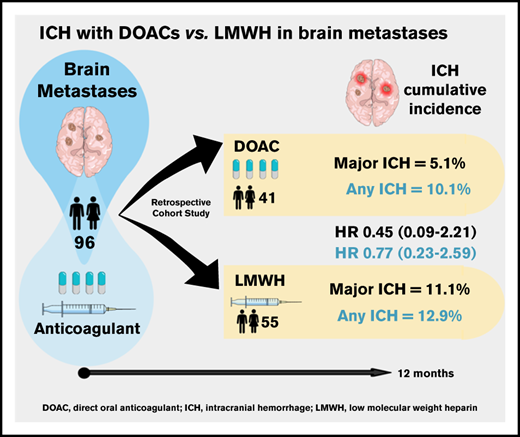
Key Points
This study suggests comparable safety of LMWH and DOACs in patients with brain metastases.
PANWARDS score was not associated with ICH risk; thus, predictors of anticoagulation-associated ICH are needed.
Abstract
Direct oral anticoagulants (DOACs) are increasingly prescribed in treatment of cancer-associated thrombosis, but limited data exist regarding safety of DOACs in patients with brain metastases. We aimed to determine the incidence of intracranial hemorrhage (ICH) in patients with brain metastases receiving DOACs or low-molecular-weight heparin (LMWH) for venous thromboembolism or atrial fibrillation. An international 2-center retrospective cohort study was designed. Follow-up started on the first day of concomitant anticoagulation and brain tumor diagnosis. At least 2 brain imaging studies were mandated. The primary outcome was the cumulative incidence of any spontaneous ICH at 12-month follow-up with death as a competing risk. Major ICH was defined as spontaneous, ≥10 mL in volume, symptomatic, or requiring surgical intervention. Imaging studies were centrally reviewed by a neuroradiologist blinded for anticoagulant type. PANWARDS (platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke) score for prediction of ICH was calculated. We included 96 patients with brain metastases (41 DOAC, 55 LMWH). The 12-month cumulative incidence of major ICH was 5.1% in DOAC-treated patients and 11.1% in those treated with LMWH (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.09-2.21). When anticoagulation was analyzed as a time-varying covariate, the risk of any ICH did not differ between DOAC- and LMWH-treated patients (HR, 0.98; 95% CI, 0.28-3.40). PANWARDS score was not associated with ICH risk. This international 2-center study suggests comparable safety of LMWH and DOACs in patients with brain metastases.
Introduction
Patients with malignancy are at risk of developing cardiovascular complications that warrant anticoagulation, including venous thromboembolism (VTE).1-3 The risk of VTE is particularly high in patients with brain tumors, whereby the cumulative incidence exceeds 10% at 6 months after diagnosis.4-6 Concurrently, high rates of intracranial hemorrhage (ICH) have been observed in patients with brain tumors (up to ∼20% in patients with brain metastases).7 In general, anticoagulation in cancer patients is associated with an increased bleeding risk, notably ICH.8 An increased risk of ICH associated with anticoagulation, as compared with no anticoagulation, has been demonstrated in patients with high-grade glioma,9 but not in patients with brain metastases.7
Low-molecular-weight heparin (LMWH) has been the standard anticoagulant in patients with cancer who develop VTE. Based on recent studies, direct oral anticoagulants (DOACs) represent an alternative, but only few patients with active brain tumors were included in these landmark trials.10-13 Limited data exist regarding the safety of DOACs in patients with metastatic brain tumors. In a recent retrospective study including 105 patients with brain metastases receiving anticoagulation for VTE treatment, DOACs did not appear to increase the risk of any ICH relative to LMWH or the risk of major ICH.14 The number of DOAC-treated patients, however, was limited (n = 21), and uncertainty regarding the safety of DOACs in patients with brain metastases remains. Furthermore, there is a paucity of data on predictors of anticoagulation-associated ICH in patients with brain cancer. The PANWARDS (platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke) score, developed to predict ICH in noncancer patients on therapeutic anticoagulation,15 predicted ICH in a retrospective cohort study including 133 glioma patients9 but has not been assessed in patients with brain metastases. Although anticoagulation-related ICH is frequent in these patients, data on clinical presentation, course, and management are also scarce.16
We aimed to assess the rates of ICH associated with DOACs and LMWH in patients with metastatic brain tumors, test the performance of the PANWARDS score, and evaluate the clinical presentation, management, and course of ICH.
