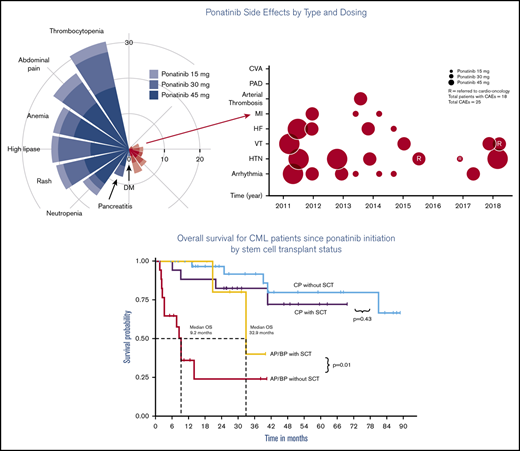
Key Points
Decrease incidence of CAEs with ponatinib may be related to timely dose adjustment and cardio-oncology support.
Ponatinib is an effective therapy for CML but allo-SCT remains an important treatment modality for those with advanced phase.
Abstract
Ponatinib is associated with cardiovascular adverse events (CAEs), and its frequency in the real world is limited. In this retrospective study, we examined the survival outcomes and associated toxicities in 78 consecutive ponatinib-treated patients with chronic myeloid leukemia (CML) at the Moffitt Cancer Center from January 2011 through December 2017. The most common non-CAE was thrombocytopenia (39.7%), occurring in a dose-dependent fashion. Eighteen patients (23.1%) experienced some form of CAE, with the most common being arrhythmia (9%) and hypertension (7.7%), whereas 3 patients experienced myocardial infarction (3.8%). Before 2014, most patients were started on ponatinib 45 mg daily. There was an inverse correlation between cardio-oncology referral and the number of CAEs (P = .0440); however, a lower ponatinib starting dose, more frequent dose reduction, and increased cardio-oncology referral all were likely to have contributed to the observed decrease in CAEs after 2014. The response rate and 5-year overall survival (OS) were higher than those observed in the Ponatinib Ph+ ALL and CML Evaluation (PACE) trial (major molecular response, 58.7% vs 40% and OS, 76% vs 73%; median follow-up of 32.5 months). Ponatinib-treated patients with chronic phase–CML did not show a significant improvement with allogeneic stem cell transplantation, whereas those with accelerated phase/blast phase–CML had a much better outcome (median OS of 32.9 months vs 9.2 months; P = .01). These results demonstrate that ponatinib is highly effective. Dose adjustments and increased awareness of the cardiotoxicities associated with ponatinib may help maximize its benefits.
Introduction
Chronic myeloid leukemia (CML) is a pluripotent hematopoietic stem cell disorder characterized by the reciprocal translocation of chromosomes 9 and 22, t(9;22)(q34;q11), also known as the Philadelphia chromosome (Ph).1 The translocation produces a chimeric BCR-ABL1 gene that encodes an oncogenic protein tyrosine kinase that underlies the malignant process. The discovery of imatinib, a tyrosine kinase inhibitor (TKI), and subsequent second-generation TKIs such as dasatinib, nilotinib, and bosutinib have allowed long-term management of this once-fatal disease.2 Despite the effectiveness of early-generation TKIs, some patients develop resistance and ultimately stop responding to these medications. Resistance can occur in pathways independent of or dependent on BCR-ABL1; however, it is most frequently caused by point mutations of the ABL1 kinase domain within the fusion protein.3 The gatekeeper T315I kinase domain mutation is the cause of resistance in ∼20% of cases, and neither first- nor second-generation TKIs have activity in these patients.4 This finding led to the development of ponatinib, a third-generation TKI that is highly potent against mutation-based resistance and inhibits mutant T315I.5
In December 2012, ponatinib gained accelerated approval by the US Food and Drug Administration (FDA) for T315I-positive CML/Ph+ acute lymphoblastic leukemia (ALL) or CML/Ph+ ALL that is resistant or intolerant to prior TKI therapy, based on the results of the pivotal Ponatinib Ph+ ALL and CML Evaluation (PACE) trial.6 Ponatinib was later suspended in October 2013 because of safety concerns arising from increased incidence of serious vascular occlusive events; however, the suspension was short lived and reversed within 2 months, after new safety measures were implemented.7 Some studies suggest that ponatinib causes endothelial dysfunction through its multikinase inhibitory properties affecting targets such as vascular endothelial growth factor receptor and can promote the expression of proatherogenic surface adhesion receptors, thereby increasing the risks of vascular occlusive events.8-11 In the final 5-year follow-up of the PACE trial (n = 449), the cumulative incidence of arterial occlusive events (AOEs), including cardiovascular, cerebrovascular, and peripheral vascular ones, was 25% overall and 31% in patients with chronic phase (CP)–CML. Overall, 13% (59 of 449) of patients had cardiovascular adverse events (CAEs) and 10% (44 of 449) had serious CAEs. In the CP-CML subgroup, 16% (42 of 270) had CAEs, and 12% (33 of 270) had serious CAEs.12 Even though the cumulative incidence of AOEs increased over time, the exposure-adjusted AOE incidence of new AOEs was stable in later years, suggesting that vascular toxicity is dose related. The median dose intensity was 32.7 mg/d for patients with events in the first year, compared with 21.1 mg/d for those with events in the fifth year. Of the 267 CP-CML patients evaluable for efficacy, 54% (144 of 267) achieved complete cytogenetic response (CCyR), 40% (108 of 267) achieved major molecular response (MMR), and 24% (64 of 267) achieved 4.5-log molecular response (MR4.5). Overall survival (OS) at 5 years was estimated at 73%.12 Outside of clinical trials, only scarce data are available regarding outcomes and safety of ponatinib treatment, which has become a point of interest, given the increasing prevalence of CML.13-15 In this single-center, retrospective study, we examined the real-life survival outcomes and associated toxicities in CML patients treated with ponatinib.
