose | |||
| 3 g/m2 | 28/28 (100) (88-100) | 25/28 (89) (72-98) | 3/28 (11) (2-28) |
| <3 g/m2 | 53/56 (95) (85-99) | 50/56 (89) (78-96) | 3/56 (5) (1-15) |
| Cumulative cytarabine dose† | |||
| ≤24 g/m2 | 52/53 (98) (90-100) | 51/53 (96) (87-100) | 1/53 (2) (0-10) |
| >24 g/m2 | 28/28 (100) (88-100) | 25/28 (89) (72-98) | 3/28 (11) (2-28) |
| . | ORR, n/N (%) (95% CI) . | CR, n/N (%) (95% CI) . | PR, n/N (%) (95% CI) . |
|---|---|---|---|
| Overall | 84/87 (97) (90-99) | 78/87 (90) (81-95) | 6/87 (7) (3-14) |
| Cohort | |||
| DFCI off trial | 47/47 (100) (92-100) | 44/47 (94) (82-99) | 3/47 (6) (1-18) |
| DFCI trial* | 22/23 (96) (78-100) | 22/23 (96) (78-100) | 0/23 (0) (0-15) |
| WUSTL trial | 15/17 (88) (64-99) | 12/17 (71) (44-90) | 3/17 (18) (4-43) |
| MIPI | |||
| Low | 47/47 (100) (92-100) | 43/47 (91) (80-98) | 4/47 (9) (2-20) |
| Intermediate | 15/16 (94) (70-100) | 15/16 (94) (70-100) | 0/16 (0) (0-21) |
| High | 14/16 (88) (62-98) | 12/16 (75) (48-93) | 2/16 (12) (2-38) |
| Ki67 | |||
| ≤30% | 48/49 (98) (89-100) | 45/49 (92) (80-98) | 3/49 (6) (1-17) |
| >30% | 19/20 (95) (75-100) | 17/20 (85) (62-97) | 2/20 (10) (1-32) |
| Histologic subtype | |||
| Other | 73/75 (97) (91-100) | 69/75 (92) (83-97) | 4/75 (5) (1-13) |
| Blastoid/pleomorphic | 10/11 (91) (59-100) | 9/11 (82) (4-98) | 1/11 (9) (0-41) |
| Starting cytarabine dose | |||
| 3 g/m2 | 28/28 (100) (88-100) | 25/28 (89) (72-98) | 3/28 (11) (2-28) |
| <3 g/m2 | 53/56 (95) (85-99) | 50/56 (89) (78-96) | 3/56 (5) (1-15) |
| Cumulative cytarabine dose† | |||
| ≤24 g/m2 | 52/53 (98) (90-100) | 51/53 (96) (87-100) | 1/53 (2) (0-10) |
| >24 g/m2 | 28/28 (100) (88-100) | 25/28 (89) (72-98) | 3/28 (11) (2-28) |
Seventy-four patients (84%) underwent planned ASCT after RB/RC induction, including 21 out of 23 (91%) in the DFCI trial, 14 out of 18 (78%) in the WUSTL trial, and 39 out of 47 (83%) in the off-trial cohort. One additional patient in the off-trial cohort was scheduled to undergo ASCT after the time of data lock, while 13 patients did not undergo ASCT due to persistent or progressive disease (n = 4), persistent cytopenias (n = 3), patient or physician preference (n = 4), inadequate stem cell collection (n = 1), and an incidental ASXL1 mutation without cytopenias (n = 1). Among patients with ≥90 days of follow-up after ASCT, 27 out of 74 (36%) received rituximab maintenance therapy. Maintenance rituximab was not mandated in either trial and was used more frequently in more recent cohorts (DFCI, 2/21 [10%]; DFCI off-trial, 16/39 [41%]; WUSTL, 9/14 [64%]).
