Key Points
Daratumumab is effective in reversing organ damage in patients with previously treated AL amyloidosis.
Abstract
Immunoglobulin light chain amyloidosis (AL amyloidosis) involves deposition of abnormally folded light chains into a wide range of tissues causing organ dysfunction, including in the heart and kidney. Daratumumab, a CD38-targeted antibody, has recently demonstrated efficacy in producing hematologic responses in previously treated disease. However, data on survival outcomes and organ responses to daratumumab are lacking. Seventy-two patients with previously treated AL amyloidosis who received daratumumab monotherapy with dexamethasone were retrospectively evaluated. With a median follow-up of 27 months, 2-year overall survival (OS) was 86.9% (median OS, not reached) and 2-year time-to-next treatment or death (TTNT)–free survival was 62% (median TTNT, not reached). Forty of 52 evaluable patients achieved a hematologic response (77%), with >60% of patients achieving a very good partial response or better; median time-to-hematologic response was 1 month. Fifty-seven patients (79%) had cardiac involvement, and 55% of evaluable patients achieved a cardiac response, with a median response time of 3.2 months among responders. Cardiac responses were associated with an improvement in OS, with landmark analysis for cardiac responses at 3 months trending toward statistical significance (100% vs 55% at 30 months, P = .051). Forty-seven patients (65%) had renal involvement, and 52% of evaluable patients achieved a renal response, with a median response time of 6 months among responders; there was no significant difference in OS between renal responders and nonresponders. This study demonstrates that daratumumab is highly effective in the treatment of previously treated AL amyloidosis, and a significant proportion of patients can achieve deep hematologic responses, as well as improvements in organ function.
Introduction
Immunoglobulin light chain amyloidosis (AL amyloidosis) is a disease that is characterized by the deposition of abnormally folded light chains into a wide range of tissues causing organ dysfunction, including in the heart, kidney, and liver. In the majority of cases, a clonal plasma cell population is the source of these amyloidogenic light chains, and treatment of AL amyloidosis has traditionally involved the use of plasma-cell directed therapies to suppress light chain production. In the front-line setting, several studies have associated the control of light chain production with improvements in organ function and demonstrated that organ responses correlate with improved survival.1-4
A commonly used front-line therapy consists of a combination of cyclophosphamide, bortezomib, and dexamethasone (CyBorD), after several retrospective studies demonstrated high overall response rates and good tolerability with this regimen.5-7 In one of the largest of these studies, Palladini et al7 reported on 230 newly diagnosed patients treated with CyBorD in the United Kingdom and Italy and found an overall hematologic response rate of 60%, with improvement in overall survival (OS) among those patients who achieved a hematologic response. However, a significant proportion of patients are refractory to, or relapse after, CyBorD; thus, effective therapies are needed for relapsed/refractory disease.
In the last several years, daratumumab, a human immunoglobulin G1κ monoclonal antibody targeting the CD38 surface antigen, has been found to be active in the treatment of AL amyloidosis. In their prospective phase 2 study, Sanchorawala et al8 reported high hematologic response rates (>80%) in 21 patients with relapsed AL amyloidosis. In our own retrospective study, we previously demonstrated daratumumab to be safe and efficacious, with a 76% overall hematologic response rate in heavily pretreated AL amyloidosis patients,9 similar to results at other institutions.8,10,11 Although many studies have reported organ response after frontline therapy, the effect of subsequent line therapy with daratumumab on organ response and recovery has not been well studied.
This study is an expansion of our original study of patients with AL amyloidosis treated with daratumumab and represents one of the largest retrospective studies on the use of daratumumab in previously treated AL amyloidosis, reporting on organ outcomes for which data are limited.
Patients and methods
This is a retrospective analysis of consecutive patients followed at Stanford University Medical Center for biopsy-proven AL amyloidosis confirmed by immunohistochemistry or mass spectrometry. Patients treated with daratumumab monotherapy (DMT) with dexamethasone between January 2016 and January 2019 were included in this study. In general, daratumumab was administered IV at 16 mg/kg weekly for 8 weeks, followed by every other week for 8 doses, and then every 4 weeks as previously described9 ; dexamethasone (20 mg) was also routinely administered with initial infusion and subsequently tapered per physician discretion. All demographic and clinical information was obtained from medical records. The study was approved by the Stanford University Institutional Review Board and was conducted in accordance with the principles of the Declaration of Helsinki.
