Key Points
SCD is associated with a high incidence of GI bleeding, particularly from the upper GI tract.
There is a twofold increased risk for death in patients with SCD who experience a bleeding event.
Abstract
Bleeding is a known complication of sickle cell disease (SCD) and includes hemorrhagic stroke, hematuria, and vitreous hemorrhage. However, the incidence of bleeding events in patients with SCD has not been well described. We present a retrospective, population-based study examining the cumulative incidence of bleeding in 6423 patients with SCD from 1991 to 2014. We also studied risk factors associated with bleeding and the effects of bleeding on mortality, using Cox proportional hazards regression models. We used California emergency department and hospitalization databases to identify patients with SCD with intracranial hemorrhage, gastrointestinal (GI) bleeding, hemophthalmos, gross hematuria, epistaxis, menorrhagia, and other bleeding events. The cumulative incidence of any first bleeding event at age 40 years was 21% (95% confidence interval [CI], 19.8%-22.3%), increasing with age to 41% by age 60 years (95% CI, 38.8%-43.1%). The majority of bleeding events were GI (41.6%), particularly from the upper GI tract. A higher bleeding risk was associated with increased frequency of hospitalization (hazard ratio [HR], 2.16; 95% CI, 1.93-2.42), venous thromboembolism 180 days before bleeding event (HR, 4.24; 95% CI, 2.86-6.28), osteonecrosis of the femoral head (HR, 1.25; 95% CI, 1.08-1.46), and ischemic stroke (HR, 1.65; 95% CI, 1.20-2.26). Bleeding was also associated with a twofold increased risk for death (HR, 2.09; 95% CI, 1.82-2.41) adjusted for other SCD-related complications. Our novel finding of a high incidence of bleeding in patients with SCD, particularly from the upper GI tract, suggests that patients with SCD may be predisposed to bleeding, with possible etiologies including increased use of nonsteroidal anti-inflammatory drugs, mucosal infarction from vascular occlusion by sickled red blood cells, and increased stress ulceration from frequent hospitalization.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobinopathy resulting in the abnormal polymerization of the β-globin protein and sickling of the red blood cell. Repeated sickling leads to a number of well-recognized acute and chronic complications, including acute chest syndrome, vaso-occlusive pain crises, and osteonecrosis of the femoral head (ONFH). SCD has also been shown to be a hypercoagulable disease state associated with an increased risk for ischemic stroke and venous thromboembolism (VTE).1-3 Although SCD is not associated with known defects in hemostasis, site-specific bleeding complications have been described.
Bleeding in patients with SCD includes neurological, renal, and ocular bleeding complications. The pathophysiology of these bleeding events likely revolves around repeated vascular occlusion and ischemic injury. For example, intracranial hemorrhage (ICH) is often the result of bleeding from abnormal neovascularization and friable vessels, which occurs as a result of arterial stenosis and ischemic stroke, or from cerebral aneurysm formation.4-6 Similarly, vitreous and retinal hemorrhage are also thought to be related to proliferative and nonproliferative retinopathy, respectively, in the setting of vascular occlusion and revascularization.7 Hematuria likewise can be a result of ischemic papillary necrosis and renal capillary rupture.8
Although patients with SCD can experience multiorgan site bleeding, existing reports primarily focus on organ-specific bleeding.4,5,7,8 To our knowledge, the burden of all bleeding events and association with mortality in patients with SCD have not been reported. To better understand the incidence of all bleeding in patients with SCD, we conducted a retrospective study of both pediatric and adult patients with SCD in California, with the aims of describing bleeding types, risk factors for bleeding, and the association of bleeding with mortality in these patients.
Methods
Cohort definition
The SCD cohort was identified using longitudinal records from the California Patient Discharge Data (PDD) and the Emergency Department Utilization (EDU) databases from the Office of Statewide Health Planning and Development, as previously reported.9-13 Since July 1990, the State of California has required that nonfederal hospitals report up to 25 diagnoses and up to 21 procedures associated with each hospitalization, coded using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). Since 2005, the EDU database of all hospital-associated emergency department (ED) encounters has also been mandated. In addition to diagnostic and procedure information, patient demographic information including age, sex, race/ethnicity, and insurance coverage is collected. An encrypted form of the social security number, called the record linkage number, is used to identify unique individuals, allowing serial linking of multiple hospitalization records over time. This administrative database does not contain laboratory or medication information. Data from 1991 to 2014 were used in this study.
As done previously, patients with SCD were identified using specific ICD-9-CM codes (282.41, 282.42, 282.60, 282.61, 282.62, 282.63, 282.64, 282.68, 282.69) for all hospitalizations available in the California PDD and EDU databases.9-14 To be included in the SCD cohort, patients had to meet 1 of the following criteria: 2 separate admissions with SCD as the principal diagnosis, or 1 admission with SCD as the principal diagnosis and 2 additional admissions with SCD as a secondary diagnosis. To increase specificity, all patients had to be younger than 65 years at entry into the cohort. This study was approved by the California Health and Human Services Agency’s Committee for the Protection of Human Subjects and the University of California, Davis, Human Research Protections Program.