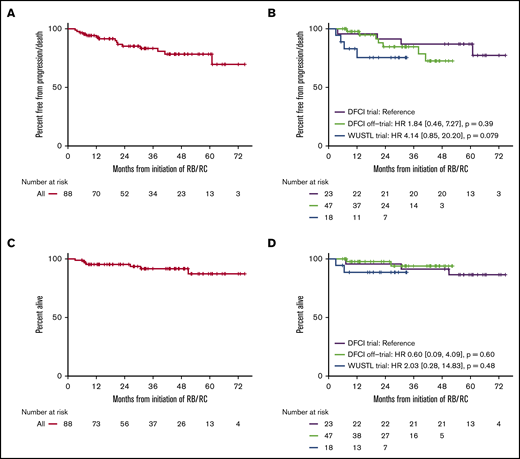
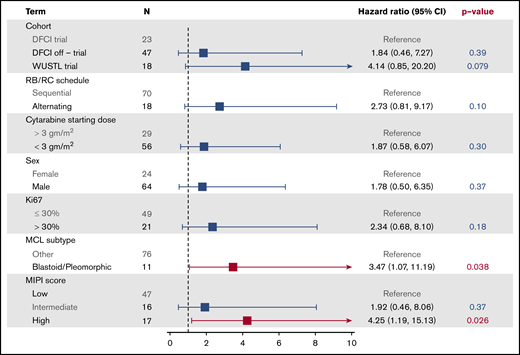
Median follow-up for all patients was 33.0 months (DFCI trial, 60.9 months; off-trial cohort, 30.9 months; WUSTL trial, 22.1 months). Among the entire study population, 3-year PFS was 83% (95% confidence interval [CI], 75% to 93%) (Figure 2). In the DFCI trial, the 1-year, 3-year, and 5-year PFS rates were 96%, 87%, and 87%, respectively. In the off-trial cohort, the 1-year and 3-year PFS rates were 98% and 85%, respectively. In the WUSTL trial, the 1-year and 2-year PFS rates were 83% and 75%, respectively. In a univariate analysis, high MIPI (hazard ratio [HR], 4.25; 95% CI, 1.19-15.13; P = .026) and blastoid/pleomorphic histology (HR, 3.47; 95% CI, 1.07-11.19; P = .038) were associated with inferior PFS (Figure 3). The 3-year PFS rates for patients with high MIPI score and blastoid/pleomorphic histologies were 76% (38% to 92%) and 66% (27% to 88%), respectively. There was no significant association between PFS and sex, Ki67 >30%, location of treatment, treatment cohort, or starting cytarabine dose (Figure 3). Among 81 patients who completed 3 cycles of RC, cumulative cytarabine dose was not a significant predictor of PFS (cumulative cytarabine dose >24 g/m2 vs ≤24 g/m2; HR, 2.20; P = .25). Among 74 patients who underwent ASCT, there was no significant difference in PFS among patients who received rituximab maintenance (HR, 0.28; 95% CI, 0.03-2.25; P = .23). Overall, the 3-year OS was 92% (95% CI, 85% to 98%) and was similar across study cohorts (Figure 2).
PFS and OS. PFS (A), PFS by cohort (B), OS (C), and OS by cohort (D).
Toxicity
A total of 81 patients (92%) completed 6 cycles of RB/RC. Seven patients (8%) discontinued therapy due to persistent cytopenias occurring during RC cycles (n = 2), progressive disease (n = 2), physician preference (n = 1), grade 3 rash attributed to bendamustine (n = 1), or grade 3 type III hypersensitivity reaction to rituximab (n = 1). Treatment discontinuation appeared to be more common in patients receiving alternating cycles compared with sequential cycles of RB/RC (17% vs 6%, P = .15). Detailed adverse event (AE) logs were maintained for patients participating in both phase 2 trials (n = 41). As expected, rates of grade 3 or 4 hematologic AEs were high, particularly during RC cycles (Table 3; supplemental Table 1). In total, 80% of patients developed grade 4 thrombocytopenia, and 68% experienced grade 4 neutropenia. Grade 3 or 4 infectious complications included febrile neutropenia in 6 patients (15%) as well as enterocolitis, pneumonia, and sepsis each in 1 patient (2% each). Other nonhematologic grade AEs were less frequent and mostly grade 1 or 2 (supplemental Tables 1 and 2). In the off-trial cohort, AEs requiring hospitalizations occurred in 14 patients (30%), including febrile neutropenia (22%), rash (2%), atrial fibrillation with rapid ventricular response (2%), tumor lysis syndrome during cycle 1 of RB in a patient with preexisting chronic kidney disease (2%), Escherichia coli sepsis (2%), and cryptogenic organizing pneumonia attributed to rituximab (2%).