Hematologic responses were determined by the change in the difference between involved and uninvolved free light chains (dFLC) and were defined per consensus guidelines.12 For patients with an initial dFLC ≥5 mg/dL, hematologic response was met if patients achieved a partial response (PR) (defined as ≥50% reduction in dFLC), very good partial response (VGPR) (defined as reduction of dFLC to <4 mg/dL), or complete response (CR) (defined as achieving a negative serum and urine immunofixation electrophoresis and normal free light chain ratio). Patients with an initial dFLC between 2 and 5 mg/dL were determined to have a hematologic response if low-dFLC PR criteria (defined as the achievement of dFLC <1 mg/dL)13 or CR was met. Patients with initial dFLC <2 mg/dL were excluded from hematologic response assessment. Hematologic responses were generally assessed monthly starting 1 month after daratumumab initiation, and the lowest dFLC achieved during follow-up prior to death or change of therapy was considered the maximal hematologic response to therapy.
Organ involvement and responses were evaluated per consensus guidelines.2,12,14 Organ response markers at daratumumab initiation were used as the initial baseline measurements.
Because of limitations in the available data, only N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was used for assessment of cardiac response; a >30% and >300 pg/mL decrease in NT-proBNP for patients with a baseline NT-proBNP ≥650 pg/mL constituted a cardiac response. A graded cardiac response metric was also explored, with PR representing a 30% to 59% reduction, VGPR representing a ≥60% reduction, and CR NT-proBNP <450 pg/mL, as reported in previous studies.4,15 Given that NT-proBNP levels are dependent on renal clearance, patients with an estimated glomerular filtration rate (eGFR) that decreased by 25%, with a creatinine level that increased by ≥0.5 mg/dL, were excluded from cardiac response assessment, as previously recommended by Palladini et al.12 For renal responses, patients were considered to have a renal response if they achieved a 30% decrease (by ≥0.5 g per day) in 24-hour urine protein without worsening in creatinine or creatinine clearance by >25% in patients with ≥0.5 g per day pretreatment. A graded renal response metric was also explored, with PR representing a 30% to 59% reduction in proteinuria, VGPR representing a ≥60% reduction, and CR ≤200 mg per 24-hour period.4 Time-to-organ response was determined from initiation of daratumumab to achievement of response criteria or best-achieved response, as specified. The best hematologic response achieved while on daratumumab was used to assess the association between hematologic response and organ response.
The Fisher’s exact test and the Mann-Whitney U test were used to determine differences between nominal and continuous variables, respectively. Survival and time-to-event data were analyzed using the Kaplan-Meier method, with the log-rank test used to compare groups when applicable. Two-sided values of P < .05 were considered statistically significant. Statistical analysis was conducted using SPSS version 25.0 (IBM, Armonk, NY).
Results
Seventy-two patients were included in the study. Baseline characteristics at the time of daratumumab initiation are shown in Table 1. The median age was 67 years old (interquartile range [IQR], 60-72). Fifty-seven patients (79%) had evidence of cardiac involvement, and 47 patients (65%) had evidence of renal involvement. The initial median dFLC prior to daratumumab was 3.45 mg/dL (IQR, 1.8-10.7 mg/dL). Median lines of prior therapy was 2 (range, 1-7); 13 patients had previous high-dose therapy with autologous stem cell transplant (ASCT). On cytogenetics and fluorescence in situ hybridization analysis, 40 patients did not have any identifiable abnormalities; the most common fluorescence in situ hybridization abnormalities identified were t(11;14) in 8 patients and 17p deletion in 3 patients. Reasons for daratumumab initiation are shown in Table 2.