Covariates
Sex, age at cohort entry, and race/ethnicity were determined from first hospitalization (PDD or EDU). Patients with Hispanic or Latino ethnicity were categorized as Hispanic. All other patients were categorized as non-Hispanic white, non-Hispanic African American, non-Hispanic Asian/Pacific Islander, and other/unknown. Frequency of hospitalization was defined using a patient’s average number of visits per year for any cause; patients with 3 or more visits per year were defined as frequent users, as this number of hospitalizations has previously been shown to be associated with an increase in mortality.9-13 For this analysis, we excluded visits with bleeding as the principal diagnosis from calculation of hospitalization frequency. Patients with fewer than 3 visits per year were defined as less frequent users. SCD-related complications, including ONFH,9 ischemic stroke, VTE including upper extremity (UE) deep vein thrombosis,12,13 and pneumonia/acute chest syndrome (ACS; coding began in 2003), were included as covariates (supplemental Table 1). Pneumonia/ACS was defined as any admission with pneumonia in the principal position of an inpatient admission, an SCD code in the principal position and pneumonia in the second position of an inpatient admission, or ACS in any diagnostic position of PDD or EDU from 2003 on. We used this combination because ACS was likely coded as pneumonia before 2003, and it is clinically difficult to differentiate the 2 entities. The Elixhauser comorbidities15 for chronic renal failure and liver failure were also included as covariates. Each SCD complication was included as a time-dependent covariate at first time it was coded for each patient.
Outcome variables
The primary outcome was incident bleeding. First admission (PDD or EDU) with a bleeding code was captured using specific ICD-9-CM codes (supplemental Table 2). Bleeding events included ICH, gastrointestinal (GI) bleeding, hemophthalmos, gross hematuria, epistaxis, menorrhagia, and other bleeding (hemarthrosis, hemoptysis, hemopericardium, and hemorrhage not otherwise specified). Bleeding events in the same admission as a trauma event were excluded. Mortality from all causes was included as a secondary outcome. Death data were obtained from hospitalization and California vital records linkage through 31 December 2013.
Statistical analysis
Univariate descriptive statistics using χ2 test were used to describe characteristics of the SCD cohort. The cumulative incidence functions of first overall bleeding event and first of each bleeding type by location were calculated using age as the scale, adjusted for the competing risk for death. The first bleeding event in each anatomic location was included, allowing for patients to have multiple bleeding events to be included in our analysis. Rates of bleeding were also calculated per 100 000 patient-years of follow-up. Gray’s K-sample test statistic was used to determine whether cumulative incidence of bleeding event differed by frequency of hospitalization.16
Multivariable Cox proportional hazards regression models were used to analyze risk factors associated with any bleeding, ICH, and GI bleeding, adjusted for the competing risk for death. To determine the effect of any bleeding on death from all causes, we used multivariable Cox proportional hazards regression models. Multivariable models were adjusted for baseline characteristics (sex, race/ethnicity, hospitalization frequency, and SCD complications). Event time was calculated from date of first entry into the SCD cohort to date of first bleeding, date of death, or the study cutoff date (31 December 2014), whichever occurred first. SCD complications (ONFH, ischemic stroke, pneumonia/ACS, VTE, renal failure, and liver failure) and bleeding (in mortality model) were included as time-dependent covariates. For all regression analyses, the proportional hazard assumption was assessed using Schoenfeld residuals.17 All covariates included met the proportional hazard assumption.
Results