Serious AEs for trial (DFCI/WUSTL) patients
| . | DFCI . | WUSTL . | Combined . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Grade 3-4 (n = 23 [56%]) . | Grade 3 . | Grade 4 . | Grade 3-4 (n = 18 [44%]) . | Grade 3 . | Grade 4 . | Grade 3-4 (n = 41) . | Grade 3 . | Grade 4 . |
| Lymphopenia | 21 (91) | — | 21 (91) | 15 (83) | 1 (6) | 14 (78) | 36 (88) | 1 (2) | 35 (85) |
| Thrombocytopenia | 19 (83) | — | 19 (83) | 16 (89) | 2 (11) | 14 (78) | 35 (85) | 2 (5) | 33 (80) |
| Neutropenia | 20 (87) | 2 (9) | 18 (78) | 14 (78) | 4 (22) | 10 (56) | 34 (83) | 6 (15) | 28 (68) |
| Leukopenia | 18 (78) | — | 18 (78) | 13 (72) | 1 (6) | 12 (67) | 31 (76) | 1 (2) | 30 (73) |
| Anemia | 11 (48) | 11 (48) | — | 7 (39) | 6 (33) | 1 (6) | 18 (44) | 17 (41) | 1 (2) |
| Febrile neutropenia | 4 (17) | 3 (13) | 1 (4) | 2 (11) | 1 (6) | 1 (6) | 6 (15) | 4 (10) | 2 (5) |
| Fever | 2 (9) | 2 (9) | — | — | — | — | 2 (5) | 2 (5) | — |
| Enterocolitis | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Pneumonia | 1 (4) | 1 (4) | — | — | — | — | 1 (2) | 1 (2) | — |
| Sepsis | 1 (4) | 1 (4) | — | — | — | — | 1 (2) | 1 (2) | — |
| Hyperglycemia | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Hyperuricemia | — | — | — | 1 (6) | — | 1 (6) | 1 (2) | — | 1 (2) |
| Infusion related reaction | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Thrombotic thrombocytopenic purpura | 1 (4) | — | 1 (4) | — | — | — | 1 (2) | — | 1 (2) |
| . | DFCI . | WUSTL . | Combined . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Grade 3-4 (n = 23 [56%]) . | Grade 3 . | Grade 4 . | Grade 3-4 (n = 18 [44%]) . | Grade 3 . | Grade 4 . | Grade 3-4 (n = 41) . | Grade 3 . | Grade 4 . |
| Lymphopenia | 21 (91) | — | 21 (91) | 15 (83) | 1 (6) | 14 (78) | 36 (88) | 1 (2) | 35 (85) |
| Thrombocytopenia | 19 (83) | — | 19 (83) | 16 (89) | 2 (11) | 14 (78) | 35 (85) | 2 (5) | 33 (80) |
| Neutropenia | 20 (87) | 2 (9) | 18 (78) | 14 (78) | 4 (22) | 10 (56) | 34 (83) | 6 (15) | 28 (68) |
| Leukopenia | 18 (78) | — | 18 (78) | 13 (72) | 1 (6) | 12 (67) | 31 (76) | 1 (2) | 30 (73) |
| Anemia | 11 (48) | 11 (48) | — | 7 (39) | 6 (33) | 1 (6) | 18 (44) | 17 (41) | 1 (2) |
| Febrile neutropenia | 4 (17) | 3 (13) | 1 (4) | 2 (11) | 1 (6) | 1 (6) | 6 (15) | 4 (10) | 2 (5) |
| Fever | 2 (9) | 2 (9) | — | — | — | — | 2 (5) | 2 (5) | — |
| Enterocolitis | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Pneumonia | 1 (4) | 1 (4) | — | — | — | — | 1 (2) | 1 (2) | — |
| Sepsis | 1 (4) | 1 (4) | — | — | — | — | 1 (2) | 1 (2) | — |
| Hyperglycemia | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Hyperuricemia | — | — | — | 1 (6) | — | 1 (6) | 1 (2) | — | 1 (2) |
| Infusion related reaction | — | — | — | 1 (6) | 1 (6) | — | 1 (2) | 1 (2) | — |
| Thrombotic thrombocytopenic purpura | 1 (4) | — | 1 (4) | — | — | — | 1 (2) | — | 1 (2) |
Data are presented as n (%) of patients.