Baseline patient characteristics
| . | DMT (n = 72) . |
|---|---|
| Age, median (IQR), y | 67 (60-72) |
| Sex, n (%) | |
| Male | 44 (61) |
| Female | 28 (39) |
| Involved free light chain, n (%) | |
| λ | 54 (75) |
| κ | 18 (25) |
| Initial dFLC, median (IQR), mg/dL | 3.45 (1.8-10.7) |
| European 2015 addition to Mayo 2004 stage, n (%) | |
| Stage I | 10 (14) |
| Stage II | 37 (51) |
| Stage IIIA | 12 (17) |
| Stage IIIB | 5 (7) |
| Unable to calculate (missing data) | 8 (11) |
| Organ involvement, n (%) | |
| Heart | 57 (79) |
| Kidney | 47 (65) |
| Heart and kidney | 35 (49) |
| Liver | 5 (7) |
| Prior lines of therapy, median (IQR) | 2 (1-3) |
| Prior therapies/exposure, n (%) | |
| ASCT | 13 (18) |
| Upfront | 11 (15) |
| Salvage | 2 (3) |
| CyBorD | 50 (70) |
| Bortezomib | 69 (96) |
| Lenalidomide | 32 (44) |
| Carfilzomib | 14 (19) |
| Pomalidomide | 10 (14) |
| Ixazomib | 8 (11) |
| . | DMT (n = 72) . |
|---|---|
| Age, median (IQR), y | 67 (60-72) |
| Sex, n (%) | |
| Male | 44 (61) |
| Female | 28 (39) |
| Involved free light chain, n (%) | |
| λ | 54 (75) |
| κ | 18 (25) |
| Initial dFLC, median (IQR), mg/dL | 3.45 (1.8-10.7) |
| European 2015 addition to Mayo 2004 stage, n (%) | |
| Stage I | 10 (14) |
| Stage II | 37 (51) |
| Stage IIIA | 12 (17) |
| Stage IIIB | 5 (7) |
| Unable to calculate (missing data) | 8 (11) |
| Organ involvement, n (%) | |
| Heart | 57 (79) |
| Kidney | 47 (65) |
| Heart and kidney | 35 (49) |
| Liver | 5 (7) |
| Prior lines of therapy, median (IQR) | 2 (1-3) |
| Prior therapies/exposure, n (%) | |
| ASCT | 13 (18) |
| Upfront | 11 (15) |
| Salvage | 2 (3) |
| CyBorD | 50 (70) |
| Bortezomib | 69 (96) |
| Lenalidomide | 32 (44) |
| Carfilzomib | 14 (19) |
| Pomalidomide | 10 (14) |
| Ixazomib | 8 (11) |
Reasons for daratumumab initiation
| . | DMT (n = 72) . |
|---|---|
| Increasing dFLC, n (%) | 42 (58) |
| dFLC at daratumumab initiation, median (IQR), mg/dL | 3.95 (2.1-10.3) |
| Meeting criteria for >50% increase from nadir and dFLC > 2 mg/dL, n | 30 |
| Meeting Pavia high-risk dFLC progression criteria,20 n | 20 |
| Meeting ISA progression criteria,14 n | 14 |
| Insufficient response to prior therapy, n (%) | 17 (24) |
| dFLC at daratumumab initiation, median (IQR), mg/dL | 8.47 (3.1-20.2) |
| Continued or worsening organ dysfunction, n (%) | 6 (8) |
| Intolerance to prior therapy, n (%) | 7 (10) |
| . | DMT (n = 72) . |
|---|---|
| Increasing dFLC, n (%) | 42 (58) |
| dFLC at daratumumab initiation, median (IQR), mg/dL | 3.95 (2.1-10.3) |
| Meeting criteria for >50% increase from nadir and dFLC > 2 mg/dL, n | 30 |
| Meeting Pavia high-risk dFLC progression criteria,20 n | 20 |
| Meeting ISA progression criteria,14 n | 14 |
| Insufficient response to prior therapy, n (%) | 17 (24) |
| dFLC at daratumumab initiation, median (IQR), mg/dL | 8.47 (3.1-20.2) |
| Continued or worsening organ dysfunction, n (%) | 6 (8) |
| Intolerance to prior therapy, n (%) | 7 (10) |
ISA, International Symposium of Amyloid and Amyloidosis.
Median follow-up was 27 months for the study population (IQR, 18-34.4). The median time from initial AL amyloid therapy to initiation of daratumumab was 21 months. The median duration from the end of the last line of therapy to initiation of daratumumab was 1.4 months, with a median of 3.3 months for the duration of last line of therapy prior to daratumumab. The majority of patients (74%) were continued on maintenance daratumumab until switched to next-line therapy; 19 patients (26%) discontinued daratumumab without progressing to next-line therapy after a median treatment duration of 5.4 months. The reasons for discontinuation included patient preference (7 patients), fatigue/body aches (4 patients), other active medical comorbidities (4 patients), infection (2 patients), physician preference (1 patient), and transition to hospice (1 patient).
