Key Points
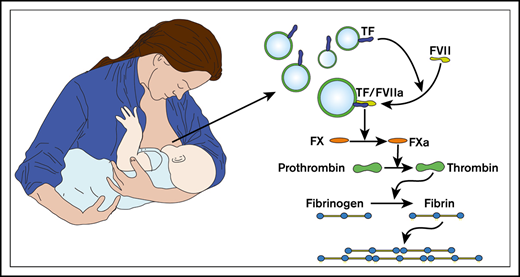
Human milk contains coagulant EVs that expose TF.
Coagulant potential of human milk is sensitive to pasteurization and preparation protocols routinely used in neonatal intensive care units.
Abstract
Almost a century ago, it was discovered that human milk activates the coagulation system, but the milk component that triggers coagulation had until now been unidentified. In the present study, we identify this component and demonstrate that extracellular vesicles (EVs) present in normal human milk expose coagulant tissue factor (TF). This coagulant activity withstands digestive conditions, mimicking those of breastfed infants, but is sensitive to pasteurization of pooled donor milk, which is routinely used in neonatal intensive care units. In contrast to human milk, bovine milk, the basis of most infant formulas, lacks coagulant activity. Currently, the physiological function of TF-exposing vesicles in human milk is unknown, but we speculate that these vesicles may be protective for infants. Another explanation could be nipple skin damage, which occurs in most breastfeeding women. Milk-derived TF-exposing EVs may seal the wound and thereby reduce bleeding and breast inflammation.
Introduction
Eighty-six years ago, pioneering pediatrician Eduard Glanzmann reported in a 1934 lecture that “no other animal milk has such blessed hemostatic properties as human milk,”1 (p458) and he recommended: “Use a tamponade soaked with human milk and press it on the bleeding spot.”1 (p458) In a systematic investigation in the same year, Alphons Solé confirmed that tamponades soaked with human milk effectively stop severe external bleeding in patients with hemophilia.2 The compound in human milk that triggers clotting, however, was never identified. Moreover, it seems that the knowledge about the hemostatic properties of human milk has faded over the years; the most recent publications on this topic are >50 years old.3-6 The present study aims to shed light on the mechanisms that underlie the unexplained hemostatic properties of human milk.
In 1961, M. W. Hess observed that the hemostatic properties of human milk resemble those of brain thromboplastin, which was used as a laboratory reagent to trigger coagulation and was derived from a crude homogenate of rabbit brain.3 Today, it is known that brain thromboplastin contains tissue factor (TF) and phospholipid-rich vesicles.7 TF is the transmembrane receptor of coagulation factor VII(a) and is not detectable in blood or plasma under physiological conditions.8 Upon vascular damage, blood becomes exposed to subendothelial TF, which triggers coagulation and thus contributes to hemostasis.9 In contrast to blood, other human body fluids, such as saliva, urine, seminal fluid, and amniotic fluid, contain coagulant TF under physiological conditions, and this TF is exclusively associated with extracellular vesicles (EVs).10-12 On the basis of these observations, we hypothesized and investigated whether human milk also contains coagulant TF-exposing EVs, which may explain the earlier observations of Glanzmann and Solé.
The American Academy of Pediatrics recommends exclusive breastfeeding for 6 months followed by continued breastfeeding as complementary foods are introduced for ≥1 year13 because of the firmly established beneficial effects of breastfeeding.14-19 We speculated that the ability of human milk to promote coagulation may contribute to these beneficial effects by, for example, triggering coagulation upon gastrointestinal vascular damage. If so, the coagulant activity of TF in human milk should be able to survive the digestive conditions encountered in an infant’s gastrointestinal system.
In the present study, we identified the component responsible for the coagulant properties of human milk and studied whether this coagulant activity could survive conditions encountered in infants’ gastrointestinal systems. Finally, we compared human milk with bovine milk, because bovine milk is the basis of most infant formulas, and we determined the effects of routinely used human milk preparation protocols in neonatal intensive care units (ICUs) on the coagulant activity of human milk.