Methods
Study design
We conducted an international 2-center retrospective cohort study in Petah Tikva, Israel and Amsterdam, The Netherlands. The study protocol was approved by the local medical ethics committees of Rabin Medical Center and the Academic Medical Center, and written informed consent was waived. Data were extracted from the electronic medical records at the respective centers from 1 January 2014 to 1 July 2019. Adult patients were eligible if the following inclusion criteria were met: (1) confirmed presence of brain metastases; (2) anticoagulation therapy prescribed at therapeutic doses in the presence of active brain cancer, for any indication and any duration; and (3) ≥2 neuroimaging studies (computed tomography or magnetic resonance imaging) from index until end of follow-up, unless death occurred prior the second imaging study. Patients with lack of follow-up data after study index, any ICH that occurred before initiation of anticoagulation, or neurosurgery within 4 weeks prior index were excluded from study participation.
Study index date was defined as the day of brain metastases diagnosis in anticoagulated patients, or start of anticoagulation in patients with known brain metastases, with a follow-up duration of 12 months. Patients with anticoagulation-related ICH were followed for an additional 90 days post-ICH, and clinical presentation, management, and outcomes of ICH were assessed. Patients were censored upon death or migration/loss to follow-up, and those discharged to receive terminal care were considered deceased at the date of the last contact.
Definitions
The diagnosis of metastatic brain cancer was defined as a pathology report confirming systemic solid cancer and an imaging report confirming brain metastases. Anticoagulation was defined as treatment with either DOACs or LMWH. Therapeutic doses included full dose and indicated dose-reductions prescribed with therapeutic intent.
Data collection
Diagnostic codes and a search engine designed to search unstructured data (CTCue) were used to identify potentially eligible patients, as detailed in the supplementary material. Using the predefined inclusion and exclusion criteria, all medical records were reviewed manually to ensure eligibility, and consecutive patients meeting the eligibility criteria were included. All available neuroimaging study reports during the study period were reviewed for each case. Imaging studies were performed due to symptoms or at routine oncological follow-up, but not at set intervals. Studies reporting any type of hemorrhage or presence of blood products were manually reviewed by a neuroradiologist blinded for type of anticoagulant. The radiologist was asked to confirm the presence of hemorrhage and assessed ICH-related outcomes, including bleeding volume, number of bleeds, location of the ICH, and presence of a midline shift and/or herniation. The bleeding volumes were calculated using the one-half ABC technique.17 In line with prior studies, ICHs were classified as “trace,” “measurable,” or “major.” Trace hemorrhages measured <1 mL in volume or were unmeasurable; measurable ICHs were classified as those that measured ≥1 mL but <10 mL in volume; and major ICH was defined as ICH that measured ≥10 mL in volume, required surgical intervention, or was associated with clinical symptoms, focal neurologic deficits, or changes in cognitive function.7,9 Only spontaneous, nontraumatic ICH was considered as a study outcome. The date of ICH was defined as the day of the first imaging study demonstrating ICH. Dates of death represent the actual dates of death based upon mortality reports (Academic Medical Center) or national mortality records (Rabin Medical Center). Fatal ICH was defined as death occurring within 30 days of ICH.
The PANWARDS prediction score, used to assess ICH risk, was calculated at study index as previously described.9,15 The score was generated only if data were available for ≥6 of the 7 variables, similar to a prior study.9 A PANWARDS score of 25 was used to discern between high-risk (≥25) and low-risk (<25) groups. This cutoff was chosen based on 100% sensitivity and 40% specificity for identifying major ICH in a prior study of patients with primary brain cancer.9 We assessed the severity of the clinical presentation and clinical course of all ICH events using prespecified criteria (see supplemental Table 1 for the definitions). The clinical presentation severity scale provides a general impression of the patient at the time of presentation with ICH. The clinical course severity scale is used to appraise the measures taken for treatment of the ICH and outcome of the bleeding event in the subsequent days.18,19
Study data were collected and managed using REDCap electronic data capture tools hosted at Rabin Medical Center using the Clalit Health Services central server.20,21
Statistical analysis
Data are presented as median (interquartile range [IQR]) for continuous variables and proportions (%) for categorical variables. Differences in categorical variables between treatment groups were analyzed using the Fisher’s exact test and the Wilcoxon rank-sum test for continuous variables. The main outcome was defined as major ICH during 12 months of follow-up. Additional outcomes included any ICH, recurrent ICH during 90 days of follow-up post-ICH (in patients with an index ICH), and VTE or arterial thromboembolism post-ICH.