From 1991 to 2014, 6423 patients with SCD were identified (Table 1). Of these, 1345 patients (20.9%) had an incident bleeding event. First bleeding events included GI bleeding (41.6%), epistaxis (19.1%), menorrhagia (15.1%), ICH (10.2%), hemophthalmos (5.1%), gross hematuria (2.6%), and other bleeds (6.3%) (Table 2). Excluding menorrhagia, there were no significant differences in sex among patients who did and did not have a bleeding event. The majority of patients (63.5%) with a bleeding event were frequently hospitalized. The median duration of follow-up was 215.7 months (95% confidence interval [CI], 212.5-219.8 months), calculated using reverse Kaplan Meier methodology.18
Baseline characteristics of sickle cell disease patients in California, 1991 to 2014
| Variables . | All . | Bleeding . | No bleeding . | P . | |||
|---|---|---|---|---|---|---|---|
| N . | % . | n . | % . | n . | % . | ||
| All | 6423 | 100.0 | 1345 | 100.0 | 5078 | 100.0 | |
| Sex | |||||||
| Male | 3041 | 47.3 | 537 | 39.9 | 2504 | 49.3 | <.0001 |
| Female | 3382 | 52.7 | 808 | 60.1 | 2574 | 50.7 | <.0001 |
| Race/ethnicity | |||||||
| African American | 5767 | 89.8 | 1241 | 92.3 | 4526 | 89.1 | .0007 |
| Non–African American | 656 | 10.2 | 104 | 7.7 | 552 | 10.9 | .0007 |
| Hospitalization frequency* | |||||||
| Less frequent | 3639 | 56.7 | 491 | 36.5 | 3148 | 62.0 | <.0001 |
| Frequent | 2784 | 43.3 | 854 | 63.5 | 1930 | 38.0 | <.0001 |
| SCD complications before bleeding | |||||||
| Incident VTE or UE DVT | 695 | 10.8 | 193 | 14.3 | 502 | 9.9 | <.0001 |
| Pneumonia/ACS | 3633 | 56.6 | 731 | 54.3 | 2902 | 57.1 | 0.0656 |
| ACS* | 1639 | 25.5 | 271 | 20.1 | 1368 | 26.9 | <.0001 |
| ONFH | 1256 | 19.6 | 292 | 21.7 | 964 | 19.0 | 0.025 |
| Ischemic stroke | 228 | 3.5 | 61 | 4.5 | 167 | 3.3 | 0.028 |
| Renal failure | 480 | 7.5 | 150 | 11.2 | 330 | 6.5 | <.0001 |
| Liver failure | 517 | 8.0 | 164 | 12.2 | 353 | 7.0 | <.0001 |
| Deceased (as of 31 December 2013) | 1096 | 17.1 | 412 | 30.6 | 684 | 13.5 | <.0001 |
| Variables . | All . | Bleeding . | No bleeding . | P . | |||
|---|---|---|---|---|---|---|---|
| N . | % . | n . | % . | n . | % . | ||
| All | 6423 | 100.0 | 1345 | 100.0 | 5078 | 100.0 | |
| Sex | |||||||
| Male | 3041 | 47.3 | 537 | 39.9 | 2504 | 49.3 | <.0001 |
| Female | 3382 | 52.7 | 808 | 60.1 | 2574 | 50.7 | <.0001 |
| Race/ethnicity | |||||||
| African American | 5767 | 89.8 | 1241 | 92.3 | 4526 | 89.1 | .0007 |
| Non–African American | 656 | 10.2 | 104 | 7.7 | 552 | 10.9 | .0007 |
| Hospitalization frequency* | |||||||
| Less frequent | 3639 | 56.7 | 491 | 36.5 | 3148 | 62.0 | <.0001 |
| Frequent | 2784 | 43.3 | 854 | 63.5 | 1930 | 38.0 | <.0001 |
| SCD complications before bleeding | |||||||
| Incident VTE or UE DVT | 695 | 10.8 | 193 | 14.3 | 502 | 9.9 | <.0001 |
| Pneumonia/ACS | 3633 | 56.6 | 731 | 54.3 | 2902 | 57.1 | 0.0656 |
| ACS* | 1639 | 25.5 | 271 | 20.1 | 1368 | 26.9 | <.0001 |
| ONFH | 1256 | 19.6 | 292 | 21.7 | 964 | 19.0 | 0.025 |
| Ischemic stroke | 228 | 3.5 | 61 | 4.5 | 167 | 3.3 | 0.028 |
| Renal failure | 480 | 7.5 | 150 | 11.2 | 330 | 6.5 | <.0001 |
| Liver failure | 517 | 8.0 | 164 | 12.2 | 353 | 7.0 | <.0001 |
| Deceased (as of 31 December 2013) | 1096 | 17.1 | 412 | 30.6 | 684 | 13.5 | <.0001 |
DVT, deep vein thrombosis.
Hospitalization frequency: patients with SCD with an average of ≥3 visits per year (inpatient or ED) were defined as frequent; patients with an average of <3 visits per year were defined as less frequent. #ACS code available from 2003 onward.
Characteristics of bleeds by location among sickle cell disease patients in California, 1991 to 2014
| Variables . | ICH . | GI . | Menorrhagia . | Epistaxis . | Hemophthalmos . | Gross hematuria . | Other . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| All* | 166 | 2.6 | 669 | 10.4 | 243 | 7.2 | 338 | 5.3 | 80 | 1.2 | 49 | 0.8 | 122 | 1.9 |
| Hospitalization frequency | ||||||||||||||
| Less frequent | 72 | 43.4 | 197 | 29.4 | 82 | 33.7 | 118 | 34.9 | 40 | 50.0 | 16 | 32.7 | 36 | 29.5 |
| Frequent | 94 | 56.6 | 472 | 70.6 | 161 | 66.3 | 220 | 65.1 | 40 | 50.0 | 33 | 67.3 | 86 | 70.5 |
| Bleeding present on admission | ||||||||||||||