Daratumumab therapy was generally well tolerated. Data for adverse events within the first cycle were available for 66 patients and were assessed using the Common Terminology Criteria for Adverse Events version 5.0. Thirty-three patients (50%) had documented infusion reactions during the first cycle of daratumumab. The majority of these (94%) were transient and resolved rapidly with supportive care and temporary infusion interruption/infusion rate modification (grade 1-2); 2 patients had grade 3 infusion reactions, with anaphylactoid reactions requiring 24-hour hospitalization for observation, but they were subsequently able to continue on daratumumab therapy without further incidents. The most common symptoms reported during infusion reactions included chest tightness/dyspnea (49%), cough (30%), nasal congestion/rhinorrhea (24%), eye irritation (18%), and nausea/vomiting (18%). Within the first cycle, 9 patients were hospitalized because of infection (3 patients), infusion reactions (2 patients), myocardial infarction (2 patients), syncope (1 patient), and volume overload (1 patient). The majority of patients (74%) were able to complete the first cycle without any treatment interruptions or delays; the most common reason for treatment delay was upper respiratory infection (7.5%).
Survival and time-to-next treatment outcomes
OS for the entire cohort is shown in Figure 1A. There were 11 deaths during follow-up. Median OS was not reached, and the 2-year OS rate was 86.9% (95% confidence interval [CI], 78.9-94.9).
Kaplan-Meier survival curves for OS and TTNT. (A) OS from initiation of daratumumab. (B) TTNT or death from initiation of daratumumab. (C) TTNT or death from initiation of daratumumab for patients with initial dFLC ≥5 mg/dL.
Kaplan-Meier survival curves for OS and TTNT. (A) OS from initiation of daratumumab. (B) TTNT or death from initiation of daratumumab. (C) TTNT or death from initiation of daratumumab for patients with initial dFLC ≥5 mg/dL.
The median time-to-next treatment or death (TTNT) was not reached, with a 2-year TTNT-free survival rate of 62% (95% CI, 48.2-73.4) for the entire cohort (Figure 1B). There were 27 events: 21 patients underwent subsequent therapies; the most common next-line therapies were daratumumab/pomalidomide/dexamethasone (7 patients), followed by daratumumab/lenalidomide/dexamethasone (3 patients). Six patients died prior to receiving subsequent-line therapy. Reasons for switching to next-line therapies are shown in Table 3. Among patients with initial dFLC ≥5 mg/dL at daratumumab initiation, the median TTNT was 20 months, with a 2-year TTNT-free survival rate of 47.3% (95% CI, 29.3-65.3) (Figure 1C).
Reasons for next-line therapy after daratumumab
| . | Events (n = 21) . |
|---|---|
| Increasing dFLC, n (%) | 10 (48) |
| dFLC at TTNT, median (IQR), mg/dL | 5.2 (3.1-9.6) |
| Meeting criteria for >50% increase from nadir and dFLC > 2 mg/dL, n | 8 |
| Meeting Pavia high-risk dFLC progression criteria,20 n | 2 |
| Insufficient hematologic response, n (%) | 8 (38) |
| dFLC at TTNT, median (IQR), mg/dL | 5.95 (3.1-16.9) |
| Continued or worsening organ dysfunction, n (%) | 2 (10) |
| Decision for consolidative autologous transplant, n (%) | 1 (5) |
| . | Events (n = 21) . |
|---|---|
| Increasing dFLC, n (%) | 10 (48) |
| dFLC at TTNT, median (IQR), mg/dL | 5.2 (3.1-9.6) |
| Meeting criteria for >50% increase from nadir and dFLC > 2 mg/dL, n | 8 |
| Meeting Pavia high-risk dFLC progression criteria,20 n | 2 |
| Insufficient hematologic response, n (%) | 8 (38) |
| dFLC at TTNT, median (IQR), mg/dL | 5.95 (3.1-16.9) |
| Continued or worsening organ dysfunction, n (%) | 2 (10) |
| Decision for consolidative autologous transplant, n (%) | 1 (5) |
Hematologic responses
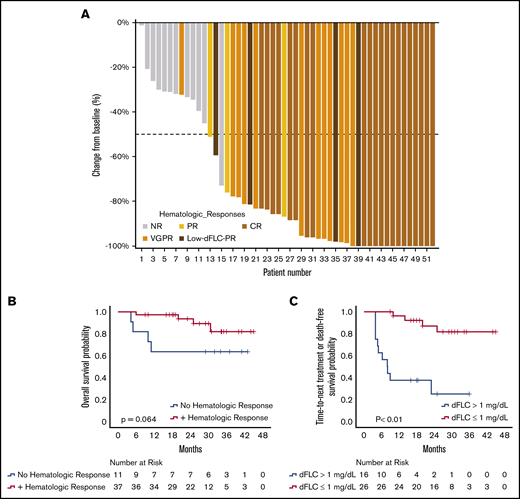
Among the 72 patients, 52 patients were evaluable for hematologic response; 20 patients were excluded from hematologic response assessment for dFLC <2 mg/dL at daratumumab initiation. Forty of 52 patients (77%) achieved a hematologic response, with 21 patients (40%) achieving CR, 12 patients (23%) achieving VGPR, 4 patients (8%) achieving a low-dFLC PR, and 3 patients (6%) achieving PR; 12 patients (23%) had no response (NR). Twenty-nine patients (56%) were able to achieve deep responses with dFLC ≤1 mg/dL. Among the 31 patients with an initial dFLC ≥5 mg/dL, a similar high response rate of 81% was observed, with 10 patients (32%) achieving CR, 12 patients (39%) achieving VGPR, 3 patients (10%) achieving PR, and 6 patients (19%) with NR. Among responders, the median maximal reduction in dFLC was 96% (IQR, 83.5-100), with a median dFLC reduction of 6.5 mg/dL (IQR, 2.7-11). Median time to response was 1 month. A waterfall plot of response rates for the evaluable patients is depicted in Figure 2A.