Patients and methods
We performed a retrospective review of CML patients at Moffitt Cancer Center from January 2011 through December 2017 to identify patients treated with ponatinib. Demographics, disease-specific variables, and clinical outcomes were collected in accordance with Institutional Review Board–approved protocol. Disease phase, response type (ie, hematologic, cytogenetic, and molecular), and treatment failure were defined according to the European LeukemiaNet recommendations.16 Quantitative reverse transcription polymerase chain reaction with a sensitivity of at least 4.5 logs has been used to measure BCR-ABL transcript levels and has been reported on the International Scale (IS) in our center since 2013. From 2011 through 2012, the same technique was used, but the IS was not reported; however, we were able to retrospectively calculate the IS by using an appropriate conversion factor for subsequent comparisons. Adverse events on ponatinib were divided into non-CAEs and CAEs, including arrhythmia, hypertension, venous thromboembolism (VTE), heart failure including left ventricular dysfunction or cardiomyopathy, myocardial infarction (MI), arterial thrombosis, peripheral arterial disease, and cerebrovascular accident. Correlations were made between CAEs, ponatinib dosage, cardio-oncology referral, and treatment with any cardiac protective medications, specifically aspirin, statin, beta-blocker, angiotensin-converting enzyme inhibitor, or calcium channel blocker. Data were summarized using mean, median, and range for continuous variables as frequency and percentage for categorical variables. P values were 2-tailed, with values less than 5% indicating statistical significance. Fisher’s exact test of independence was used to determine significance for categorical variables. OS since ponatinib initiation to the date of last follow-up or death was further classified based on phase of disease and allogeneic stem cell transplant (allo-SCT) status. Probabilities of survival and comparisons were estimated using the Kaplan-Meier method with the log-rank test. All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).17
Results
Patient characteristics
A total of 78 patients (38 females/40 males) with a median age of 42.5 years (range, 14-70), who were treated with ponatinib, were included in the analysis (Table 1). At the time of ponatinib initiation, 51 patients were in CP, 9 in AP, and 18 in BP. The most common vascular risk factor at baseline was hypertension (17.6% in CP patients, 11.1% in AP, and 33.3% in BP). Ponatinib was the third- or fourth-line therapy in 65%, 77.8%, and 72.2% of CP, AP, and BP patients, respectively. In CP patients, 86.2% had been treated with imatinib, 84.3% with dasatinib, and 72.5% with nilotinib before starting ponatinib (Table 2). The mean starting dose was 39.65 mg/d in CP patients, similar to patients in other phases of CML (Table 1). Dose reduction occurred in 64.7% of patients with CP-CML, and the median duration of ponatinib was 14.6 months. The reason for ponatinib initiation was the presence of a T315I mutation in 23.5% of the patients and treatment failure without T315I mutation in 76.5% of the patients.