Methods
Collection and preparation of human milk
Human milk was collected from 6 healthy breastfeeding adult women (median age, 33 years; range, 30-39 years), with approval from the Ethics Committee of the Medical University of Vienna (#1721/2015). According to the study inclusion criteria, each participant had delivered a healthy full-term infant, and infants had been breastfeeding for at least 6 weeks (median, 115 days; range, 63-225 days). Milk was collected in the morning ∼2 hours after previous breastfeeding using a manual breast pump (SCF330/20 pump; Philips Avent, Glemsford, United Kingdom). Milk was collected atraumatically with a low-pressure hand pump. To assure atraumatic milk collection, every donor was asked to report any pain or discomfort during or after milk collection (no donor reported this). A minimum of 10 mL of milk was collected into sterile polyethylene collection tubes. Collected milk was kept at room temperature and centrifuged twice at 3000 g (Rotanta 460R; Hettich, Tuttlingen, Germany) for 10 minutes within 30 minutes after collection. After each centrifugation, the supernatant fat was skimmed, and the pellet was discarded to remove the cells as described previously.20 Aliquots were stored at −80°C until measurements were performed.
Isolation of EVs from human milk
Milk was thawed in a water bath at 37°C. For some experiments, milk was centrifuged at 154 000 g (Beckman Coulter, Indianapolis, IN) for 1 hour at 4°C. After centrifugation, the supernatant was transferred into a new tube, and the pellet was resuspended in the original volume in buffer. Alternatively, milk was fractionated by Sepharose CL-2B (GE Health Care, Pittsburgh, PA) size-exclusion chromatography (SEC) Telos column (15 mL; Kinesis, Vernon Hills, IL) to isolate EVs as described previously.21 Briefly, 500 µL of human milk was thawed in a water bath at 37°C and diluted with 500 µL of saline before loading into the SEC column. In total, 26 fractions of each 0.5 mL were collected. Because SEC separates EVs efficiently from soluble proteins, the protein concentration of the EV-containing peak fractions was very low. Therefore, the total protein present in each SEC fraction was precipitated using trichloroacetic acid and pelleted at 21 000 g for 10 minutes, and pellets were resuspended in 20 μL of phosphate-buffered saline. The pH was adjusted to ∼7.0 in each fraction, and proteins were dissolved in reducing sample buffer as described in the supplemental Methods. By putting the total protein content of SEC fractions on blot, the absolute presence of a protein of interest can be compared between fractions as described earlier.22 Both CD63 and CD9 were below the detection limit in milk, the starting material (Figure 2A-B, respectively), whereas TF was clearly detectable (Figure 3A). The supernatant and resuspended pellet together with SEC fractions were used for flow cytometry, surface plasmon resonance imaging, western blot, and clotting assay, respectively.
Clotting assay
A clotting assay was performed to determine the coagulant potential of milk or fractions thereof as described previously.10 EV-depleted plasma was prepared by centrifuging citrated human pooled normal plasma at 18 890 g at 20°C for 1 hour. Milk was diluted in buffer (total volume, 20 µL) to a final concentration of 1% for most experiments (except those in which milk was fractionated by SEC) and added to 70 μL of the EV-depleted pooled normal plasma with or without anti-TF (clone HTF-1; eBiosciences, San Diego, CA; final concentration, 30 μg/mL). The mixture was incubated at 37°C for 5 minutes. Clotting was initiated by addition of 15 µL of calcium chloride (stock 100 mmol/L). Fibrin generation (ie, clot formation) was monitored for 1 hour at 37°C by measuring the optical density at the wavelength of 405 nm using a SpectraMax i3 microplate detector (Molecular Devices, Sunnyvale, CA).
Supplemental Methods
Detailed methods describing cryogenic electron microscopy (cryo-EM), flow cytometry, surface plasmon resonance imaging, western blot analysis, human milk in vitro digestive models, bovine milk and bovine plasma collection procedures, human milk collection procedures, and clinical characteristics of preterm infants and nursing mothers in the neonatal ICU are provided in the supplemental Methods.
Statistics
Data were analyzed by 2-tailed Student t test (SPSS software, version 25.0; SPSS, Inc., Chicago, IL). A probability value of <.05 was considered statistically significant. Continuous variables are shown as mean ± standard error of the mean, unless stated otherwise.
Results
Procoagulant activity of human milk
To investigate whether human milk triggers clotting, milk was added to pooled normal plasma as described in “Methods.” Milk shortened the clotting time (CT) of plasma, and this shortening was dose dependent (Figure 1A).