| Yes | 112 | 67.5 | 359 | 53.7 | 174 | 71.6 | 122 | 36.1 | 46 | 57.5 | 29 | 59.2 | 67 | 54.9 |
| No | 28 | 16.9 | 73 | 10.9 | 4 | 1.6 | 106 | 31.4 | 2 | 2.5 | 9 | 18.4 | 23 | 18.9 |
| Unknown (prior to 1996) | 23 | 13.9 | 112 | 16.7 | 26 | 10.7 | 29 | 8.6 | 14 | 17.5 | — | — | 16 | 13.1 |
| ED | 3 | 1.8 | 125 | 18.7 | 39 | 16.0 | 81 | 24.0 | 18 | 22.5 | 11 | 22.4 | 16 | 13.1 |
| Bleed admission type | ||||||||||||||
| PDD | 163 | 98.2 | 544 | 81.3 | 204 | 84.0 | 257 | 76.0 | 62 | 77.5 | 38 | 77.6 | 106 | 86.9 |
| ED | 3 | 1.8 | 125 | 18.7 | 39 | 16.0 | 81 | 24.0 | 18 | 22.5 | 11 | 22.4 | 16 | 13.1 |
| Age of first bleed, y | ||||||||||||||
| Mean age (standard deviation) | 35.9 (15.8) | 35.9 (14.9) | 33.8 (9.3) | 29.6 (16.2) | 37.2 (11.0) | 26.8 (13.1) | 32.2 (13.1) | |||||||
| Median age (25th, 75th percentiles) | 37 (23, 48) | 35 (24, 47) | 35 (26, 41) | 27 (17, 42) | 38 (30.5, 45.5) | 24 (19, 34) | 31 (23, 39) | |||||||
| Variables . | ICH . | GI . | Menorrhagia . | Epistaxis . | Hemophthalmos . | Gross hematuria . | Other . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| All* | 166 | 2.6 | 669 | 10.4 | 243 | 7.2 | 338 | 5.3 | 80 | 1.2 | 49 | 0.8 | 122 | 1.9 |
| Hospitalization frequency | ||||||||||||||
| Less frequent | 72 | 43.4 | 197 | 29.4 | 82 | 33.7 | 118 | 34.9 | 40 | 50.0 | 16 | 32.7 | 36 | 29.5 |
| Frequent | 94 | 56.6 | 472 | 70.6 | 161 | 66.3 | 220 | 65.1 | 40 | 50.0 | 33 | 67.3 | 86 | 70.5 |
| Bleeding present on admission | ||||||||||||||
| Yes | 112 | 67.5 | 359 | 53.7 | 174 | 71.6 | 122 | 36.1 | 46 | 57.5 | 29 | 59.2 | 67 | 54.9 |
| No | 28 | 16.9 | 73 | 10.9 | 4 | 1.6 | 106 | 31.4 | 2 | 2.5 | 9 | 18.4 | 23 | 18.9 |
| Unknown (prior to 1996) | 23 | 13.9 | 112 | 16.7 | 26 | 10.7 | 29 | 8.6 | 14 | 17.5 | — | — | 16 | 13.1 |
| ED | 3 | 1.8 | 125 | 18.7 | 39 | 16.0 | 81 | 24.0 | 18 | 22.5 | 11 | 22.4 | 16 | 13.1 |
| Bleed admission type | ||||||||||||||
| PDD | 163 | 98.2 | 544 | 81.3 | 204 | 84.0 | 257 | 76.0 | 62 | 77.5 | 38 | 77.6 | 106 | 86.9 |
| ED | 3 | 1.8 | 125 | 18.7 | 39 | 16.0 | 81 | 24.0 | 18 | 22.5 | 11 | 22.4 | 16 | 13.1 |
| Age of first bleed, y | ||||||||||||||
| Mean age (standard deviation) | 35.9 (15.8) | 35.9 (14.9) | 33.8 (9.3) | 29.6 (16.2) | 37.2 (11.0) | 26.8 (13.1) | 32.2 (13.1) | |||||||
| Median age (25th, 75th percentiles) | 37 (23, 48) | 35 (24, 47) | 35 (26, 41) | 27 (17, 42) | 38 (30.5, 45.5) | 24 (19, 34) | 31 (23, 39) | |||||||
First of each bleeding type by location; patients can have multiple bleeds.
Percentage of all patients with SCD.
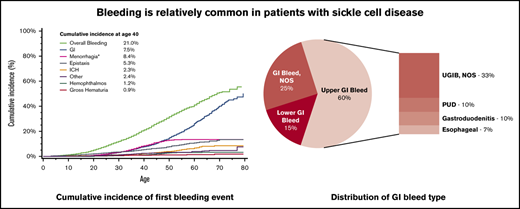
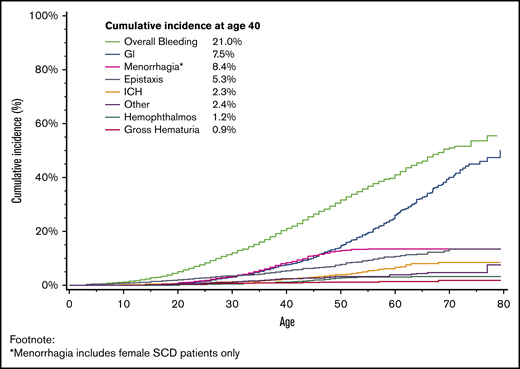
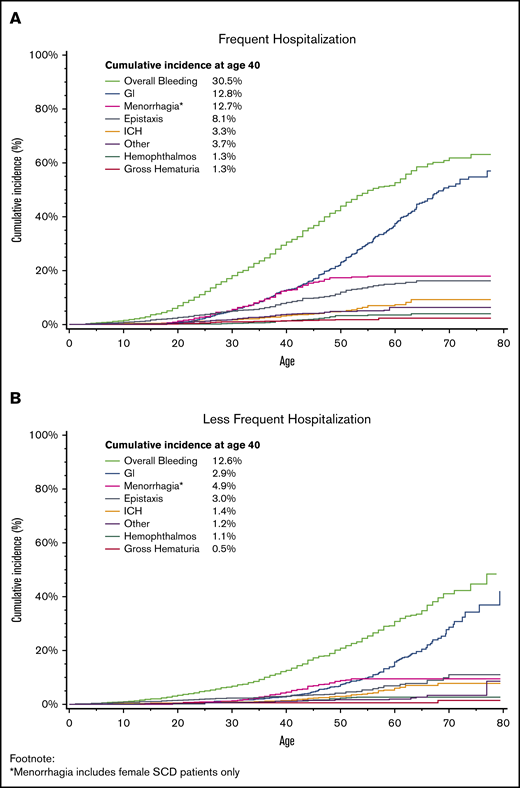
The mean age at first bleeding event was 32.9 years (standard deviation, ±14.6 years). The cumulative incidence of a first bleeding event by age 40 years was 21.0% (Figure 1; 95% CI, 19.8%-22.3%), with an incidence rate of 1603 per 100 000 person-years. The 2 most common bleeding events were GI and menorrhagia, with 7.5% of patients experiencing a GI bleed by age 40 years (95% CI, 6.7%-8.4%) and 8.4% of women with menorrhagia by age 40 years (95% CI, 7.2%-9.6%). The cumulative incidence of all bleeding increased with age, rising to 41.0% by age 60 years (95% CI, 38.8%-43.1%); 26.0% (95% CI, 23.9%-28.1%) of all patients with SCD had a GI bleed, and 13.4% (95% CI, 11.8%-15.1%) of female patients with SCD had menorrhagia by age 60 years. This was also seen in ICH, as 2.27% of patients had experienced an incident ICH by age 40 years (95% CI, 1.8%-2.8%), increasing to 6.6% by age 60 years (95% CI, 5.4%-7.9%). The cumulative incidence of a first bleed differed by frequency of hospitalization (Figure 2), with 30.5% (95% CI, 28.5%-32.6%) of patients with frequent hospitalization and 12.6% (95% CI, 11.3%-14.1%) with less frequent hospitalization experiencing an incident bleed by age 40 years. This effect was also seen with GI bleeding, with 12.8% (95% CI, 11.3%-14.4%) of patients with frequent hospitalization and 2.9% (95% CI, 2.2%-3.6%) of patients with less frequent hospitalization having a GI bleed by age 40 years.