The cumulative incidence of major ICH and any ICH over 12 months and corresponding 95% confidence intervals (CIs) was calculated for each treatment group, with death as competing risk using the Aalen-Johansen estimator. A Cox proportional hazards model was used to calculate hazard ratios (HRs) and corresponding 95% CIs for major ICH and any ICH between the LMWH and DOAC cohorts, with death as a competing risk (Fine and Gray model). These analyses were repeated with anticoagulation as a time-dependent covariate, taking into account changes in or discontinuation of anticoagulation occurring before end of follow-up. The performance of the PANWARDS prediction score was assessed by calculating cumulative incidence of any ICH at 12 months in the low- and high-risk groups and determining HR (95% CI) for any ICH between the 2 risk groups, with death as a competing risk. In patients with anticoagulation-associated ICH, a descriptive analysis of presentation, course, management, and 90-day outcomes was performed. All statistical analyses were carried out in SAS version 9.4.
Results
Patient characteristics
The study cohort included 96 patients with brain metastases, of whom 41 were treated with a DOAC and 55 received LMWH at the index date. Patient characteristics, stratified for anticoagulation class at index, are shown in Table 1. Lung cancer was the most common primary tumor site, followed by esophageal cancer in the DOAC-treated patients and breast cancer in the LMWH group. The majority of patients received anticoagulation for VTE treatment (49 of 55 [89.1%] in the LMWH group and 22 of 41 [53.7%] in the DOAC group, respectively). DOACs were started a median of 115 days before brain metastases were diagnosed (IQR, 419 days before to 106 after), while LMWH was initiated a median of 36 days after diagnosis (IQR, 7 days before to 190 after).
Patient characteristics at baseline, stratified for anticoagulation class
| Variable (at study index) . | Anticoagulation class at index . | |
|---|---|---|
| DOAC* . | LMWH† . | |
| All patients, n | 41 | 55 |
| Age, median (IQR), y | 66 (58-74) | 64 (57-71) |
| Sex | ||
| Male | 27 (65.9) | 28 (50.9) |
| Female | 14 (34.1) | 27 (49.1) |
| Primary cancer type | ||
| Lung | 23 (56.1) | 31 (56.4) |
| Renal cell | 3 (7.3) | 2 (3.6) |
| Breast | 1 (2.4) | 8 (14.6) |
| Esophageal | 7 (17.1) | 4 (7.3) |
| Other‡ | 7 (17.1) | 10 (18.2) |
| Cancer status | ||
| Newly diagnosed | 2 (4.9) | 6 (10.9) |
| Relapsed/refractory | 39 (95.1) | 49 (89.1) |
| Medical anticancer treatment (yes) | 23 (56.1) | 44 (80) |
| Radiotherapy | ||
| Stereotactic radiosurgery | 20 (48.8) | 23 (41.8) |
| Whole brain radiotherapy | 6 (14.6) | 13 (23.6) |
| None | 14 (34.2) | 17 (30.9) |
| Missing | 1 (2.4) | 2 (3.6) |
| Prior neurosurgery (yes) | 4 (9.8) | 5 (9.1) |
| Anticoagulation dose | ||
| Full | 37 (90.2) | 48 (87.3) |
| Intermediate | 0 | 7 (12.7) |
| Secondary prophylaxis | 4 (9.8) | 0 |
| Indication for anticoagulation | ||
| VTE | 22 (53.7) | 49 (89.1) |
| Atrial fibrillation | 19 (46.3) | 6 (10.9) |
| Comorbidities | ||
| Hypertension | 20 (48.8) | 15 (27.3) |
| Chronic kidney disease | 8 (19.5) | 4 (7.3) |
| Prior ischemic stroke§ | 4 (9.8) | 5 (9.1) |
| Aspirin therapy (yes) | 2 (4.9) | 2 (3.6) |
| PANWARDS score|| | ||
| High ICH risk (≥25) | 26 (63.4) | 25 (45.5) |
| Low ICH risk (<25) | 14 (34.2) | 28 (50.9) |
| Missing | 1 (2.4) | 2 (3.6) |
| Study center | ||
| Amsterdam | 24 (58.5) | 21 (38.2) |
| Rabin | 17 (41.5) | 34 (61.8) |