Coagulant activity of human milk (HM). (A) Dilutions of HM (n = 6) were added to human plasma as described in “Methods.” Percentages shown are the final concentrations of HM (vol/vol). (B) Images of EVs from milk labeled with annexin V–gold nanoparticles at 2 magnifications: scale bar 1 µm and 100 nm. At higher magnification, the characteristic lipid bilayer of EVs is visible. (C) Milk was fractionated by ultracentrifugation, and presence of TF was determined by western blot for all donors. Human brain lysate was used as positive control. TF was detectable in the pellet and below the detection limit in the supernatant. (D) Milk-, pellet-, and supernatant-induced (1% final concentration; vol/vol) clotting of plasma in the absence and presence of anti-TF. *P < .05, **P < .01, ***P < .001. CF, carbon film; n.s., nonsignificant.
Coagulant activity of human milk (HM). (A) Dilutions of HM (n = 6) were added to human plasma as described in “Methods.” Percentages shown are the final concentrations of HM (vol/vol). (B) Images of EVs from milk labeled with annexin V–gold nanoparticles at 2 magnifications: scale bar 1 µm and 100 nm. At higher magnification, the characteristic lipid bilayer of EVs is visible. (C) Milk was fractionated by ultracentrifugation, and presence of TF was determined by western blot for all donors. Human brain lysate was used as positive control. TF was detectable in the pellet and below the detection limit in the supernatant. (D) Milk-, pellet-, and supernatant-induced (1% final concentration; vol/vol) clotting of plasma in the absence and presence of anti-TF. *P < .05, **P < .01, ***P < .001. CF, carbon film; n.s., nonsignificant.
Because we suspected that the unidentified milk component that induces clotting may result from the presence of EVs, we performed cryo-EM. Cryo-EM demonstrated the presence of EVs in human milk (Figure 1B).
We subjected milk to ultracentrifugation to pellet most EVs. After ultracentrifugation, both TF protein and TF-mediated clotting activity were associated with the pellets (Figure 1C-D, respectively). TF protein was below the detection limit in the supernatant (Figure 1C), but some coagulant activity remained (Figure 1D). On the basis of an Innovin titration curve, we estimated the concentration of TF activity in human milk at 80 pM of TF (n = 6; range, 25-147 pM; supplemental Figure 3).
Because human milk contains multiple soluble and insoluble components, we also isolated EVs by SEC. As published previously,21 EVs eluted mainly in fractions 8 to 10 as confirmed by the presence of the common EV-associated tetraspanins CD9 and CD63 (Figure 2A-B, respectively), flow cytometry (Figure 2C), and surface plasmon resonance imaging (Figure 2D), respectively. The EV containing fractions 8 to 10 had detectable levels of TF protein (Figure 3A) and TF-dependent clotting activity (Figure 3B-C), confirming that both TF protein and TF activity in human milk are associated with EVs.
Isolation of EVs from human milk. Human milk was fractionated by Sepharose 2B SEC, and all fractions were blotted for common EV markers tetraspanins CD63 (A) and CD9 (B). CD63 blot was reused for CD9 staining. Starting material is shown, as well as platelet lysate (positive control) and human brain lysate (negative control). EV-containing fractions 8 and 9 were pooled, and presence of CD9 and CD63 were determined on single EVs by flow cytometry (C) and on bulk EVs by surface plasmon resonance imaging (D). Pooled fractions 25 and 26 were used as negative and procedural controls in panels C and D, respectively. Immunoglobulin G1 (IgG1) was used to correct for binding of antibodies to Fc receptors exposed on EVs. ***P < .001. LALS, large angle light scatter; SPRi, surface plasmon resonance imaging.
Isolation of EVs from human milk. Human milk was fractionated by Sepharose 2B SEC, and all fractions were blotted for common EV markers tetraspanins CD63 (A) and CD9 (B). CD63 blot was reused for CD9 staining. Starting material is shown, as well as platelet lysate (positive control) and human brain lysate (negative control). EV-containing fractions 8 and 9 were pooled, and presence of CD9 and CD63 were determined on single EVs by flow cytometry (C) and on bulk EVs by surface plasmon resonance imaging (D). Pooled fractions 25 and 26 were used as negative and procedural controls in panels C and D, respectively. Immunoglobulin G1 (IgG1) was used to correct for binding of antibodies to Fc receptors exposed on EVs. ***P < .001. LALS, large angle light scatter; SPRi, surface plasmon resonance imaging.