Cumulative incidence of first bleeding event, adjusted for the competing risk for death, among patients with SCD in California, 1991 to 2014.
Cumulative incidence of first bleeding event, adjusted for the competing risk for death, among patients with SCD in California, 1991 to 2014.
Cumulative incidence of first bleeding event by frequency of hospitalization, adjusted for the competing risk for death, among patients with SCD in California, 1991 to 2014.
Cumulative incidence of first bleeding event by frequency of hospitalization, adjusted for the competing risk for death, among patients with SCD in California, 1991 to 2014.
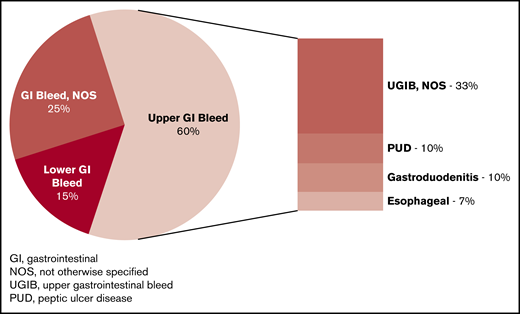
Of the 669 patients with a first-time GI bleed, 401 (60%) had an upper GI bleed. Among the upper GI bleeds, 17.2% had peptic ulcer disease (PUD), 17.0% had gastroduodenitis, 10.7% had esophageal bleeds, and 55% did not have a specific diagnosis (ICD-9 code of hematemesis or melena; Figure 3). Ninety-eight of the 669 patients (14.7%) with GI bleed had an unspecified lower GI bleed, all with ICD-9 code “rectal and anal hemorrhage.” No patients had a diverticular bleed as their incident bleeding event. The incidence rate of any GI bleed was 751.7/100 000 person-years, whereas the rate was 450.5/100 000 person-years for an upper GI bleed and 110.1/100 000 for a lower GI bleed. The 166 patients with nontraumatic ICH had diagnoses of intracerebral hemorrhage (47%), subarachnoid hemorrhage (30.1%), subdural hemorrhage (10.8%), extradural hemorrhage (3.0%), and ICH not otherwise specified (9%). Six of the 166 patients (3.6%) with ICH had an ICD-9 code for “cerebral aneurysm” during the same admission or in a prior admission. Five of these 6 patients with a cerebral aneurysm had a subarachnoid hemorrhage.
Distribution of GI bleeding types among California patients with SCD, 1991 to 2014.
Distribution of GI bleeding types among California patients with SCD, 1991 to 2014.
We determined the location and frequency of subsequent bleeds after an index bleeding event (supplemental Table 3). Of the 1345 patients with an initial bleeding event, 333 (24.8%) went on to have another bleed in the same location as the incident bleed. Thirty-five percent of patients with menorrhagia went on to experience a subsequent episode of menorrhagia. Gastrointestinal bleeds and hemophthalmos were the next most likely to have a subsequent bleed in the same organ system, with 32% of patients with GI and hemophthalmos experiencing another GI or ocular bleed. Approximately 9% of patients with an index ICH had a recurrent ICH.
Using Cox proportional hazards models, we identified several risk factors associated with any first bleeding event, first ICH, and first GI bleed (Table 3). Frequent hospitalization increased the risk for all bleeds (hazard ratio [HR], 2.16; 95% CI, 1.93-2.42) and GI bleeds (HR, 2.73; 95% CI, 2.29-3.26). A VTE within 180 days of the bleeding event, but not a VTE more than 180 days before the bleeding event, was associated with an increased risk for all bleeds (HR, 4.24; 95% CI, 2.86-6.28), ICH, menorrhagia, epistaxis, and GI bleeding. The strongest associations between bleeding and VTE within 180 days were with epistaxis and menorrhagia, with HRs of 6.00 (95% CI, 3.18-11.34) and 5.10 (95% CI, 2.25-11.57), respectively (data not shown in tables). An incident ICH was strongly associated with a prior ischemic stroke (HR, 6.63; 95% CI, 4.06-10.82). An association between ONFH and GI bleeding was also noted (HR, 1.25; 95% CI, 1.02-1.54; Table 3).