Seventy-four of 75 patients (99%) in whom stem cell mobilization was attempted successfully collected CD34+ stem cells for ASCT (median 3.6 × 106 cells/kg). Patients required a mean of 1.96 days for collection (median, 2 days; range, 1-6 days). Plerixafor was used for mobilization in 44 out off 75 patients (59%), including 12 out of 21 (57%) in the DFCI trial, 14 out of 14 (100%) in the WUSTL trial, and 18 out of 40 (45%) in the off-trial cohort. Delayed platelet engraftment after ASCT was seen more often in patients receiving alternating cycles of RB/RC compared with sequential RB/RC (day 30 platelets <50 × 109/L, 58% vs 14%, P = .002; day 100 platelets <100 × 109/L, 67% vs 18%, P = .002). An initial cytarabine dose of 3 g/m2 was also associated with impaired platelet recovery (day 30 platelets <50 × 109/L, 38% vs 10%, P = .007).
There were no treatment-related deaths during RB/RC induction. Following ASCT, there were 2 transplant-related deaths. One patient died suddenly from an unknown cause 13 days after ASCT. No autopsy was performed. A second patient succumbed to a respiratory syncytial virus infection and respiratory failure 56 days after ASCT. A third patient was diagnosed with progressive multifocal leukoencephalopathy ∼6 months after ASCT and 4 months after initiating rituximab maintenance and is currently in stable condition.
MRD
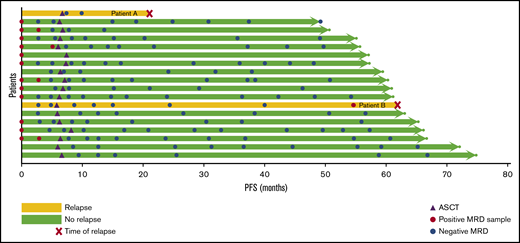
Among 23 patients enrolled in the DFCI trial, tumor clonotypes could be identified for MRD assessment in 20 patients (87%). Eighteen of these patients had blood samples at ≥1 time point for MRD analysis, including 12 samples at baseline, 11 after 3 cycles of RB, 12 at EOI, and 112 at various time points after ASCT. All baseline samples were positive for ctDNA in PBMCs or plasma. MRD was detected in 3 out of 11 (27%) samples after 3 cycles of RB, 1 out of 12 EOI (8%) samples, and 0 out of 15 (0%) samples collected 3 months after ASCT. There was no correlation between interim or EOI MRD positivity and PFS (Figure 4). Among 17 patients with post-ASCT blood samples, 2 patients relapsed (patient A, 21 months after ASCT; and patient B, 62 months after ASCT). Patient B had 5 initial negative MRD samples, but ctDNA was detected in both PBMCs and plasma 7.2 months before clinical relapse. Patient A had 2 MRD assessments, collected 13.5 and 11 months prior to relapse, which were both negative. A sample at the time of relapse was not available for assessment. All other post-ASCT samples have been negative for MRD.
MRD analysis. When feasible, patients in the DFCI trial cohort had samples collected at baseline, after 3 cycles of induction therapy, after 6 cycles of induction therapy, and at various time points following ASCT for MRD analysis using Ig-NGS with ClonoSeq (Adaptive Biotechnologies). In this swimmer’s plot, each horizontal arrow represents an individual patient. Yellow lines denote patients who have relapsed, and the time of relapse is marked by a red x. Green patients are progression-free. Purple triangles represent the timing of ASCT, red circles are MRD-positive samples, and blue circles are MRD-negative samples.