Hematologic responses with DMT. (A) Waterfall plot of best hematologic responses. (B) OS stratified by hematologic responses at 3 months. (C) TTNT or death among hematologic responders stratified by depth of response at 3 months. Panels B and C show 3-month landmark analyses; the 0 time point represents initiation of daratumumab.
Hematologic responses with DMT. (A) Waterfall plot of best hematologic responses. (B) OS stratified by hematologic responses at 3 months. (C) TTNT or death among hematologic responders stratified by depth of response at 3 months. Panels B and C show 3-month landmark analyses; the 0 time point represents initiation of daratumumab.
Fifty-one patients were evaluable for early responses 1 month after starting therapy. Six patients (12%) achieved CR, 16 patients (31%) achieved VGPR, 8 patients (16%) achieved low-dFLC PR, 7 patients (14%) achieved PR, and 14 patients (27%) had NR. Among the 14 patients with NR after 1 month, only 2 patients subsequently achieved a hematologic response (1 VGPR and 1 CR) with continuing DMT, all within 3 months of starting daratumumab.
At a 3-month landmark analysis, there was a trend toward improved OS among hematologic responders vs nonresponders (P = .064) (Figure 2B). Among responders, those achieving a deep hematologic response (dFLC ≤1 mg/dL) at 3 months had significantly improved TTNT-free survival, with a 3-year TTNT-free survival of 82% compared with 25% for responders who did not achieve a deep hematologic response (median, not reached vs 8 months; P < .01) (Figure 2C).
Cardiac responses
Fifty-seven patients were determined to have cardiac involvement. Median NT-proBNP, troponin I, and dFLC prior to daratumumab was 2840 pg/mL (IQR, 624-7432), 0.03 ng/mL (IQR, 0-0.1), and 4.86 mg/dL (IQR, 2.1-13.2 mg/dL), respectively.
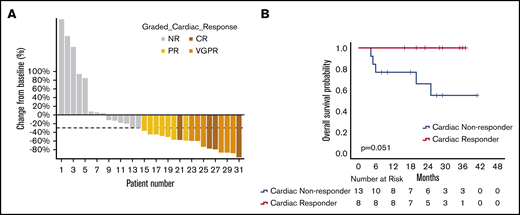
Among these patients, 31 were evaluable for cardiac response; the other patients were excluded because of NT-proBNP <650 ng/mL (13 patients), incomplete data (7 patients), worsening eGFR > 25% with an increase in creatinine > 0.5 mg/dL (4 patients), and prior heart transplant (2 patients). Seventeen of the 31 patients (55%) achieved a cardiac response with improvement of NT-proBNP by ≥30% and 300 ng/mL. In terms of cardiac responses, 4 patients (13%) were able to achieve CR, 6 patients (20%) achieved VGPR, and 7 patients (23%) achieved PR. Among responders, the median reduction was 60% (IQR, 50-83), and the median absolute reduction was 2333 pg/mL (IQR, 1027-4056). A waterfall plot depicting cardiac responses is shown in Figure 3A. Of note, among the cardiac responders, 8 patients had never achieved a cardiac response with prior therapies. Four patients who had responded previously had further improvements in NT-proBNP with starting daratumumab after their NT-proBNP levels had plateaued or began to rise prior to starting daratumumab.
Cardiac responses with DMT. (A) Waterfall plot of best cardiac responses. (B) OS of cardiac responders and cardiac nonresponders at 3 months (3-month landmark analysis with 0 time point representing initiation of daratumumab).