Baseline characteristics and descriptive analysis of ponatinib usage
| . | CP (n = 51) . | AP (n = 9) . | BP (n = 18) . |
|---|---|---|---|
| Patients, n | 51 | 9 | 18 |
| Sex, male/female, n | 27/24 | 3/6 | 10/8 |
| Median age (range), y | 42 (17-70) | 38 (14-54) | 46.5 (20-69) |
| Vascular risk factors before ponatinib, n (%) | |||
| HTN | 9 (17.6) | 1 (11.1) | 6 (33.3) |
| CVA | 0 (0) | 0 (0) | 0 (0) |
| DM | 0 (0) | 1 (11.1) | 3 (16.7) |
| PAD | 1 (2.0) | 1 (11.1) | 0 (0) |
| MI | 5 (9.8) | 1 (11.1) | 1 (5.5) |
| Lines of therapy, n | |||
| Second | 4 | 1 | 0 |
| Third | 17 | 2 | 7 |
| Fourth | 16 | 5 | 6 |
| Fifth | 6 | 0 | 3 |
| ≥Sixth | 8 | 1 | 2 |
| Mean ponatinib starting dose, mg/d | 39.65 | 41.95 | 38.59 |
| Had dose reduction, n (%) | 33 (64.7) | 7 (77.8) | 8 (44.4) |
| Median ponatinib duration, mo | 14.60 | 10.47 | 5.02 |
| Reason for ponatinib, n (%) | |||
| T315I mutation | 12 (23.5) | 1 (11.1) | 3 (16.7) |
| Failure without T315I | 39 (76.5) | 8 (88.9) | 15 (83.3) |
| . | CP (n = 51) . | AP (n = 9) . | BP (n = 18) . |
|---|---|---|---|
| Patients, n | 51 | 9 | 18 |
| Sex, male/female, n | 27/24 | 3/6 | 10/8 |
| Median age (range), y | 42 (17-70) | 38 (14-54) | 46.5 (20-69) |
| Vascular risk factors before ponatinib, n (%) | |||
| HTN | 9 (17.6) | 1 (11.1) | 6 (33.3) |
| CVA | 0 (0) | 0 (0) | 0 (0) |
| DM | 0 (0) | 1 (11.1) | 3 (16.7) |
| PAD | 1 (2.0) | 1 (11.1) | 0 (0) |
| MI | 5 (9.8) | 1 (11.1) | 1 (5.5) |
| Lines of therapy, n | |||
| Second | 4 | 1 | 0 |
| Third | 17 | 2 | 7 |
| Fourth | 16 | 5 | 6 |
| Fifth | 6 | 0 | 3 |
| ≥Sixth | 8 | 1 | 2 |
| Mean ponatinib starting dose, mg/d | 39.65 | 41.95 | 38.59 |
| Had dose reduction, n (%) | 33 (64.7) | 7 (77.8) | 8 (44.4) |
| Median ponatinib duration, mo | 14.60 | 10.47 | 5.02 |
| Reason for ponatinib, n (%) | |||
| T315I mutation | 12 (23.5) | 1 (11.1) | 3 (16.7) |
| Failure without T315I | 39 (76.5) | 8 (88.9) | 15 (83.3) |
AP, accelerated phase; BP, blast phase; CVA, cerebrovascular accident; DM, diabetes; HTN: hypertension; PAD, peripheral arterial disease.
Prior lines of treatments in CP CML cohort
| Prior treatment . | Lines of therapy at ponatinib initiation, n . | Total, n (%) (N = 51) . | ||||
|---|---|---|---|---|---|---|
| 2nd (n = 4) . | 3rd (n = 17) . | 4th (n = 16) . | 5th (n = 6) . | >6th (n = 8) . | ||
| Imatinib | 2 | 15 | 13 | 6 | 8 | 44 (86.2) |
| Dasatinib | 1 | 11 | 17 | 6 | 8 | 43 (84.3) |
| Nilotinib | 1 | 6 | 15 | 6 | 9 | 37 (72.5) |
| Bosutinib | 0 | 0 | 0 | 1 | 0 | 1 (2.0) |
| Other* | 0 | 2 | 3 | 5 | 15 | 25 (49.0) |
| Prior treatment . | Lines of therapy at ponatinib initiation, n . | Total, n (%) (N = 51) . | ||||
|---|---|---|---|---|---|---|
| 2nd (n = 4) . | 3rd (n = 17) . | 4th (n = 16) . | 5th (n = 6) . | >6th (n = 8) . | ||
| Imatinib | 2 | 15 | 13 | 6 | 8 | 44 (86.2) |
| Dasatinib | 1 | 11 | 17 | 6 | 8 | 43 (84.3) |
| Nilotinib | 1 | 6 | 15 | 6 | 9 | 37 (72.5) |
| Bosutinib | 0 | 0 | 0 | 1 | 0 | 1 (2.0) |
| Other* | 0 | 2 | 3 | 5 | 15 | 25 (49.0) |
Chemotherapy, immunotherapy, or clinical trial.