TF is associated with EVs in human milk. (A) Human milk was fractionated by Sepharose 2B SEC, and all fractions were blotted for TF. Blots also show human brain lysate (positive control) and platelet lysate (negative control). All fractions were tested for their ability to trigger clotting of human plasma in the absence and presence of anti-TF (panels B and C, respectively).
TF is associated with EVs in human milk. (A) Human milk was fractionated by Sepharose 2B SEC, and all fractions were blotted for TF. Blots also show human brain lysate (positive control) and platelet lysate (negative control). All fractions were tested for their ability to trigger clotting of human plasma in the absence and presence of anti-TF (panels B and C, respectively).
Cellular origin of EVs in human milk
We isolated EVs from human milk by SEC and pooled the EV-containing fractions 8 and 9 to investigate the cellular origin of EVs by flow cytometry. We detected EVs from epithelial origin (EpCAM+) but not EVs from granulocytes (CD66b+), monocytes (CD14+), lymphocytes (CD4+, CD20+), or platelets (CD6; supplemental Figure 2).
Impact of digestion on coagulant activity of human milk
Next, we determined whether the coagulant activity of human milk resists the digestive conditions encountered in an infant’s gastrointestinal tract. Figure 4A shows that simulated gastric fluid impaired the coagulant activity of human milk at pH 1 and pH 2 but not at pH ≥3 (supplemental Table 1). Figure 4B shows that the coagulant activity was not inhibited by incubation with simulated intestinal fluid (supplemental Table 2). Therefore, the coagulant activity of milk seems to withstand both an infant’s gastric fluid (in infants, the expected pH is ∼6; see “Discussion”) and intestinal fluid.
Effect of digestion on coagulant activity of human milk (HM). HM (1% final concentration; vol/vol) was incubated in simulated gastric fluid (SGF) for 1 hour at 37°C at pH from 1 to 7 (A) and in simulated intestinal fluid (SIF) for 1 hour at 37°C with increasing concentrations of pancreatic enzymes (pancreatin) (B). After incubation of HM with SGF or SIF, samples were ultracentrifuged. Supernatant was removed, and pellets containing TF-exposing EVs were resuspended in the original volume to perform the clotting assay. **P < .01, ***P < .001.
Effect of digestion on coagulant activity of human milk (HM). HM (1% final concentration; vol/vol) was incubated in simulated gastric fluid (SGF) for 1 hour at 37°C at pH from 1 to 7 (A) and in simulated intestinal fluid (SIF) for 1 hour at 37°C with increasing concentrations of pancreatic enzymes (pancreatin) (B). After incubation of HM with SGF or SIF, samples were ultracentrifuged. Supernatant was removed, and pellets containing TF-exposing EVs were resuspended in the original volume to perform the clotting assay. **P < .01, ***P < .001.
Bovine milk is not coagulant
According to Solé,2 bovine milk lacks coagulant activity. Addition of bovine milk to bovine plasma or human plasma indeed failed to trigger coagulation and thus did not shorten the plasma CT (Figure 5). As a control, adding human milk to bovine plasma shortened the CT (supplemental Table 3), indicating that the factor VIIa–mediated pathway of coagulation is functional. Therefore, whereas human milk is highly coagulant, bovine milk completely lacks this activity. Accordingly, we detected TF protein in human milk but not in bovine milk (supplemental Figure 1).
Comparison of coagulant activity of human (HM) and bovine milk (BM). Coagulant activity of HM and BM (both 1% final concentration; vol/vol) was tested in both human plasma (left) and bovine plasma (right). ***P < .001.
Comparison of coagulant activity of human (HM) and bovine milk (BM). Coagulant activity of HM and BM (both 1% final concentration; vol/vol) was tested in both human plasma (left) and bovine plasma (right). ***P < .001.