| Variable (at study index) . | Anticoagulation class at index . | |
|---|---|---|
| DOAC* . | LMWH† . | |
| All patients, n | 41 | 55 |
| Age, median (IQR), y | 66 (58-74) | 64 (57-71) |
| Sex | ||
| Male | 27 (65.9) | 28 (50.9) |
| Female | 14 (34.1) | 27 (49.1) |
| Primary cancer type | ||
| Lung | 23 (56.1) | 31 (56.4) |
| Renal cell | 3 (7.3) | 2 (3.6) |
| Breast | 1 (2.4) | 8 (14.6) |
| Esophageal | 7 (17.1) | 4 (7.3) |
| Other‡ | 7 (17.1) | 10 (18.2) |
| Cancer status | ||
| Newly diagnosed | 2 (4.9) | 6 (10.9) |
| Relapsed/refractory | 39 (95.1) | 49 (89.1) |
| Medical anticancer treatment (yes) | 23 (56.1) | 44 (80) |
| Radiotherapy | ||
| Stereotactic radiosurgery | 20 (48.8) | 23 (41.8) |
| Whole brain radiotherapy | 6 (14.6) | 13 (23.6) |
| None | 14 (34.2) | 17 (30.9) |
| Missing | 1 (2.4) | 2 (3.6) |
| Prior neurosurgery (yes) | 4 (9.8) | 5 (9.1) |
| Anticoagulation dose | ||
| Full | 37 (90.2) | 48 (87.3) |
| Intermediate | 0 | 7 (12.7) |
| Secondary prophylaxis | 4 (9.8) | 0 |
| Indication for anticoagulation | ||
| VTE | 22 (53.7) | 49 (89.1) |
| Atrial fibrillation | 19 (46.3) | 6 (10.9) |
| Comorbidities | ||
| Hypertension | 20 (48.8) | 15 (27.3) |
| Chronic kidney disease | 8 (19.5) | 4 (7.3) |
| Prior ischemic stroke§ | 4 (9.8) | 5 (9.1) |
| Aspirin therapy (yes) | 2 (4.9) | 2 (3.6) |
| PANWARDS score|| | ||
| High ICH risk (≥25) | 26 (63.4) | 25 (45.5) |
| Low ICH risk (<25) | 14 (34.2) | 28 (50.9) |
| Missing | 1 (2.4) | 2 (3.6) |
| Study center | ||
| Amsterdam | 24 (58.5) | 21 (38.2) |
| Rabin | 17 (41.5) | 34 (61.8) |
Values represent n (%) of patients unless otherwise indicated.
NSAID, nonsteroidal anti-inflammatory drug.
Specific DOAC types included apixaban (n = 11), dabigatran (n = 5), edoxaban (n = 8), and rivaroxaban (n = 17).
Specific LMWH types included enoxaparin (n = 34), nadroparin (n = 15), and tinzaparin (n = 6).
Other cancer types (n ≤ 2) include cervical, colon, diffuse large B-cell lymphoma, germ-cell tumor, ovarian, sarcoma, sarcomatoid carcinoma, squamous cell carcinoma of parotid, urothelial carcinoma, thymic neuroendocrine, or unknown origin.
Evidence of prior ischemic stroke on baseline neuroimaging studies.
PANWARDS risk score for predicting ICH on therapeutic anticoagulation15 classified as either high (score ≥25) or low (score <25) ICH risk.
The median duration of follow-up was 136 days (IQR, 63-320 days) in the DOAC group and 175 days (IQR, 63-365 days) in LMWH-treated patients. Four patients (4.2%) were lost to follow-up. The 12-month mortality rates were comparable between the DOAC and LMWH groups (63.4% [n = 26] and 63.6% [n = 35], respectively). A median of 3 neuroimaging studies were performed in both the DOAC (IQR, 2-4) and LMWH (IQR, 2-6) groups during follow-up. Four patients in the DOAC group (9.8%) and 7 patients in the LMWH group (12.7%) had severe thrombocytopenia (ie, platelets <50 × 109/L) documented at any time during follow-up.
ICH incidence
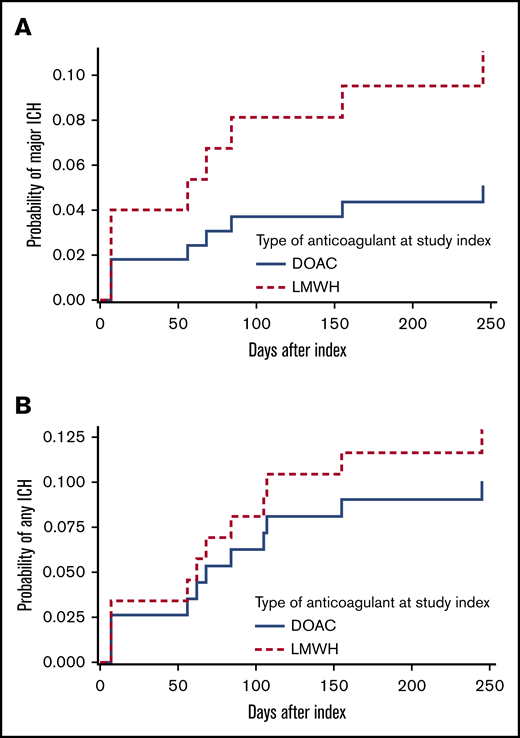
The 12-month cumulative incidence of major and any ICH in the DOAC group was 5.1% and 10.1%, respectively, compared with 11.1% and 12.9% in LMWH-treated patients (Figure 1; HR, 0.45 [95% CI, 0.09-2.21] and HR, 0.77 [95% CI, 0.23-2.59]). This did not materially change when anticoagulation was analyzed as a time-varying covariate; the HR for major ICH was 0.57 (95% CI, 0.12-2.87), and the HR for any ICH was 0.98 (95% CI, 0.28-3.4). The cumulative incidence of major ICH did not differ between the 2 study centers (HR, 1.16; 95% CI, 0.3-4.52).