Safety profile
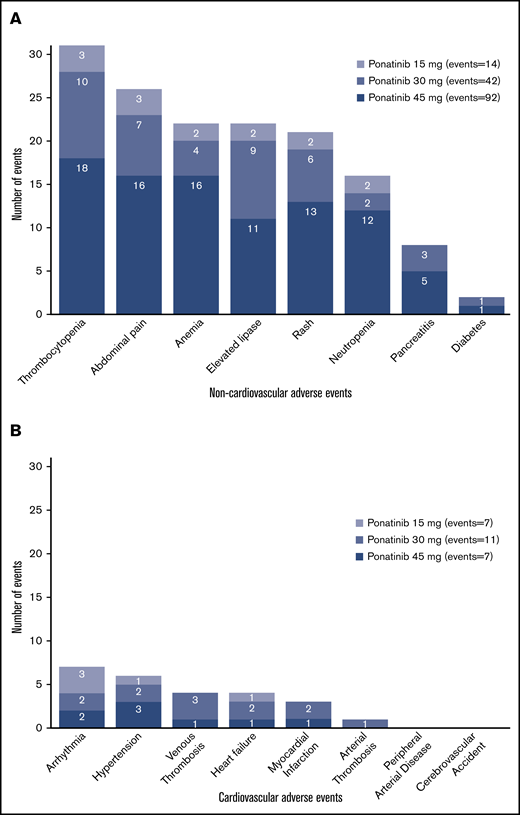
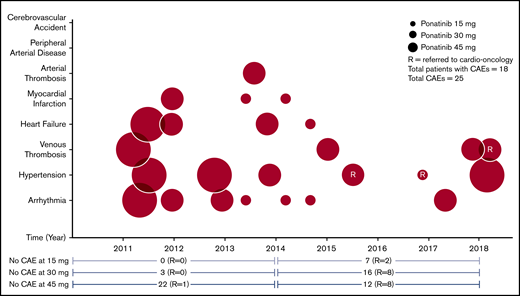
The most common non-CAEs were thrombocytopenia (39.7%), abdominal pain (33.3%), anemia (28.2%), elevated lipase (28.2%), and rash (26.9%), occurring in a dose-dependent fashion (Table 3; Figure 1A). At the lowest dose of ponatinib (15 mg daily), no patients developed pancreatitis or diabetes mellitus. The most common new-onset CAEs included arrhythmia (9%) and hypertension (7.7%; Table 4; Figure 1B). One patient who was previously on nilotinib developed renal arterial thrombosis (1.3%) while on her sixth line of therapy with ponatinib 30 mg daily. Three patients experienced MI (3.8%): 1 had significant cardiovascular risk factors at baseline, 2 were in AP, and all 3 had received nilotinib. The first patient had a history of hypertension and MI before being placed on ponatinib 15 mg daily for mutated T315I CP-CML. He had had 4 prior lines of therapy, including nilotinib. The second patient also had baseline hypertension and nilotinib in the past before starting ponatinib 30 mg daily for BP-CML. Finally, the third patient, who also had nilotinib, was on her fifth line of therapy for AP-CML at ponatinib initiation. No other arterial thrombotic events were reported. Eighteen patients (23.1%) experienced at least 1 CAE, and 4 patients had more than 1. One-third of those patients (6 of 18; 33.3%) had at least 1 vascular risk factor at baseline. Eleven of 18 (61.1%) patients were receiving cardiac protective medication(s), including aspirin, statins, beta-blockers, angiotensin-converting enzyme inhibitors, and calcium channel blockers, at the time of the CAE, and about half of all patients (45 of 78; 57.7%) were taking these medication(s) at baseline. Before 2014, a majority of the patients were started on ponatinib 45 mg daily (32 of 37; 86.5%) with the remaining started on ponatinib 30 mg. Our center was an early adopter of the lower dose, recognizing the potential cardiotoxicities that may be associated with the higher dose. After 2014, less than one-third of the patients (12 of 41; 29.3%) were started on ponatinib 45 mg daily, with the rest having an initial dose of 30 mg (22 of 41; 53.7%) or 15 mg (7 of 41; 17.1%) daily, depending on their clinical characteristics. More patients in treatment before 2014 had dose reductions compared with those in treatment after 2014 (24 of 37; 64.9% vs 24 of 41; 58.5%), but overall, a large number of all patients had dose reduction at 1 point during their course of therapy. The frequency and proportion of CAEs were higher for patients receiving ponatinib 45 mg daily before 2014 (6 of 13; 46.2% CAEs) vs after 2014 (1 of 11; 9.1% CAEs). Ten of 37 (27.0%) patients experienced CAEs before 2014 compared with 8 of 41 (19.5%) patients after 2014, yielding a decrease of 7.5% (P = .5913). There was an even greater percentage decrease (11.1%; P = .0961) in patients with serious CAEs (before 2014: 5 of 37; 13.5% vs after 2014: 1 of 41; 2.4%). The number of CAEs also decreased slightly, from 14 of 296 (4.7%) in the pre-2014 era to 11 of 328 (3.4%) in the post-2014 era (P = .4186). Similarly, serious CAEs decreased over time (before 2014: 6 of 296; 2.0% vs after 2014: 2 of 328; 0.6%; P = .1588). Before 2014, only 1 of 37 (2.7%) patients was referred to cardio-oncology whereas 21 of 41 (51.2%) were referred after 2014 (P = .0001) at the time of ponatinib initiation, to help mitigate cardiovascular risks. There was a statistically significant association between cardio-oncology referral and the number of CAEs (P = .0440), although we recognize that the dosage may be confounding. Figure 2 summarizes the cardiac safety profile of ponatinib with its associated dosing and frequency of cardio-oncology referral.