Effect of neonatal ICU procedures on human milk coagulant activity
We collected human milk from 5 breastfeeding mothers of preterm infants at a neonatal ICU to investigate the impact of procedures routinely applied in neonatal ICUs on the coagulant potential of human milk.23 These procedures include fortification and different storage conditions. In parallel, we also investigated the coagulant activity of pasteurized pooled donor milk. Figure 6 shows that fortification and cooling did not affect the coagulant potential of mothers’ own milk. Compared with untreated mothers’ own milk, freeze thawing increased the procoagulant potential (P = .002). In contrast, pasteurized pooled donor milk had a decreased coagulant potential (P < .001; supplemental Table 4).
Coagulant activity of human milk preparations used in neonatal ICUs. Human milk from breastfeeding mothers of preterm infants at a neonatal ICU (n = 5) was collected to investigate the impact of routine fortification and storage procedures. For comparison, pasteurized pooled donor milk is shown. **P < .01, ***P < .001.
Coagulant activity of human milk preparations used in neonatal ICUs. Human milk from breastfeeding mothers of preterm infants at a neonatal ICU (n = 5) was collected to investigate the impact of routine fortification and storage procedures. For comparison, pasteurized pooled donor milk is shown. **P < .01, ***P < .001.
Discussion
In the present study, we revisited the pioneering studies on the hemostatic properties of human milk, and we show that human milk contains highly coagulant TF-exposing EVs. We further demonstrate that such TF-exposing EVs are absent from bovine milk, indicating that such EVs are not required for the lactation process per se. Why then is human milk coagulant?
Although we cannot provide direct evidence for a role of TF-exposing EVs in human milk, an explanation could be the occurrence of substantial nipple skin damage in most breastfeeding women.24 Milk-derived TF-exposing EVs may seal these wounds, thereby preventing bleeding as well as breast inflammation resulting from pathogen invasion, securing the mother’s health and the infant’s alimentation. Another possible explanation for the coagulant potential of human milk may be vitamin K deficiency at birth, which predisposes newborns to vitamin K–deficiency bleeding,25 which was observed frequently until vitamin K administration immediately after birth became widely established.
To be functional in the gastrointestinal tract, the coagulant activity of human milk should be able to resist digestion. Human milk effectively buffers gastric fluid,26,27 with a mean postprandial gastric pH of ∼6.28,29 Because infants feed at 1- to 3-hour intervals,30,31 a relatively high gastric pH is probably normal. In the present study, we show that the coagulant activity of human milk withstands conditions resembling those encountered in an infant’s gastrointestinal system, thus making it likely that the coagulant activity of human milk is functional in the whole digestive tract of infants.
In contrast to human milk, bovine milk completely failed to trigger coagulation. This may be clinically relevant, because bovine milk is the basis of most infant formulas and fortifiers, which are added to human milk to increase caloric intake.32 Infants fed with a bovine milk–based formula have a substantially increased rate of gastrointestinal complications compared with infants fed with human milk.16,18,33,34 Although EVs have been studied in bovine milk, plasma, and serum,35,36 to our knowledge, no studies other than that by Solé2 in 1934 and our current study have investigated the coagulant properties of any bovine body fluid.
At neonatal ICUs, feeding with mothers’ own milk is the current standard of care that improves outcomes of preterm infants.37 Shandler et al38 showed that extremely preterm infants fed with >50 mL/kg per day of mothers’ own milk had a 50% reduction in the rate of necrotizing enterocolitis and/or sepsis and a shortened length of hospital stay compared with infants fed with mothers’ own milk plus formula or formula alone. The use of donor milk is recommended at neonatal ICUs if mothers’ own milk is not available.39 However, mothers’ own milk and donor milk are not the same, because donor milk is pasteurized and stored and is donated by women later in lactation.40 Therefore, we studied the coagulant potential of freshly prepared human milk and milk samples of the same donors that were cooled, freeze thawed, and fortified and pasteurized pooled donor milk. Freeze thawing increased the coagulant potential of unprocessed milk, which was most probably due to freezing-induced cell damage and fragmentation.20 Only pasteurized pooled donor milk had a strongly impaired coagulant potential compared with untreated mothers’ own milk, which may be due to prolonged storage and pasteurization.41 Interestingly, infants with very low birth weight fed with pasteurized pooled donor milk have an increased risk of necrotizing enterocolitis compared with infants fed with mothers’ own milk.42,43 One explanation could be that pasteurization reduces the presence of beneficial proteins in human milk.44 Therefore, our results support the observation that mothers’ own milk is best for infants.