Cardiac responses with DMT. (A) Waterfall plot of best cardiac responses. (B) OS of cardiac responders and cardiac nonresponders at 3 months (3-month landmark analysis with 0 time point representing initiation of daratumumab).
Among the 17 patients achieving a cardiac response, 7 patients achieved hematologic CR, 3 patients achieved hematologic VGPR, 2 patients achieved hematologic PR, 1 patient achieved low-dFLC PR, and 1 patient had NR (3 patients were not assessable for hematologic response because of an initial dFLC <2 mg/dL). There was a higher rate of cardiac response among patients with hematologic responses compared with those without hematologic responses (68% vs 16%, P = .056), bordering on statistical significance.
For those achieving a cardiac response, the median time to achieve at least a cardiac PR was 3.2 months (IQR, 1.2-5.4) and 6.9 months (IQR, 3.1-18.3) for achieving a cardiac VGPR or CR; median time-to-best cardiac response was 16.8 months (IQR, 4-26.7). With time-to-event analysis via the Kaplan-Meier method for all evaluable patients, the estimated median time-to-cardiac response was 6 months (95% CI, 2.5-9.5), and the estimated median time-to-maximal response was 24 months (95% CI, 12.4-35.5).
Cardiac response was associated with statistically significant longer OS (P = .001). At a 3-month landmark analysis, all of the patients who achieved a cardiac response at 3 months were alive at 30 months compared with only 55% of those without a cardiac response at 30 months; this trended toward statistical significance (P = .051; Figure 3B).
Renal responses
Forty-seven patients were determined to have renal involvement. The median creatinine, eGFR, 24-hour urine protein, and initial dFLC were 1.9 mg/dL (IQR, 1.18-3.54), 35 mL/min per 1.73 m2 (IQR, 17-63), 3853 mg per 24 hours (IQR, 2348-6886), and 3.5 mg/dL (IQR, 2-10.4), respectively. Six patients (12.8%) were on dialysis prior to initiating daratumumab.
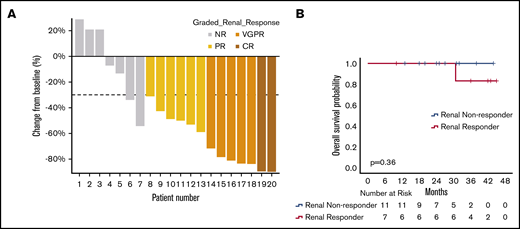
Among the 47 patients, 25 patients were evaluable for response. Among the 22 excluded patients, 12 patients did not have sufficient data, 6 patients were previously on dialysis, and 4 patients had minimal residual proteinuria (<0.5 g per 24 hours) at the time of initiating daratumumab. Thirteen of 25 patients (52%) achieved a renal response based on a >30% reduction in 24-hour proteinuria. Using the new proposed renal criteria, 2 patients (8%) achieved renal CR, 5 patients (20%) achieved renal VGPR, 6 patients (24%) achieved renal PR, and 12 patients had NR, worsening creatinine, or were subsequently started on hemodialysis (54%). Among responders, the median percentage reduction in 24-hour urine protein, median absolute reduction in 24-hour urine protein, and median percentage improvement in eGFR were 71.7% (IQR, 49.5-83.7), 3.2 g (IQR, 1.8-5.7), and 11% (IQR, −1 to 52), respectively. Renal responses are shown in the waterfall plot in Figure 4A. Of the renal responders, 5 patients had not achieved a renal response prior to daratumumab therapy, and 2 patients had a previous renal response but then developed increasing proteinuria, which subsequently responded to daratumumab therapy. Of the renal nonresponders, 2 patients achieved a reduction in proteinuria by ≥30% after starting daratumumab, despite a >25% increase in creatinine above baseline. Three patients were started on hemodialysis.
Renal responses with DMT. (A) Waterfall plot of best renal responses. Patients who were started on hemodialysis or had evidence of worsening creatinine clearance > 25% without available 24-hour urine protein measurements were excluded from the plot. (B) OS of renal responders and renal nonresponders at 6 months (6-month landmark analysis with 0 time point representing initiation of daratumumab).
Renal responses with DMT. (A) Waterfall plot of best renal responses. Patients who were started on hemodialysis or had evidence of worsening creatinine clearance > 25% without available 24-hour urine protein measurements were excluded from the plot. (B) OS of renal responders and renal nonresponders at 6 months (6-month landmark analysis with 0 time point representing initiation of daratumumab).