MRD analysis. When feasible, patients in the DFCI trial cohort had samples collected at baseline, after 3 cycles of induction therapy, after 6 cycles of induction therapy, and at various time points following ASCT for MRD analysis using Ig-NGS with ClonoSeq (Adaptive Biotechnologies). In this swimmer’s plot, each horizontal arrow represents an individual patient. Yellow lines denote patients who have relapsed, and the time of relapse is marked by a red x. Green patients are progression-free. Purple triangles represent the timing of ASCT, red circles are MRD-positive samples, and blue circles are MRD-negative samples.
Discussion
In our pooled analysis, RB/RC induction followed by ASCT was associated with high rates of durable remissions in transplant-eligible patients with MCL. Prior studies suggest that deeper remissions before ASCT assessed either radiographically13 or molecularly5,14-17 are associated with improved long-term outcomes. In our study, 90% of patients achieved a CR at the EOI. In addition, MRD testing from a subset of patients suggests that RB/RC induction achieves high rates of MRD negativity that persists for years following ASCT in most patients. High-risk patient groups (high MIPI, blastoid/pleomorphic histology, Ki67 >30%) each achieved CRRs ≥75%, but remissions appeared to be less durable for patients with high MIPI scores and blastoid/pleomorphic histology. Differences in frequencies of these patients appeared to explain the lower response rates and trend toward inferior PFS observed in the WUSTL trial, which included more high-risk patients.
RB/RC induction was generally well tolerated with expected, manageable rates of hematologic and infectious AEs. RB/RC did not impair the successful collection of autologous stem cells for transplantation, but delayed platelet recovery was observed in some patients after ASCT, particularly among those who received 3 g/m2 cytarabine and/or alternating cycles of RB/RC. Since neither higher cytarabine doses nor alternating cycles of RB/RC were associated with improvement in response rates or PFS, we would recommend sequential cycles of RB/RC with 2 g/m2 dosing of cytarabine for clinical practice and for future trials that adopt this regimen.
Cross-trial comparisons are inherently challenging due to differences in patient populations and response assessment. Acknowledging these limitations, the 3-year PFS of 83% compares favorably with that seen with other cytarabine-based induction regimens.5,13,18 Approximately half of the patients in this study were treated outside of a clinical trial. These patients had low rates of treatment discontinuation due to toxicity and achieved similarly durable remissions, suggesting that RB/RC is an effective choice of induction therapy for transplant-eligible patients outside of a clinical trial.
By pooling data from 2 phase 2 trials and a cohort of nontrial patients, we were able to compare safety across different dosing schedules, assess real-world outcomes outside of a clinical trial, and examine outcomes among high-risk patient subsets. However, pooling data from different cohorts has inherent limitations. In this case, the pooled phase 2 trials had different primary end points, dosing schedules, dose modifications, and response assessments. In addition, among patients treated outside of a clinical trial, the starting dose cytarabine was lower than that used among trial patients. These limitations should be considered when interpreting the results, particularly when comparing data across patient cohorts.
While inclusion of off-trial patients provides important information about real-world outcomes with this regimen, it also introduces the possibility of selection bias. In this case, the possibility of bias is minimized by the fact that RB/RC was the institutional standard practice during the study timeframe. Detailed toxicity results from the 2 clinical trials suggest that RB/RC can be delivered safely. While toxicity data for nontrial patients are less robust, limited AE information, rates of treatment discontinuation, treatment-related mortality, and post-ASCT safety were similar in the off-trial cohort. EOI response assessment was not uniform across study cohorts, reflecting the relatively recent implementation of PET as the preferred imaging modality in MCL. This limits our ability to compare across study cohorts, but RB/RC appears to have excellent activity based on either PET or CT imaging. TP53 deletion/mutation testing was not performed for either clinical trial or as part of standard practice for off-trial patients, so we cannot comment on its prognostic significance. Finally, longer follow-up will be helpful in confirming that the favorable outcomes observed with RB/RC are durable.