Risk factors associated with first bleeding event among patients with SCD in California, 1991 to 2014
| Variables . | Any bleeding . | Intracranial hemorrhage . | Gastrointestinal bleed . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Sex | |||||||||
| Female | 1.26 | 1.13-1.42 | <.0001 | 0.81 | 0.60-1.11 | .193 | 1.00 | 0.86-1.18 | .954 |
| Male | Reference | Reference | Reference | ||||||
| Race/ethnicity | |||||||||
| African American | Reference | ||||||||
| Non–African American | 0.92 | 0.75-1.12 | .383 | 0.65 | 0.33-1.26 | .2035 | 1.07 | 0.80-1.42 | .6481 |
| Hospitalization frequency | |||||||||
| Less frequent | Reference | Reference | Reference | ||||||
| Frequent | 1.87 | 1.67-2.09 | <.0001 | 1.17 | 0.85-1.62 | .332 | 2.16 | 1.82-2.56 | <.0001 |
| SCD complications | |||||||||
| Venous thrombosis/upper extremity* | |||||||||
| No | Reference | Reference | Reference | ||||||
| VTE <180 d prior of bleeding | 4.24 | 2.86-6.28 | <.0001 | 4.82 | 1.72-13.45 | .0027 | 3.19 | 1.80-5.66 | <.0001 |
| VTE ≥180 d prior of bleeding | 1.41 | 1.16-1.72 | .0006 | 1.14 | 0.68-1.91 | .6188 | 1.21 | 0.92-1.58 | .1709 |
| Prior bleeding* | |||||||||
| Yes | NA | 0.91 | 0.56-1.47 | .6946 | 1.62 | 1.26-2.09 | .0002 | ||
| No | NA | Reference | Reference | ||||||
| ONFH* | |||||||||
| Yes | 1.25 | 1.08-1.46 | .0034 | 0.81 | 0.52-1.26 | .3425 | 1.25 | 1.02-1.54 | .0345 |
| No | Reference | Reference | Reference | ||||||
| Ischemic stroke* | |||||||||
| Yes | 1.65 | 1.20-2.26 | .0022 | 6.63 | 4.06-10.82 | <.0001 | 1.25 | 0.83-1.89 | .2882 |
| No | Reference | Reference | Reference | ||||||
| Pneumonia/ACS* | |||||||||
| Yes | 1.23 | 1.09-1.40 | .0012 | 1.47 | 1.02-2.11 | .0385 | 1.23 | 1.03-1.47 | .0197 |
| No | Reference | Reference | Reference | ||||||
| Renal failure* | |||||||||
| Yes | 2.18 | 1.76-2.70 | <.0001 | 2.31 | 1.31-4.06 | .0038 | 2.23 | 1.71-2.90 | <.0001 |
| No | Reference | Reference | Reference | ||||||
| Liver failure* | |||||||||
| Yes | 1.82 | 1.50-2.22 | <.0001 | 1.72 | 1.04-2.85 | .0347 | 1.92 | 1.49-2.47 | <.0001 |
| No | Reference | Reference | Reference | ||||||
| Variables . | Any bleeding . | Intracranial hemorrhage . | Gastrointestinal bleed . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Sex | |||||||||
| Female | 1.26 | 1.13-1.42 | <.0001 | 0.81 | 0.60-1.11 | .193 | 1.00 | 0.86-1.18 | .954 |
| Male | Reference | Reference | Reference | ||||||
| Race/ethnicity | |||||||||
| African American | Reference | ||||||||
| Non–African American | 0.92 | 0.75-1.12 | .383 | 0.65 | 0.33-1.26 | .2035 | 1.07 | 0.80-1.42 | .6481 |
| Hospitalization frequency | |||||||||
| Less frequent | Reference | Reference | Reference | ||||||
| Frequent | 1.87 | 1.67-2.09 | <.0001 | 1.17 | 0.85-1.62 | .332 | 2.16 | 1.82-2.56 | <.0001 |
| SCD complications | |||||||||
| Venous thrombosis/upper extremity* | |||||||||
| No | Reference | Reference | Reference | ||||||
| VTE <180 d prior of bleeding | 4.24 | 2.86-6.28 | <.0001 | 4.82 | 1.72-13.45 | .0027 | 3.19 | 1.80-5.66 | <.0001 |
| VTE ≥180 d prior of bleeding | 1.41 | 1.16-1.72 | .0006 | 1.14 | 0.68-1.91 | .6188 | 1.21 | 0.92-1.58 | .1709 |
| Prior bleeding* | |||||||||
| Yes | NA | 0.91 | 0.56-1.47 | .6946 | 1.62 | 1.26-2.09 | .0002 | ||
| No | NA | Reference | Reference | ||||||
| ONFH* | |||||||||
| Yes | 1.25 | 1.08-1.46 | .0034 | 0.81 | 0.52-1.26 | .3425 | 1.25 | 1.02-1.54 | .0345 |
| No | Reference | Reference | Reference | ||||||
| Ischemic stroke* | |||||||||
| Yes | 1.65 | 1.20-2.26 | .0022 | 6.63 | 4.06-10.82 | <.0001 | 1.25 | 0.83-1.89 | .2882 |
| No | Reference | Reference | Reference | ||||||
| Pneumonia/ACS* | |||||||||
| Yes | 1.23 | 1.09-1.40 | .0012 | 1.47 | 1.02-2.11 | .0385 | 1.23 | 1.03-1.47 | .0197 |
| No | Reference | Reference | Reference | ||||||
| Renal failure* | |||||||||
| Yes | 2.18 | 1.76-2.70 | <.0001 | 2.31 | 1.31-4.06 | .0038 | 2.23 | 1.71-2.90 | <.0001 |
| No | Reference | Reference | Reference | ||||||
| Liver failure* | |||||||||
| Yes | 1.82 | 1.50-2.22 | <.0001 | 1.72 | 1.04-2.85 | .0347 | 1.92 | 1.49-2.47 | <.0001 |
| No | Reference | Reference | Reference | ||||||
*Adjusted for competing risk for death, age, and year at entry into the SCD cohort.
Incident bleeding was found to be an independent predictor of mortality (HR, 2.10; 95% CI, 1.82-2.43), after adjusting for other previously shown risk factors for mortality in SCD (Table 4). Of the individual types of bleeding, ICH was more strongly associated with mortality (HR, 2.79; 95% CI, 2.17-3.59) than other bleeding types (data not shown in tables), although GI bleeding also had an association with mortality (HR, 1.74; 95% CI, 1.48-2.05). The in-hospital case fatality rate for ICH was 24.7% (95% CI, 18.1-31.3), whereas GI bleeding had a case fatality rate of 5.68% (95% CI, 3.9-7.4). There was no increased risk for mortality noted with menorrhagia, hemophthalmos, or hematuria.