Frequency of non-CAEs in ponatinib-treated patients
| Non-CAEs . | Cumulative incidence, n (%) (n = 78) . | Ponatinib 45 mg/d, n (%) . | Ponatinib 30 mg/d, n (%) . | Ponatinib 15 mg/d, n (%) . |
|---|---|---|---|---|
| Thrombocytopenia | 31 (39.7) | 18 (23.1) | 10 (12.8) | 3 (3.8) |
| Abdominal pain | 26 (33.3) | 16 (20.5) | 7 (9.0) | 3 (3.8) |
| Anemia | 22 (28.2) | 16 (20.5) | 4 (5.1) | 2 (2.6) |
| Elevated lipase | 22 (28.2) | 11 (14.1) | 9 (11.5) | 2 (2.6) |
| Rash | 21 (26.9) | 13 (16.7) | 6 (7.7) | 2 (2.6) |
| Neutropenia | 16 (20.5) | 12 (15.4) | 2 (2.6) | 2 (2.6) |
| Pancreatitis | 8 (10.3) | 5 (6.4) | 3 (3.8) | 0 (0.0) |
| Diabetes Mellitus | 2 (2.6) | 1 (1.3) | 1 (1.3) | 0 (0.0) |
| Non-CAEs . | Cumulative incidence, n (%) (n = 78) . | Ponatinib 45 mg/d, n (%) . | Ponatinib 30 mg/d, n (%) . | Ponatinib 15 mg/d, n (%) . |
|---|---|---|---|---|
| Thrombocytopenia | 31 (39.7) | 18 (23.1) | 10 (12.8) | 3 (3.8) |
| Abdominal pain | 26 (33.3) | 16 (20.5) | 7 (9.0) | 3 (3.8) |
| Anemia | 22 (28.2) | 16 (20.5) | 4 (5.1) | 2 (2.6) |
| Elevated lipase | 22 (28.2) | 11 (14.1) | 9 (11.5) | 2 (2.6) |
| Rash | 21 (26.9) | 13 (16.7) | 6 (7.7) | 2 (2.6) |
| Neutropenia | 16 (20.5) | 12 (15.4) | 2 (2.6) | 2 (2.6) |
| Pancreatitis | 8 (10.3) | 5 (6.4) | 3 (3.8) | 0 (0.0) |
| Diabetes Mellitus | 2 (2.6) | 1 (1.3) | 1 (1.3) | 0 (0.0) |
Frequency of adverse events in ponatinib-treated patients. (A) New-onset non-CAEs increased in a ponatinib dose-dependent fashion. (B) New-onset CAEs were fewer in comparison with non-CAEs. The most common CAEs were arrhythmia and hypertension.
Frequency of adverse events in ponatinib-treated patients. (A) New-onset non-CAEs increased in a ponatinib dose-dependent fashion. (B) New-onset CAEs were fewer in comparison with non-CAEs. The most common CAEs were arrhythmia and hypertension.
Frequency of CAEs in ponatinib-treated patients
| New-onset CAE . | Cumulative incidence, n (%) (n = 78)* . | Ponatinib 45 mg/d, n (%) . | Ponatinib 30 mg/d, n (%) . | Ponatinib 15 mg/d, n (%) . |
|---|---|---|---|---|
| Arrhythmia | 7 (9.0) | 1 (1.3) | 3 (3.8) | 3 (3.8) |
| Hypertension | 6 (7.7) | 3 (3.8) | 2 (2.6) | 1 (1.3) |
| Venous thrombosis | 4 (5.1) | 1 (1.3) | 3 (3.8) | 0 (0.0) |
| Heart failure | 4 (5.1) | 1 (1.3) | 2 (2.6) | 1 (1.3) |
| Myocardial infarction | 3 (3.8) | 0 (0.0) | 1 (1.3) | 2 (2.6) |
| Arterial thrombosis† | 1 (1.3) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| Peripheral arterial disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cerebrovascular accident | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New-onset CAE . | Cumulative incidence, n (%) (n = 78)* . | Ponatinib 45 mg/d, n (%) . | Ponatinib 30 mg/d, n (%) . | Ponatinib 15 mg/d, n (%) . |
|---|---|---|---|---|
| Arrhythmia | 7 (9.0) | 1 (1.3) | 3 (3.8) | 3 (3.8) |
| Hypertension | 6 (7.7) | 3 (3.8) | 2 (2.6) | 1 (1.3) |
| Venous thrombosis | 4 (5.1) | 1 (1.3) | 3 (3.8) | 0 (0.0) |
| Heart failure | 4 (5.1) | 1 (1.3) | 2 (2.6) | 1 (1.3) |
| Myocardial infarction | 3 (3.8) | 0 (0.0) | 1 (1.3) | 2 (2.6) |
| Arterial thrombosis† | 1 (1.3) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| Peripheral arterial disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cerebrovascular accident | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Four patients had >1 CAE.