Among the 13 patients achieving a renal response, 5 patients achieved a hematologic CR, 1 patient achieved a hematologic PR, 1 patient achieved a low-dFLC response, and 2 patients had NR (4 patients were not assessable for hematologic response because of initial dFLC <2 mg/dL). There was no significant difference in renal response rates among evaluable hematologic responders vs nonresponders (54% vs 66%, P = .6).
For patients who achieved a renal response, median time to renal response was 6 months (IQR, 4.3-7), and it was 11.4 months (IQR, 7.7-22.9) for renal VGPR or CR; median time-to-best renal response was 12.9 months (IQR, 6.2-26.2). With time-to-event analysis via the Kaplan-Meier method for all evaluable patients, the estimated median time-to-initial renal response was 20.6 months (95% CI, 0-47), and the estimated median time-to-maximal response was 25.9 months (95% CI, 20.3-31.6).
There was no significant difference in OS among renal responders vs renal nonresponders for all evaluable patients (P = .75) and on 6-month landmark analyses (P = .36; Figure 4B).
Discussion
Our study highlights the efficacy of daratumumab in terms of hematologic and organ responses. This is the largest study to report on organ responses after daratumumab treatment and builds on our original pivotal study highlighting the safety and efficacy of daratumumab in heavily pretreated AL amyloidosis.
In our cohort, OS with daratumumab therapy was excellent, with a 2-year OS rate of 86.9% and a median OS that was not reached with a median follow-up of 27 months. A high hematologic response rate of 77% was observed, including >60% of patients achieving a VGPR or better, despite patients being heavily pretreated, consistent with other studies.8,10,11,16
The median TTNT or death was not reached for the study cohort. Of note, we observed that the majority of treatment changes or deaths occurred within the first 6 months; subsequently, a notable proportion was able to achieve longer-term responses, as demonstrated by the plateau in the survival curve tails. To explore the relationship between depth of response and duration of response, we stratified hematologic responders by whether “stringent dFLC response” criteria were met, recently defined by Manwani et al17 as achieving a dFLC level < 1 mg/dL. Similar to their study, we observed that achieving a stringent dFLC response at 3 months was associated with a significantly improved TTNT, with 82% of those achieving a stringent dFLC response not having progressed to next-line therapy or dead at 3 years, perhaps providing early evidence that durable responses can be achieved with daratumumab in a subset of patients.
In our cohort, >50% of evaluable patients treated with daratumumab-based therapies achieved a measurable cardiac and renal response, with a median time-to-initial response of 3.2 and 6 months, respectively, among responders. These results demonstrate that daratumumab can induce rapid organ responses and, most notably, our cardiac response rates are faster than those reported previously. Muchtar et al reported a median time from initiation of therapy to first cardiac response of 9.4 months among organ responders in the front-line setting at the Mayo Clinic.4 One possible explanation for the faster responses observed in our study is that our responses were assessed in the subsequent-line setting, in which prior therapies may have reduced the burden of disease, facilitating more rapid organ responses compared with initial treatment. Our median time-to-best cardiac response was 16.8 months, which is similar to the median time-to-best cardiac response of 13.5 months described by the Mayo Clinic group in the subsequent-line setting with daratumumab.10
Our findings support the notion that organ damage and dysfunction can be reversed by effective plasma cell–directed therapies. Consistent with other studies that have shown an association with hematologic responses and organ responses,2,3,18,19 we found that a high proportion of organ responders had achieved a hematologic response. Muchtar et al4 demonstrated that achieving deeper cardiac and renal responses with plasma cell–directed therapies translated to improved OS in newly diagnosed patients. In our study, cardiac response was associated with improved OS and, on a formal 3-month landmark analysis, all patients who achieved a cardiac response 3 months after starting daratumumab were alive at 30 months compared with 55% of patients without a cardiac response. We did not observe a difference in OS with renal responses. Although this may be due to insufficient power in our study, other studies have shown a mixed association between renal response and OS. Muchtar et al4 observed a survival benefit in those with deep renal responses (eg, renal VGPR or CR), but no significant difference in OS was observed in those with partial renal responses. In a larger study by Palladini et al,2 there was also no significant association with OS in those who met the conventional renal response criteria.