Predictors of mortality from all causes among California patients with SCD, 1991 to 2014
| Variables . | HR . | 95% CI . | P . |
|---|---|---|---|
| SCD complications | |||
| Bleeding* | |||
| Yes | 2.09 | 1.82-2.41 | <.0001 |
| No | Reference | ||
| Ischemic stroke* | |||
| Yes | 2.41 | 1.92-3.02 | <.0001 |
| No | Reference | ||
| VTE/UE* | |||
| Yes | 2.16 | 1.85-2.53 | <.0001 |
| No | Reference | ||
| Pneumonia/ACS* | |||
| Yes | 1.99 | 1.72-2.29 | <.0001 |
| No | Reference | ||
| ONFH* | |||
| Yes | 1.38 | 1.20-1.58 | <.0001 |
| No | Reference | ||
| Renal failure* | |||
| Yes | 5.21 | 4.45-6.10 | <.0001 |
| No | Reference | ||
| Liver failure* | |||
| Yes | 3.20 | 2.76-3.71 | <.0001 |
| No | Reference | ||
| Sex | |||
| Female | 0.96 | 0.85-1.09 | .5498 |
| Male | Reference | ||
| Race/ethnicity | |||
| African American | Reference | ||
| Non–African American | 1.02 | 0.80-1.29 | .8876 |
| Variables . | HR . | 95% CI . | P . |
|---|---|---|---|
| SCD complications | |||
| Bleeding* | |||
| Yes | 2.09 | 1.82-2.41 | <.0001 |
| No | Reference | ||
| Ischemic stroke* | |||
| Yes | 2.41 | 1.92-3.02 | <.0001 |
| No | Reference | ||
| VTE/UE* | |||
| Yes | 2.16 | 1.85-2.53 | <.0001 |
| No | Reference | ||
| Pneumonia/ACS* | |||
| Yes | 1.99 | 1.72-2.29 | <.0001 |
| No | Reference | ||
| ONFH* | |||
| Yes | 1.38 | 1.20-1.58 | <.0001 |
| No | Reference | ||
| Renal failure* | |||
| Yes | 5.21 | 4.45-6.10 | <.0001 |
| No | Reference | ||
| Liver failure* | |||
| Yes | 3.20 | 2.76-3.71 | <.0001 |
| No | Reference | ||
| Sex | |||
| Female | 0.96 | 0.85-1.09 | .5498 |
| Male | Reference | ||
| Race/ethnicity | |||
| African American | Reference | ||
| Non–African American | 1.02 | 0.80-1.29 | .8876 |
Cox model is stratified by entry year and adjusted for age at entry.
Included as time-dependent covariate.
Discussion
This report, to our knowledge, is the first study of the incidence of bleeding events in patients with SCD. We found a 21.0% cumulative incidence of any bleeding by age 40 years, with GI bleeding being the most common (41.6% of bleeding events). Risk factors for bleeding included recent VTE, prior ischemic stroke, frequent hospitalization, and ONFH. In addition, bleeding was associated with a twofold increased risk for mortality, with each individual bleeding type associated with increased mortality with the exception of menorrhagia, hemophthalmos, and hematuria. Incidence of bleeding nearly doubled as patients aged, suggesting that bleeding in SCD is a result of accumulated organ damage rather than an inherent bleeding risk
Patients with SCD have a high incidence rate of GI bleeding, estimated at 751.5/100 000 person-years. The incidence rate of upper GI bleeds, the most common type of GI bleeding in our study, was 450.5/100 000 person-years. Estimates of the incidence of upper GI bleeds in hospitalized patients from the general population range from 102/100 000 person-years in 199119 to from 47 to 60.6/100 000 person-years more recently.20-22 Thus, patients with SCD appear to have more than a fourfold increased risk for upper GI bleed compared with the general hospitalized population. The incidence rate of lower GI bleeds was 110.1/100 000 in our study compared with a rate of from 20.5 to 35.7/100 000 person-years in the general hospitalized population,20,21,23 representing a potential three- to fivefold higher incidence in patients with SCD compared with the general population. It is important to note that our study included ED visits in addition to inpatient admissions, although 81% of patients with a GI bleed were hospitalized. In 69% of these admissions, GI bleeding occupied 1 of the top 3 hospital discharge diagnoses.
No prior studies, to our knowledge, have considered the incidence of GI bleeding in patients with SCD. There have been a small number of case reports of GI bleeding in patients with SCD, with etiologies including hemorrhagic duodenal ulcer,24 Helicobacter pylori–associated peptic ulcer disease,25 and variceal bleeding.26,27 In one of our previous studies, 2.2% of patients experienced GI bleeding after incident VTE, which was attributed, in part, to use of anticoagulation.12
Of the GI bleeds in our study population, 60% were from an upper GI source, with the most common etiologies being PUD and gastroduodenitis (each accounting for 10% of all GI bleeds). The prevalence of PUD in patients with SCD has previously been estimated to be between 3.0% and 7.7%.28,29 One proposed etiology for PUD in patients with SCD is mucosal infarction resulting from vascular congestion with sickled red blood cells, as this was demonstrated via histology in 2 case studies of duodenal ulcer and intestinal necrosis in SCD.24,30 Other potential explanations for the high incidence of upper GI bleeds include frequent nonsteroidal anti-inflammatory drug (NSAID) usage in patients with SCD. NSAIDs increase the risk for both upper GI bleed (odds ratio, 1.92),21 and PUD specifically (odds ratio, 2.21),31 in the general population. Because NSAIDs are a recommended modality of pain control for both vaso-occlusive pain crises and ONFH,32 increased usage of these medications may predispose patients with SCD to developing ulcers and bleeding. Patients with SCD may also be at risk for stress ulceration, given frequent hospitalizations for vaso-occlusive pain crises.33,34 It is likely that these multiple risk factors in combination create a high risk for upper GI bleeding in patients with SCD and partially explains the association with hospitalization frequency and ONFH.