Other than myocardial infarction or cerebrovascular accident.
Cardiac safety profile of ponatinib with its associated dosing and frequency of cardio-oncology referral over time. The reason for the decrease in incidence of CAEs since 2014 is multifactorial, including heightened awareness of cardiovascular risks, with ponatinib treatment leading to increased cardio-oncology referrals, shorter time to ponatinib dose reduction when clinically indicated, and lower starting dose. Pre-2014 patients with CAE(s): 10 of 37 (28.0%); post-2014 CAE(s): 8 of 14 (19.5%).
Cardiac safety profile of ponatinib with its associated dosing and frequency of cardio-oncology referral over time. The reason for the decrease in incidence of CAEs since 2014 is multifactorial, including heightened awareness of cardiovascular risks, with ponatinib treatment leading to increased cardio-oncology referrals, shorter time to ponatinib dose reduction when clinically indicated, and lower starting dose. Pre-2014 patients with CAE(s): 10 of 37 (28.0%); post-2014 CAE(s): 8 of 14 (19.5%).
Clinical outcomes
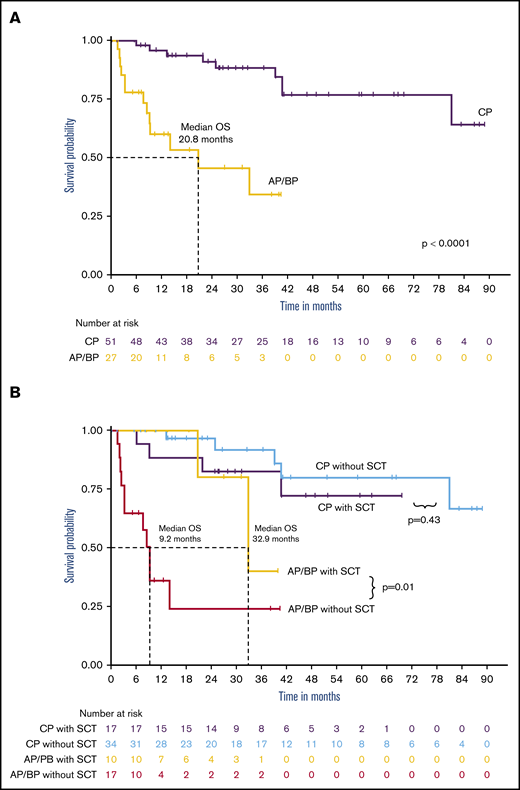
Of the patients with CP-CML, 69.6% achieved a CCyR, 58.7% had an MMR, 41.3% had a 4-log reduction of BCR-ABL1 transcripts (MR4.0), and 37% achieved MR4.5 (Table 5). Patients with AP and BP disease achieved CCyR in 37.5% and 68.8%, respectively. Among the BP patients who achieved at least a CCyR (n = 11), 81.1% had received a TKI with chemotherapy at the time of BP. OS since ponatinib initiation at 5 years for CP-CML patients was 76.8%, with a median follow-up of 40.8 months (Figure 3A). AP/BP CML patients had a median OS of 20.8 months with a median follow-up of 18.7 months. About a third of patients with CP (17 of 51; 33.3%) or AP/BP (10 of 27; 37%) had undergone allo-SCT. Since ponatinib initiation, OS at 5 years for CP-CML was estimated at 72.1% with allo-SCT and at 79.7% without allo-SCT (Figure 3B). Median OS for AP/BP disease with allo-SCT was 32.9 months compared with 9.2 months without allo-SCT (P = .01).
Overall responses with ponatinib
| Phase of CML . | CCyR, n (%) . | MMR, n (%) . | MR 4.0, n (%) . | MR 4.5, n (%) . |
|---|---|---|---|---|
| CP (n = 51) | 32/46 (69.6) | 27/46 (58.7) | 19/46 (41.3) | 17/46 (37.0) |
| AP (n = 9) | 3/8 (37.5) | 0/8 (0.0) | 0/8 (0.0) | 0/8 (0.0) |
| BP (n = 18) | 11/16 (68.8) | 10/16 (62.5) | 7/16 (43.8) | 7/16 (43.8) |
| Phase of CML . | CCyR, n (%) . | MMR, n (%) . | MR 4.0, n (%) . | MR 4.5, n (%) . |
|---|---|---|---|---|
| CP (n = 51) | 32/46 (69.6) | 27/46 (58.7) | 19/46 (41.3) | 17/46 (37.0) |
| AP (n = 9) | 3/8 (37.5) | 0/8 (0.0) | 0/8 (0.0) | 0/8 (0.0) |
| BP (n = 18) | 11/16 (68.8) | 10/16 (62.5) | 7/16 (43.8) | 7/16 (43.8) |
In CP, the response was unknown in 5 patients; in AP, in 1 patient; and in BP, in 2 patients.