Because induction and consolidative ASCT are not curative and are associated with significant morbidity, many trials are seeking to improve outcomes by incorporating novel agents, like Bruton tyrosine kinase inhibitors, into first-line regimens. An upcoming US Intergroup randomized trial (EA4181) will determine if the addition of acalabrutinib to an RB/RC-based chemotherapy backbone can improve EOI MRD negativity in untreated patients with MCL. This trial and others are increasingly using MRD as a primary measure of efficacy and a tool to tailor the intensity of frontline treatment. MRD testing using allele-specific oligonucleotide polymerase chain reaction has previously been successfully used to track depth of response following ASCT and initiate pre-emptive treatments in high-risk patients.14,15 In our study, MRD testing using IgNGS (ClonoSeq) was feasible for nearly all patients, had a high negative predictive value, and identified the presence of ctDNA in a relapsing patient 7.2 months prior to clinical recurrence. Future trials should consider mandatory long-term sample collection for MRD assessment to further evaluate the operating characteristics of IgNGS MRD testing following ASCT. While additional events and longer follow-up are necessary, our results suggest that IgNGS could be a useful tool for tracking MRD following ASCT and predicting impending recurrence.
In conclusion, induction therapy with RB/RC followed by ASCT was well tolerated and achieved high rates of durable remissions in 2 phase 2 clinical trials and among a cohort of off-trial patients. RB/RC is therefore an effective option for induction therapy in transplant-eligible patients with MCL.
For data sharing, please e-mail the corresponding author, R.M. (reid_merryman@dfci.harvard.edu).
Acknowledgments
The authors are grateful for the support of DFCI OncDRS database, which helped to identify off-trial patients. They are also indebted to the nursing and research staff involved in these studies and to all the participants and their families.
Partial funding was provided by Otsuka; ClonoSeq testing was provided free of charge by Adaptive/Sequenta. R.W.M. gratefully acknowledges support from an ASH Research Training Award for Fellows, the ASH Clinical Research Training Institute, an ASBMT New Investigator Award, and the LRF Lymphoma Clinical Research Mentoring Program. P.A. gratefully acknowledges the support of the Harold and Virginia Lash Foundation, as well as the Leukemia and Lymphoma Society.
Authorship
Contribution: R.W.M. designed the research, collected and analyzed data, and wrote the manuscript; N.E. designed the research, collected data, and edited the manuscript; R.R. designed the research, analyzed data, and edited the manuscript; N.E., J.B., M.C., A.L., A.F., C.J., D.F., S.N., J.C., A.K., O.O., M.D., J.R.B., A.C., N.L.B., N.M.-S., A.G., and R.J. collected data and edited the manuscript; H.J. collected data, analyzed data, and edited the manuscript; and B.K., P.A., and E.J. designed the research, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: A.L. received consulting fees from Seattle Genetics, DSMB BMS, and Research to Practice for Speakers’ Bureau. J.R.B. has served as a consultant for Abbvie, Acerta, Astra-Zeneca, Beigene, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, Novartis, Octapharma, Pfizer, Pharmacyclics, Sunesis, TG Therapeutics, and Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun, and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. B.K. received consulting fees from Genetech and Roche and research support from Genentech. N.M-S. received consulting fees from Kiowa Hakka Kirin and institutional research funding from Genentech, BMS, Verastem, and Celgene. P.A. received consulting fees from Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, and Miltenyi; research funding (institutional) from Merck, BMS, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, and IGM; and honoraria from Merck and BMS. E.J. received consulting fees from Merck and Acerta; honoraria from Takeda, Astra-Zeneca, and Merck; and research funding from Novartis, Seattle Genetics, Celgene, Merck, Hoffmann-LaRoche, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Reid W. Merryman, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: reid_merryman@dfci.harvard.edu.
References
Author notes
P.A. and E.J. contributed equally to this study.
The full-text version of this article contains a data supplement.