Twelve-month cumulative incidence of ICH, stratified for anticoagulation class (DOAC vs LMWH). (A) Major ICH. (B) Any ICH. Death was considered a competing risk (Aalen-Johansen estimator), and type of anticoagulant was a fixed variable.
Twelve-month cumulative incidence of ICH, stratified for anticoagulation class (DOAC vs LMWH). (A) Major ICH. (B) Any ICH. Death was considered a competing risk (Aalen-Johansen estimator), and type of anticoagulant was a fixed variable.
Table 2 shows the ICH characteristics and anticoagulation management during follow-up. The median time (range) to any ICH was 35 (7-84) days in the DOAC group and 84 (56-155) days in the LMWH group. In addition to the 11 spontaneous ICH events, 2 patients (both in the LMWH group) had traumatic ICH (major). No ICH occurred in patients who crossed anticoagulant groups during follow-up, as detailed in Table 2. Anticoagulation was stopped in 1 patient prior to ICH.
ICH and anticoagulation management during 12 months of follow-up
| Variable . | Anticoagulation class at index . | |
|---|---|---|
| DOAC . | LMWH . | |
| All patients, n | 41 | 55 |
| Anticoagulation management* | ||
| Anticoagulants stopped | 3 (7.3) | 4 (7.3) |
| Anticoagulant group changed† | 2 (4.9) | 4 (7.3) |
| Spontaneous ICH (any) | 4 (10) | 7 (13) |
| ICH classification | ||
| Major‡ | 2 (4.9) | 6 (10.9) |
| Measurable§ | 2 (4.9) | 0 |
| Trace|| | 0 | 1 (1.8) |
| Radiological characteristics of ICH | ||
| Midline shift | 1 (2.4) | 1 (1.8) |
| Herniation | 0 | 2 (3.6) |
| Volume of largest bleed, median (IQR), mL | 1.4 (0.73-1.8) | 0.7 (0.5-5) |
| Number of distinct bleeds, median (IQR) | 1 (1-2) | 1 (1-2) |
| Fatal ICH | 0 | 3 (5.5) |
| Variable . | Anticoagulation class at index . | |
|---|---|---|
| DOAC . | LMWH . | |
| All patients, n | 41 | 55 |
| Anticoagulation management* | ||
| Anticoagulants stopped | 3 (7.3) | 4 (7.3) |
| Anticoagulant group changed† | 2 (4.9) | 4 (7.3) |
| Spontaneous ICH (any) | 4 (10) | 7 (13) |
| ICH classification | ||
| Major‡ | 2 (4.9) | 6 (10.9) |
| Measurable§ | 2 (4.9) | 0 |
| Trace|| | 0 | 1 (1.8) |
| Radiological characteristics of ICH | ||
| Midline shift | 1 (2.4) | 1 (1.8) |
| Herniation | 0 | 2 (3.6) |
| Volume of largest bleed, median (IQR), mL | 1.4 (0.73-1.8) | 0.7 (0.5-5) |
| Number of distinct bleeds, median (IQR) | 1 (1-2) | 1 (1-2) |
| Fatal ICH | 0 | 3 (5.5) |
Values represent n (%) of patients unless otherwise indicated.
During the 12-month follow-up, before censorship for death or ICH.
Defined as a change from LMWH to DOAC or vice versa.
ICH ≥10 mL in volume, required surgical intervention, or was associated with symptoms. All the patients with major ICH were symptomatic. One LMWH-treated patient also had ICH volume ≥10 mL.
ICH volume ≥1 mL but <10 mL.
ICH volume <1 mL or unmeasurable.
ICH risk assessment with PANWARDS score
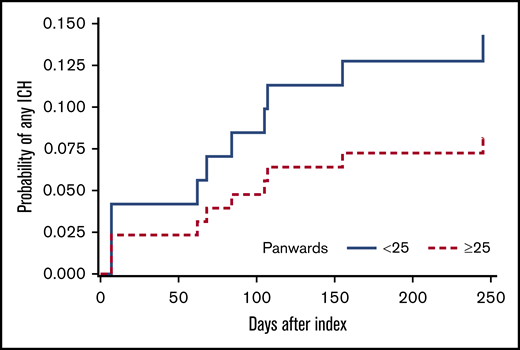
The PANWARDS score could be calculated in 93 patients (96.9%) using either 6 (n = 63) or 7 (n = 30) of the full set of 7 variables. The full set of PANWARDS variables was available more often in DOAC-treated patients (n = 16; 39%) than in LMWH-treated ones (n = 14; 25%). The median PANWARDS score (range) was 28 (23-36) in the DOAC group and 24 (6-31) in the LMWH group. More DOAC-treated patients had a high PANWARDS risk score than LMWH patients (26 of 41 [63.1%] and 25 of 55 [45.5%], respectively; P = .097). The 12-month cumulative incidence of any ICH was 14.3% in the low-risk group and 8.2% in the high-risk group (Figure 2). The incidence of any ICH did not differ between the PANWARDS risk groups (HR, 1.81; 95% CI, 0.52-6.35).