Outcomes of CML patients treated with ponatinib. (A) OS since ponatinib initiation was significantly better for CP-CML patients compared with AP patients, with a 5-year OS of 76.8% and median OS of 20.8 months, respectively. (B) Overall survival since ponatinib initiation, by SCT status. allo-SCT conferred a significant benefit in AP patients, but the response was not found in CP-CML patients.
Outcomes of CML patients treated with ponatinib. (A) OS since ponatinib initiation was significantly better for CP-CML patients compared with AP patients, with a 5-year OS of 76.8% and median OS of 20.8 months, respectively. (B) Overall survival since ponatinib initiation, by SCT status. allo-SCT conferred a significant benefit in AP patients, but the response was not found in CP-CML patients.
Discussion
Patients with CP-CML are living longer in most cases, matching the healthy population, because of effective TKI therapies.18 Although there are many ongoing efforts to prolong treatment-free remission and find a cure, for now, most patients will require life-long therapy to control their disease.2 Adverse events become especially important in this setting, not only because they lead to comorbidities, but they can also affect compliance and therefore disease state and eligibility for discontinuation in the future. Ponatinib is the latest generation TKI with efficacy, even in heavily pretreated CML patients, and is the only US Food and Drug Administration–approved TKI for patients harboring the T315I mutation. The potency and broad inhibition of numerous tyrosine kinases, many responsible for vascular biology, are thought to contribute to the increased incidence of CAEs.19 In the real-world setting, there are very few studies on ponatinib outcomes and toxicities in this patient population.13-15 In our study, we analyzed the safety and effectiveness of ponatinib in the treatment of 78 patients with CML at our institution.
In our cohort, 23.1% (18 of 78) of patients experienced 1 or more CAEs, whereas in the PACE trial 30.1% (111 AOEs + 27 VTEs of 449 patients) were observed.12 Rates of VTEs were similar in both studies (4 of 78 patients; 5.1% vs 27 of 449 patients; 6%). Cerebrovascular and peripheral vascular events were reported in the PACE trial at 9% (41 of 449) and 11% (48 of 449) of patients, respectively. In our present study, there were 3 cases of MI, 1 case of renal arterial thrombosis, and no cases of cerebrovascular or peripheral vascular events. Several factors can influence and may explain these results. More patients were started on ponatinib 45 mg daily before 2014 (32 of 37; 86.5%) compared with after 2014 (12 of 41; 29.3%). It is well-documented that there is a dose-dependent relationship between ponatinib and CAEs. Therefore, many of our patients had dose reductions and lower starting doses. In recent years, there has been an increased awareness of the cardiotoxicities associated with TKI therapy for CML. Disease management is shifting toward a more multidisciplinary approach, including referral of patients to a cardio-oncology service for expertise in optimizing primary and secondary prevention of cardiac disease, especially in high-risk patients treated with ponatinib.19 As a result, significantly more patients were referred to cardio-oncology in our center after 2014 when the risk of CAEs with ponatinib was realized (before 2014: 1 of 37; 2.7% referred vs after 2014: 21 of 41; 51.2% referred; P = .0001). The number of patients with CAEs diagnosed after 2014 (8 of 41; 19.5%) was numerically less compared with that before 2014 (10 of 37, 27%; P = .5913), and there was a significant inverse correlation between cardio-oncology referral and CAEs, keeping in mind, however, the lower dosage that was used during that same time frame (P = .0440). About half of all patients (45 of 78; 57.7%) were receiving cardiac protective medications at baseline and 61.1% (11 of 18) were taking them at the time of CAE. No association was found between treatment with these medications and CAEs in this cohort. This was also suggested in an observational study by Heiblig et al13 (n = 48; CP-CML patients), but they were assessing only antiaggregants or anticoagulants. It is important to note that, although there has been speculation, the exact underlying mechanism of how ponatinib increases the risk of CAEs remains incompletely understood; therefore, it is unclear whether typical medications used to prevent atherosclerotic disease would have any efficacy in this setting.