It is important to note that most of the previous large studies assessing organ responses in AL amyloidosis have involved patients treated predominantly with melphalan-based therapies and ASCT, which are more aggressive treatment options and may not be feasible in a large proportion of AL amyloidosis patients, who tend to be older and have significant medical comorbidities. In our study, >80% of our patients were not previously or subsequently treated with ASCT compared with the Mayo Clinic cohort, in which >50% of their patients were previously treated with ASCT.10 The positive findings in our study support the use of daratumumab in previously treated AL amyloidosis, and the favorable outcomes in the absence of preceding ASCT are especially reassuring for patients who are unable to or choose not to undergo early ASCT. For transplant-eligible patients, given the known risks of transplant-related morbidity and mortality, further studies are warranted to determine whether early ASCT confers a significant benefit over systemic therapy alone, especially because many patients are able to achieve deep and potentially durable responses with upfront chemotherapy and now, daratumumab in the subsequent-line setting.
Given its retrospective nature, there are several limitations to our study. Because the Stanford University Medical Center does not routinely wait until formal International Symposium of Amyloid and Amyloidosis progression criteria are met before changing therapy, the TTNT or death end point was used as a real-world surrogate for progression-free survival, similar to other reported studies.7,17 However, we acknowledge that this end point is subjective, because the decision to initiate next-line therapy is dependent on different clinical practice patterns; although there are emerging criteria to guide these decisions (eg, Pavia high-risk criteria20 ), there is no definitive consensus on the appropriate time to restart or change therapies. Second, given that the highest risk for mortality in AL amyloidosis is during initial front-line therapy, selection bias for patients with less aggressive disease should be considered, because the patients included in this study had to have survived front-line therapy. Furthermore, the median initial dFLC was relatively low in our study, potentially inflating survival outcomes; however, we continued to observe favorable outcomes when analysis was restricted to those with dFLC >5 mg/dL at daratumumab initiation. Additionally, systematic collection of end points was not preplanned; therefore, incomplete data reduced the number of evaluable patients for organ responses. Because intervals for organ assessments were not prespecified, exact time-to-response outcomes are likely affected by the timing of testing in our study. Furthermore, given that organ responses are time-dependent variables, there may be a bias toward evaluating responses more regularly in those patients clinically responding to treatment. Although our study is one of the largest to report on daratumumab in AL amyloidosis, the sample size and low number of events did limit the power of our study to detect statistically significant differences in our exploratory analyses. Longer follow-up would also be beneficial.
In summary, our study demonstrates that daratumumab-based therapy is a very effective treatment option for previously treated AL amyloidosis. In addition to producing deep hematologic responses, significant improvements in cardiac and renal organ responses can be observed, potentially translating into reduced morbidity and mortality. Given that daratumumab is efficacious and well-tolerated, it should be considered an early treatment option for relapsed/refractory AL amyloidosis; the ongoing multicenter ANDROMEDA study (NCT 03201965) is assessing its utility in the frontline setting in newly diagnosed disease in combination with CyBorD. Further studies are needed to investigate whether durable responses can be achieved, the optimal duration of daratumumab therapy, and whether daratumumab combinations can improve outcomes for those patients with early treatment failures or extend the duration of response.
Data sharing requests should be sent to Michaela Liedtke (mliedtke@stanford.edu).
Acknowledgments
The authors thank Patricia Ulloa and Marie Lugtu for caring for AL amyloidosis patients at Stanford University Medical Center and helping to extract data.
Authorship
Contribution: A.C. and G.P.K. collected data; A.C. analyzed results and created the figures; A.C., G.P.K., and M.L. designed the research; and A.C., G.P.K., S.S., E.E., S.L.S., R.A.L, S.A., R.M.W., and M.L. wrote the manuscript.
Conflict-of-interest disclosure: G.P.K. has received travel, lodging, and meal reimbursement, as well as research support, from Janssen. M.L. has acted as a consultant for Adaptive, Amgen/Onyx, and IQVIA/Jazz pharmaceuticals; has received research funding from Agios, Amgen/Onyx, bluebird bio, Caelum, Celator Pharmaceuticals, Celgene, Genentech/Roche, Gilead, Janssen, Novartis, Pfizer, Prothena, and Takeda; has received honoraria from Amgen/Onyx, Caelum, Pfizer, and Prothena; and has served on advisory committees for Caelum, Gilead, Pfizer, Prothena, and Takeda. The remaining authors declare no competing financial interests.
Stanley L. Schrier died on 16 August 2019.
Correspondence: Michaela Liedtke, Stanford Cancer Institute, 875 Blake Wilbur Dr, Room 2327, Stanford, CA 94305; e-mail: mliedtke@stanford.edu.