Twelve-month cumulative incidence of ICH, stratified for high-risk (≥25) vs low-risk (<25) PANWARDS score.15 Death was considered a competing risk (Aalen-Johansen estimator). PANWARDS score was calculated at index using platelet count, albumin, history of congestive heart failure, age, race, diastolic blood pressure, and previous history of stroke or transient ischemic attack.15
Twelve-month cumulative incidence of ICH, stratified for high-risk (≥25) vs low-risk (<25) PANWARDS score.15 Death was considered a competing risk (Aalen-Johansen estimator). PANWARDS score was calculated at index using platelet count, albumin, history of congestive heart failure, age, race, diastolic blood pressure, and previous history of stroke or transient ischemic attack.15
Presentation and management of anticoagulation-associated ICH
Ten patients had anticoagulation-associated spontaneous ICH during 12 months of follow-up. The clinical presentation, course, and management of each case are summarized in Table 3, and the cases are detailed in the supplementary material. The ICH was classified as major in 7 out of 10 cases, primarily to due neurological symptoms. Accordingly, the ICH presentation18 was category 3 in all these cases, with the remainder graded as category 1. The immediate clinical course was variable, with only 2 patients in category 3, both of whom were alive at 90 days after ICH, while the others had a category 1 or 2 clinical course.
Clinical presentation, course, and management of anticoagulation-associated ICH
| Case . | Cancer type . | AC type* . | ICH class† . | ICH presentation‡ . | ICH course‡ . | PCC . | Surgical management . | ICU . | AC management§ . | Death|| . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lung | Apixaban | Measurable | 1 | 1 | No | No | No | Continue | No |
| 2 | Lung | Apixaban | Major | 3 | 3 | Yes | No | Yes | Stop | No |
| 3 | RCC | Apixaban | Measurable | 1 | 1 | No | No | No | Continue | No |
| 4 | RCC | Rivaroxaban | Major | 3 | 3 | Yes | No | Yes | Stop | No |
| 5 | Lung | Nadroparin | Major | 3 | 1 | No | No | No | Stop | Yes (+62) |
| 6 | Lung | Enoxaparin | Trace | 1 | 1 | No | No | No | Continue | No |
| 7¶ | Lung | Enoxaparin | Major | 3 | 2 | No | Yes | No | Hold (9 d) | No |
| 8 | Lung | Enoxaparin | Major | 3 | — | — | — | — | — | Yes (+14) |
| 9 | Thymic NEC | Enoxaparin | Major | 3 | 1 | No | No | No | Hold (2 d) | Yes (+21) |
| 10¶ | Lung | Enoxaparin | Major | 3 | — | — | — | — | — | Yes (+32) |
| Case . | Cancer type . | AC type* . | ICH class† . | ICH presentation‡ . | ICH course‡ . | PCC . | Surgical management . | ICU . | AC management§ . | Death|| . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lung | Apixaban | Measurable | 1 | 1 | No | No | No | Continue | No |
| 2 | Lung | Apixaban | Major | 3 | 3 | Yes | No | Yes | Stop | No |
| 3 | RCC | Apixaban | Measurable | 1 | 1 | No | No | No | Continue | No |
| 4 | RCC | Rivaroxaban | Major | 3 | 3 | Yes | No | Yes | Stop | No |
| 5 | Lung | Nadroparin | Major | 3 | 1 | No | No | No | Stop | Yes (+62) |
| 6 | Lung | Enoxaparin | Trace | 1 | 1 | No | No | No | Continue | No |
| 7¶ | Lung | Enoxaparin | Major | 3 | 2 | No | Yes | No | Hold (9 d) | No |
| 8 | Lung | Enoxaparin | Major | 3 | — | — | — | — | — | Yes (+14) |
| 9 | Thymic NEC | Enoxaparin | Major | 3 | 1 | No | No | No | Hold (2 d) | Yes (+21) |
| 10¶ | Lung | Enoxaparin | Major | 3 | — | — | — | — | — | Yes (+32) |
AC, anticoagulation; ICU, intensive care unit; NEC, neuroendocrine carcinoma; PCC, prothrombin complex concentrate; RCC, renal cell carcinoma.