The median time to CAE onset was 5.7 months, which is shorter than the reported 13.4 months for an AOE in the PACE trial.12 The difference in definition (AOEs did not include VTEs) and longer median time to onset for cerebrovascular AOEs (20.1 months) and peripheral AOEs (19.9 months), of which there was none in this cohort, may account for some of the differences observed. Early event onset argues for an active, preemptive approach to help minimize cardiac risks. Barber et al20 proposed an algorithm for cardiovascular care in these patients. Those with high cardiac risks (diabetes, hypertension, age >60 years, hyperlipidemia, and tobacco abuse) should follow a modified guideline of monitoring (every 3-6 months: cardiovascular assessment, blood pressure check, electrocardiogram, lipid panel, and ankle-brachial index) and consider drug modification when needed.20 One study showed that the Systematic Coronary Risk Evaluation (SCORE) assessment may be a useful tool for identifying those with a higher risk of developing AOEs while on ponatinib treatment.15 Jain et al21 recently published a study using data from several different clinical trials with CP-CML patients who were treated with TKIs, to evaluate their cardiovascular impact, and found that ponatinib was associated with the highest incidence of CAEs/AOEs. Most of the patients in this cohort were at least in their third line of therapy upon starting ponatinib and >70% of the CP-CML patients were on nilotinib, which has been shown to increase the risks of cardiac ischemic events and peripheral arterial occlusive disease.22,23 The PACE trial also had a large number of patients who were heavily pretreated with nilotinib. PACE patients on ponatinib who had 2 or more cardiovascular risk factors had an estimated relative risk of 2.2 (95% confidence interval, 1.5-3.3) for development of serious AOEs.24 In our study, about one-third of the patients had cardiovascular risks at baseline. Non-CAEs, both hematologic and nonhematologic, increased in a dose-dependent fashion, as expected, and were comparable overall to the those found in the PACE trial.
Among the patients with CP-CML, response rates were higher than those observed in the PACE trial, with 27 of 46 (58.7%) vs 40% and 17 of 46 (37%) vs 24% achieving MMR and MR4.5, respectively. The estimated 5-year OS since initiation of ponatinib was also slightly higher at 76% vs 73%. About one-third of the patients with CP at diagnosis eventually underwent allo-SCT, and their survival, when stratified by allo-SCT status, showed no significant differences in OS at 5 years for those without allo-SCT (79.7% vs 72.1%; P = .43). This result suggests that ponatinib without allo-SCT may be a reasonable treatment option for patients with CP-CML, especially for those who are borderline candidates for transplantation. Even with improved supportive care and advances in immunosuppression, treatment-related mortality of allo-SCT is a major concern. This observation is somewhat consistent with a recent study published by Nicolini et al25 in which they found that 48-month OS rates were significantly higher in patients with T315I-mutated CP-CML who received ponatinib, compared with those who had allo-SCT (72.7% vs 55.8%; P = .013). In contrast, advanced phase patients, especially those with BP, have very poor outcomes, with a median OS of 20.8 months. Patients who received allo-SCT had a better outcome than those who did not (median OS of 32.9 months vs 9.2 months; P = .01). Restated, allo-SCT is an important treatment modality for patients with AP CML.
We recognize there are several limitations to our study. CML in general is a rare disease, and the subset of patients requiring ponatinib is even smaller, allowing for only limited sample sizes, especially in the advanced phase. The observed decrease in CAEs after cardio-oncology referral could be confounded by factors including that some patients may have been already seeing a cardiologist outside of our institution who may or may not have been specifically trained to manage this specialized population. It is also difficult to decipher exactly how much the dosage of ponatinib contributed to these events, given the small number of CAEs observed in this cohort, but historically there has been a positive correlation as mentioned before. Dorer et al26 conducted multivariate analyses from a pooled population (3 clinical trials; n = 671) to assess the impact of dose intensity of ponatinib on various adverse events and showed a significant association between dose intensity and the risk of AOEs.
In conclusion, in our series, the incidence of new arterial thrombotic events and hypertension are lower than reported in the PACE trial, keeping in the mind, however, that a direct comparison is difficult because of the differences in the CAEs included. The incidence of CAEs declined beginning in 2014 when a lower starting dose, early dose reductions, and increased cardio-oncology referral became standard practice at our institution. Ponatinib without allo-SCT may be a very reasonable treatment option for patients with CP-CML, but transplantation remained an important treatment modality for those with advanced phase CML. Further studies analyzing the impact of specific interventions on mitigating cardiovascular events are needed.
Acknowledgment
The authors thank the CML patients and their caregivers for allowing us to partner with them in their health care.
Authorship
Contribution: J.P.-I. and K.S. designed the study; O.C., C.T., L.I., and S.S. collected the data; O.C., C.T., and L.I. analyzed the data with supervision by J.P.-I. and K.S.; O.C. wrote the first draft; and all authors including C.T., L.I., S.S., L.N., M.F., K.S., and J.P.-I. critically reviewed the first draft and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.P.-I. has received honoraria for consulting from Novartis and Bristol-Myers Squibb and is on the speakers bureau of Takeda. K.S. has received honoraria from Novartis for consulting and is on its speakers bureau. L.N. is on the speakers bureau of Novartis and has received a consulting fee from Pfizer. M.F. has received honoraria from Novartis for consulting. The remaining authors declare no competing financial interests.
Correspondence: Kendra Sweet, Department of Malignant Hematology, Moffitt Cancer Center, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: kendra.sweet@moffitt.org.
References
Author notes
K.S. and J.P.-I. contributed equally to this study, and both are senior authors.
For the original data, please e-mail the corresponding author.