A limitation of this study is that human milk was collected at a single time point. The coagulant activity of human milk may have a diurnal rhythm, as has been observed for saliva,45 and may also change significantly during the course of lactation. Results from our present study do not allow such speculation, but a longitudinal study to investigate the coagulant activity in human milk according to time of day and during the course of lactation is currently scheduled. It should also be mentioned that although we show that TF is clearly associated with EVs in human milk, we were unable to demonstrate the presence of TF in individual EVs by cryo-EM or flow cytometry (data not shown), which is likely explained by low TF protein density.
Regarding the cellular origin of EVs in milk, we detected EVs exposing EpCAM, that is, EVs of an epithelial origin, but no EVs originating from granulocytes, monocytes, lymphocytes, or platelets (supplemental Figure 2). One should be cautious in concluding from these data that TF is present on epithelial EVs, because double-labeling experiments could not be performed. Even so, speculating on the cellular origin of EVs, it seems likely that TF-exposing EVs are released directly from the membrane of lactocytes during lactation, because human milk is synthesized by apocrine secretion. Accordingly, in an immunohistochemical study, Sturm et al46 detected ample TF protein in pregnancy-stimulated mammary myoepithelial ducts and proliferating acini, whereas hardly any TF protein was detected in ductal epithelia or acini in resting mammary glands. The effect of gastric fluid on human milk may have been overestimated in our simulated gastric fluid model, because lower pepsin concentrations have been reported in some clinical studies.47 In our simulated intestinal fluid model, we did not add bile salts, because very low levels have been observed in newborns.48,49 Therefore, on the basis of our investigations, we cannot exclude an effect of bile salts on coagulant EVs in older infants.
In conclusion, human milk triggers coagulation via TF-exposing EVs, which may be protective for mothers and infants. In contrast, bovine milk, which is the basis of most infant formulas, is not coagulant. Conditions mimicking digestion in vivo have no effect on the coagulant potential of human milk, indicating that human milk–derived TF-exposing EVs are likely to be functional in the entire gastrointestinal tracts of infants. Compared with mothers’ own milk, the coagulant potential of pasteurized pooled donor milk is strongly impaired, which may put some infants at increased risk of developing complications.
Requests for data sharing should be e-mailed to the corresponding author, Johannes Thaler (e-mail: johannes.thaler@meduniwien.ac.at).
Acknowledgments
The authors thank Martin Zbiral (Medical University of Vienna, Vienna, Austria) for sample collection at the neonatal ICU of the Medical University of Vienna, sample preparation, and performance of fibrin generation experiments; Wolfram Ruf (Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Germany) for providing TF antibodies 5B7, 5G9, and 10H10; Leonie de Rond (Laboratory of Experimental Clinical Chemistry, Vesicle Observation Center, Biomedical Engineering & Physics, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands) for providing CD63 antibody; and Sisareuth Tan (UMR-5248-CBMN, CNRS-University of Bordeaux-IPB, Pessac, France) for help with cryo-EM.
Y.H. was supported by a scholarship from the China Scholarship Council. J.T. was supported by an unrestricted travel grant from the International Society on Thrombosis and Haemostasis. L.H. was supported by the Austrian Science Fund Special Research Program SFB-F54.
Authorship
Contribution: Y.H., A.v.D., L.H., and R.A.K. designed and performed experiments; R.N. and J.T. designed experiments; N.H. and C.H. performed experiments; Y.H., J.T., R.N., L.H., R.A.K., R.J.B., A.v.D., A.B., I.P., C.A., L.W., and A.R. analyzed data; J.T., A.R., and R.A.K. acquired samples; R.N. and J.T. supervised the study; Y.H., J.T., and R.N. drafted the manuscript; L.H., N.H., C.H., R.J.B., I.P., C.A., L.W., and A.R. provided administrative, technical, or material support; and all authors took part in reviewing and editing the entire manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johannes Thaler, Clinical Division of Hematology and Hemostaseology, Department of Medicine I, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: johannes.thaler@meduniwien.ac.at.
References
Author notes
R.N. and J.T. contributed equally to this study.
The full-text version of this article contains a data supplement.