The anticoagulation dose at the time of ICH was therapeutic in 9 out of 10 cases (prophylactic in case 8), and no patients were receiving antiplatelet therapy.
Major indicates ICH ≥10 mL in volume, required surgical intervention, or was associated with symptoms; Measurable indicates ICH volume ≥1 mL but <10 mL; trace indicates ICH volume <1 mL or unmeasurable.
Based on prespecified criteria as shown in supplemental Table 1, as reported by Bleker et al.18
Anticoagulation management at ICH. The number in parentheses indicates the number of days until anticoagulation was resumed after temporarily holding.
Death by 90 days post-ICH. The number in parentheses indicates the number of days between ICH and death.
Incomplete follow-up data post-ICH.
Two patients with major ICH received prothrombin complex concentrate. No patient received specific reversal agents or other prohemostatic agents. Anticoagulation was continued without disruption in the 3 patients with nonmajor ICH. No patient had a thromboembolic event post-ICH, and 1 patient (case 5) had a recurrent ICH that was not associated with anticoagulation. Four patients, all with major ICH, died within 90 days. There was consensus among the 2 adjudicators (G.S. and H.R.B.) in all cases for clinical presentation and 9 out of 10 cases for clinical course.18 Consensus was achieved in the discordant case after consulting with a referee (S.M.).
Discussion
Results from this international 2-center retrospective cohort study on 96 patients with brain metastases receiving anticoagulation suggest comparable safety of LMWH and DOACs in patients with brain metastases. The risk of any or major nontraumatic ICH at 12 months did not differ statistically between 41 DOAC-treated and 55 LMWH-treated patients, with anticoagulation as a fixed or time-varying covariate and death as a competing risk. The overall 12-month mortality was high and comparable between the 2 groups, whereas ICH-related fatalities only occurred in the LMWH group (3 of 55; 5.5%). While in noncancer patients with ICH, mortality is reduced with DOACs compared with vitamin K antagonists,22,23 reduced ICH-related mortality with DOACs remains to be demonstrated in this context.
Our findings are in concordance with results from another recent retrospective single-center cohort study including 105 patients with metastatic brain tumors, which showed a comparable 12-month cumulative incidence of major ICH in the DOAC (21 patients; 11.1% [95% CI, 0.5% to 40.6%]) and LMWH (84 patients; 17.8% [95% CI, 10.2% to 27.2%]) cohorts.14 The ICH incidence was higher in the prior study than in the current one; nonetheless, there was still no statistically significant difference in ICH between the DOAC and LMWH groups, suggesting generalizability of our findings in populations with variable bleeding risk.
The absolute 12-month incidence of ICH, however, remains high in this population. Whether this is driven by anticoagulation, in addition to patient characteristics, remains to be determined.7 Accordingly, predictors of anticoagulation-associated ICH are warranted to enable assessment of the risk-benefit ratio of anticoagulation. We did not find the PANWARDS risk classification (using a cutoff of 25) to be associated with the risk of ICH in our cohort of anticoagulated patients with brain metastases, in contrast to its performance in glioma patients.10,19 Due to incomplete scores (6 of 7 variables) in two-thirds of patients and the low number of ICH events, the PANWARDS score could be still evaluated in future studies including receiver-operating curve analyses identifying alternative cutoffs. Nonetheless, we propose that it is unlikely to find a positive correlation between the PANWARDS score and ICH, given the lack of a signal using the 25 cutoff. Therefore, an alternative risk-assessment model for anticoagulation-associated ICH developed separately for metastatic and primary brain tumors is needed. This could be particularly relevant for patients with atrial fibrillation or even remote VTE, whose bleeding risk may exceed the risk of thrombosis.
Clinical decision-making regarding resumption of anticoagulation post-ICH and timing thereof remains challenging, as data to support an optimal strategy are lacking. A recent study of 79 patients with brain tumors demonstrated a high incidence of ICH recurrence and associated mortality after resumption of anticoagulation post-ICH.16 Additionally, the severity of the initial ICH appeared to directly correlate with the risk of recurrent ICH. In the current study, anticoagulation was continued in ∼50% of patients with ICH (Table 3), which is most likely a reflection of the nonsevere clinical presentation and course of these patients. This emphasizes the importance of reporting the severity of clinical presentation and course in relation to the ICH event. The adjudication process highlighted a number of important unresolved issues with the classification criteria, such as whether holding anticoagulation should be considered an intervention or if we can differentiate between symptoms caused by ICH and those caused by brain metastases in the presence of a minor bleed (eg, case 8).