We also demonstrate the incidence of ICH to be 178.9 per 100 000 person-years. Although this incidence is not directly comparable to prior studies in patients with SCD, which are stratified by age group, it appears to be consistent with existing studies. The Cooperative Study of Sickle Cell Disease estimates an incidence of 170/100 000 person-years in patients younger than 10 years, and 440/100 000 person-years in ages 20 to 29 years.1 Another study also using the California PDD estimated incidences of 32/100 000 person-years in pediatric patients, 330/100 000 in patients aged 35 to 64 years, and 1100/100 000 in adults aged 65 years and older.4 The Baltimore-Washington Cooperative Stroke Study estimated an incidence of 47.5/100 000 person-years in patients aged 1 to 14 years.5 Previously noted risk factors for ICH include renal disease, coagulopathy, hypertension, prior transfusion, steroid use, low steady-state hemoglobin, and high leukocyte count.1,4,35 We also report an association between renal disease and ICH, although we were unable to assess for the other aforementioned risk factors. We additionally found that prior ischemic stroke is predictive of future ICH. Although Cook et al did not find an association of hemorrhagic stroke with a prior ischemic stroke,33 this study was performed in pediatric patients, and it is possible the association between the 2 stroke types arises as patients age, given other studies showing the highest incidence of hemorrhagic stroke in adults.1,4 Ischemic stroke may be linked to ICH as a consequence of progressive central nervous system vasculopathy, stenosis, and cerebral infarction years after the incident ischemic event.36 It is also of note that 8.8% of patients with an initial ICH went on to have a subsequent ICH.
Menorrhagia is a common complaint in female patients with SCD, with a cumulative incidence of 8.4% by age 40 years and 13.4% by age 60 years in female patients. Prior studies evaluating menstrual bleeding in patients with SCD have had mixed results, with some showing higher incidence of heavy menses while others show no difference compared with women without SCD.37-39 Although we do not know whether the patients in this study met the formal definition of menorrhagia (>80 mL of blood loss per cycle, or lasting longer than 7 days), 84% of patients with menorrhagia were admitted to the hospital from the ED. Of these patients, 44% had menorrhagia coded as the principal, second, or third diagnosis for their hospitalization, suggesting the bleeding was clinically significant enough to warrant admission. It is unclear what contributes to menorrhagia in patients with SCD.
A VTE within 180 days of bleed was also a risk factor for bleeding, which we interpret to be the result of anticoagulation. This was similarly seen in a study by Patel et al, in which 15% of patients with SCD undergoing treatment of VTE experienced a clinically significant bleed.40 This issue is of considerable clinical importance, given the well-established increased incidence of VTE in patients with SCD.2,12,41 The decreased incidence of bleeding (particularly ICH) with use of direct oral anticoagulants in treating VTE among patients with SCD should be noted in consideration for anticoagulation selection.40
A bleeding event was independently associated with a twofold increase in mortality in patients with SCD. Although increased mortality may be related to the bleeding event itself, it is also possible that bleeding events are more likely in patients with SCD with more complications that are more casually related to death. However, this increased association was maintained even after adjusting for other complications of SCD, including ACS, VTE, organ failure, and ischemic stroke.
Several limitations to this study exist. The identification of bleeding events was dependent on accurate ICD-9 coding, creating the possibility of coding inaccuracies. As a result of a lack of laboratory data available in the patient database, the severity of some bleeding events is not known. However, the majority of patients in all bleeding categories were admitted to the hospital, suggesting a significant enough bleed warranting observation and/or treatment. For example, 81% of patients with a GI bleed were admitted into the hospital, 46% of whom had GI bleeding listed in the principal or second diagnosis, suggesting a clinically significant bleed. A subset of patients with SCD may have also been missed from our analysis, suggesting a possible underestimation of bleeding events. For example, the used datasets include only patients in California, and therefore patients who migrated into or out of California before a bleed may have been missed. The ED dataset also began in 2005, and therefore only hospital data for bleeding are available between 1990 and 2005. The patient population also included ED or hospitalized patients, and therefore, patients whose bleeding was managed on an outpatient basis would have also been missed. Furthermore, we evaluated only incident bleeding events and the first of each type of subsequent bleeding event. For example, after an incident bleed, the first subsequent GI bleed and first subsequent ICH would be captured, but not any additional GI bleeds or additional ICH. Therefore, the total number of bleeding events may be underestimated, and the overall natural history of all subsequent bleeding events is not described here. An examination of the lifetime burden of bleeding events may be an area of interest for future studies.
To our knowledge, this is the only study to examine bleeding events in patients with SCD. We demonstrated a high risk of bleeding, with the novel finding of a high incidence of GI bleeding compared with the general population, and a twofold increased risk for mortality associated with bleeding. This raises consideration of empiric ulcer prophylaxis for all hospitalized patients with SCD, as well as attempts to minimize usage of NSAIDs whenever possible. Furthermore, the significant association of a prior ischemic stroke with future hemorrhagic stroke warrants further study on the temporality between the two, as well as investigation into possible preventive measures.
Presented, in part, at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
For original data, please contact Ted Wun (twun@ucdavis.edu).
Acknowledgment
This work was supported by a grant from the National Center for Advancing Translational Science, National Institutes of Health (UL1 00001860) (T.W.).
Authorship
Contribution: A.B. and T.W. analyzed the data; N.H., A.B., and T.W. drafted the manuscript; and all authors designed the study, made revisions, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ted Wun, Division of Hematology Oncology, UC Davis Comprehensive Cancer Center, 4501 X St, Sacramento, CA 95817; e-mail: twun@ucdavis.edu.
References
Author notes
The full-text version of this article contains a data supplement.