Strengths of this study include a blinded radiology review to minimize classification bias and the use of strict criteria to define ICH. To the best of our knowledge, we here present the largest cohort of DOAC-treated patients with metastatic brain cancer to date, and we provide novel data on the PANWARDS score in this context. We acknowledge this retrospective study has several limitations. The differences at baseline between the anticoagulation groups suggest confounding by indication. Interestingly, most DOACs were started prior to brain metastases diagnosis, and most LMWH treatments were initiated thereafter. We speculate that physicians may be more likely to continue DOACs than initiate DOAC treatment in this setting. More LMWH-treated patients recently started anticoagulant treatment, a period associated with the highest bleeding risk,24 which may indicate the physicians’ preference of LMWH for acute events that warrant anticoagulation. On the other hand, comorbidities were more common in the DOAC group. Importantly, the 12-month overall survival did not differ between the anticoagulation groups. In addition, it is known that the ICH risk varies greatly according to tumor type, with the highest risk associated with renal cell carcinoma and melanoma.7 Our study included little or none of these tumors, affecting generalizability to tumors with high bleeding risk. Finally, DOAC-treated patients were overrepresented in the PANWARDS ≥25 group and had a nominally lower numerical rate of any ICH, which might have contributed to the poor performance of the PANWARDS score.
DOACs are an appealing option from the perspective of a patient with an indication for long-term anticoagulation, provided efficacy and safety are not compromised.25 Although our data did not demonstrate an increased risk of ICH with DOACs, the CIs were wide. Assuming an average 12-month major ICH rate of 15% in patients treated with LMWH, ∼1452 subjects would be required (484 DOAC treated and 968 LMWH treated) to detect a ≥5% increase in the incidence of major ICH in the DOAC group at 80% power and a 2-sided significance level of .05. Therefore, this study and a prior one14 were not powered to detect an increased bleeding risk with DOACs, meaning that further research is warranted. Randomized trials of DOACs vs LMWH in patients with brain tumors are currently not expected, and the required sample size and intricacy of such a study appear to be prohibitive. The current pilot study enabled and drove the design of an ongoing sufficiently powered multinational observational study similarly addressing the multiple aspects of anticoagulation management in patients with brain cancer.26 Such a study could confirm whether DOACs are an acceptable option in patients with metastatic brain tumors.
In conclusion, this study suggests comparable safety of LMWH and DOACs in patients with brain metastases. Research into clinically relevant predictors of anticoagulation-associated ICH is warranted.
Presented in abstract form at the 28th International Society of Thrombosis and Haemostasis Congress and 66th Annual Scientific and Standardization Committee Meeting (virtual conferences; https://academy.isth.org/isth/2020/milan/297215/avi.leader.intracranial.hemorrhage.with.direct.oral.anticoagulants.in.patients.html), 11 July 2020.
For original data, please contact avileader@yahoo.com.
Acknowledgments
The authors thank Tzippy Shochat and Uri Rozovski for their help in the statistical analyses.
Authorship
Contribution: A.L., E.N.H., H.R.B., J.I.Z., and G.S. participated in all aspects of the study and authored the manuscript; B.J.C. and J.I.Z. previously developed the methods and provided the data extraction form; M.A., O.I., and J.J.K. retrieved patient data, and S.R. and L.F.M.B. performed radiologic review of imaging in the respective centers; H.R.B., G.S., and S.M. adjudicated clinical presentation and course of all bleeding events; and all authors interpreted data, reviewed drafts and approved the final draft of the manuscript.
Conflict-of-interest disclosure: A.L. reports personal fees from Pfizer and personal fees from Bayer Healthcare outside the submitted work. S.Y.-K. reports grants from BMS and personal fees from Novartis, AstraZeneca, and Teva outside the submitted work. S.M. reports grants and personal fees from Bayer, BMS Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, and Portola during the conduct of the study, as well as grants and personal fees from GSK and Aspen and personal fees from Sanofi outside the submitted work. H.R.B. reports grants and personal fees from Sanofi-Aventis, Bayer Healthcare, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, and Novartis outside the submitted work. J.I.Z. reports grants from Incyte during the conduct of the study and grants from Incyte and Quercegen, personal fees from CSL, Merck, Parexel, Sanofi, Daiichi, Pfizer/BMS, and Portola outside the submitted work. G.S. reports personal fees from Pfizer, Bayer, Boehringer Ingelheim, and Sanofi outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Avi Leader, Institute of Hematology, Rabin Medical Centre, 39 Jabotinsky, Petah Tikva 4941492, Israel; e-mail: avileader@yahoo.com.
References
Author notes
A.L. and E.N.H. contributed equally to this study.
The full-text version of this article contains a data supplement.