Key Points
Despite a more unfavorable baseline profile, CAR T-cell outcomes were not inferior to alloHCT outcomes, whether measured by ITT or from CI.
Further comparison of CAR T cells vs alloHCT will require effective matching to compensate for basic risk profile and selection differences.
Abstract
CD19-directed chimeric antigen receptor (CAR) T-cell treatment has evolved as standard of care (SOC) for multiply relapsed/refractory (R/R) large B-cell lymphoma (LBCL). However, its potential benefit over allogeneic hematopoietic cell transplantation (alloHCT) remains unclear. We compared outcomes with both types of cellular immunotherapy (CI) by intention to treat (ITT). Eligble were all patients with R/R LBCL and institutional tumor board decision recommending SOC CAR T-cell treatment between July 2018 and February 2020, or alloHCT between January 2004 and February 2020. Primary end point was overall survival (OS) from indication. Altogether, 41 and 60 patients for whom CAR T cells and alloHCT were intended, respectively, were included. In both cohorts, virtually all patients had active disease at indication. CI was recommended as part of second-line therapy for 21 alloHCT patients but no CAR T-cell patients. Median OS from indication was 475 days with CAR T cells vs 285 days with alloHCT (P = .88) and 222 days for 39 patients for whom alloHCT beyond second line was recommended (P = .08). Of CAR T-cell and alloHCT patients, 73% and 65%, respectively, proceeded to CI. After CI, 12-month estimates for nonrelapse mortality, relapse incidence, progression-free survival, and OS for CAR T cells vs alloHCT were 3% vs 21% (P = .04), 59% vs 44% (P = .12), 39% vs 33% (P = .97), and 68% vs 54% (P = .32), respectively. In conclusion, CAR T-cell outcomes were not inferior to alloHCT outcomes, whether measured by ITT or from CI administration, supporting strategies preferring CAR T cells over alloHCT as first CI for multiply R/R LBCL.
Introduction
The prognosis for patients with large B-cell lymphoma (LBCL), such as diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements/double-hit lymphoma, and primary mediastinal B-cell lymphoma (PMBCL), for whom 2 lines of chemoimmunotherapy have failed is poor.1-4 Until recently, allogeneic hematopoietic cell transplantation (alloHCT) was considered the only cellular immunotherapy (CI) with curative potential for patients with multiply relapsed/refractory (R/R) LBCL.5,6 However, alloHCT is associated with substantial treatment-related mortality and morbidity because of acute and chronic graft-versus-host disease, limiting its use in elderly and comorbid patients with lymphoma.7,8 Moreover, despite some evidence for effective graft-versus-lymphoma activity in LBCL compared with other lymphoma subtypes, relatively many patients relapse or progress early after transplantation or experience treatment failure during preparation for alloHCT. As a result, only 30% to 40% of allotransplanted patients with LBCL achieve long-term disease-free survival,6,9-11 and this percentage is lower if analyzed by intention to treat (ITT).12
The role of alloHCT in the treatment of R/R LBCL has been challenged by the recent advent of CD19-directed chimeric antigen receptor (CAR)–engineered T cells as a more targeted form of CI in the clinical routine.13-16 Because of their favorable toxicity profile and their efficacy in active disease, CAR T cells have rapidly become the preferred source of CI for treatment of R/R LBCL.17-19 The aim of the present study was to provide evidence for whether this preference is justified. For this purpose, CAR T cells and alloHCT were compared in a standard-of-care (SOC) setting by ITT.
Patients and methods
Study design and patient eligibility
All adult patients with R/R LBCL referred to our institution who had a tumor board decision recommending treatment with SOC CAR T cells between July 2018 and February 2020 were eligible for this retrospective single-center analysis. These patients were retrospectively compared with all consecutive patients with R/R LBCL who had an institutional tumor board decision recommending allogeneic donor search between January 2004 and February 2020. The latter largely overlapped with the DLBCL group of a recent study on alloHCT by ITT12 but were analyzed here with different end points and much higher granularity. Patients with DLBCL transformed from chronic lymphocytic leukemia were excluded. Diagnoses were made or confirmed by an experienced hematopathologist. Primary end point was overall survival (OS) measured from tumor board decision. Secondary end points included probability of proceeding to CI; best response to CI; OS, progression-free survival (PFS), nonrelapse mortality (NRM), and incidence of relapse/progression (IR) measured from CI, both collectively and stratified by alloHCT treatment line; OS after post-CI relapse/progression; and prognostic factor analyses.
Baseline characteristics, including secondary International Prognostic Index20,21 and HCT comorbidity index,22 treatment details, and outcome data, were extracted from chart review. The study was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent to data collection and scientific evaluation. Data analysis was approved by the institutional review board.
Procedures
Tumor board decisions for SOC CAR T cells were based on the European Medicines Agency label (ie, patients with DLBCL, including transformed follicular lymphoma and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements/double-hit lymphoma, or PMBCL with 2 failed lines of systemic therapy). In addition, performance status (PS), comorbidity, and tumor dynamics were taken into account, but eligibility was not generally restricted to the inclusion criteria of the ZUMA-1 approval trial for axicabtagene cilocleucel (axi-cel).23 Patients were exclusively assigned to axi-cel until August 2019, when our center became qualified for tisagenlecleucel. Thereafter, both constructs were used alternately if not restricted by label (PMBCL).
Tumor board decisions for alloHCT took into account general transplantation eligibility and were based on currently effective European Society for Medical Oncology, European Society for Blood and Marrow Transplantation, and national and institutional guidelines.12 The latter varied over time, but basically, patients for whom autologous HCT (autoHCT) and/or ≥2 lines of systemic therapy had failed were considered for allogeneic transplantation. However, between 2007 and 2017, institutional policies also suggested alloHCT as second-line consolidation for transplantation-eligible patients who were primary refractory or had relapsed after dose-dense first-line therapy.24 With the availability of SOC CAR T cells at our center in July 2018, alloHCT was recommended only for those patients with LBCL who had progressed after CAR T-cell treatment or who had a late relapse after autoHCT along with low transplantation risk.6
AlloHCT and selection of conditioning regimens were protocol driven10,25 or followed valid institutional standard operating procedures. Conditioning intensity was categorized according to the working group definitions.26 Graft-versus-host disease prophylaxis and supportive care for alloHCT were performed as previously described.27 Specifically, all recipients of unrelated-donor transplants received ex-vivo T-cell depletion with antithymocyte globulin, whereas all haplotransplantations were performed with posttransplantation cyclophosphamide. Supportive measures after CAR T-cell administration consisted of anti-infectious prophylaxis with rifaximin and fluconazole until neutrophil recovery as well as aciclovir and cotrimoxazole until CD4 recovery and endothelial prophylaxis until day +14.28
Statistical analysis
Kaplan-Meier product-limit estimates were used to assess the probability of OS and PFS. Events for OS were defined as death resulting from any cause and events for PFS as disease relapse/progression or death resulting from any cause. Survival curves were compared using log-rank tests. Patients were allowed to cross over (ie, to undergo the alternate CI in case of relapse after the first CI). However, they only appeared in both cohorts if both tumor board decisions were made in Heidelberg, Germany. In that case, patients crossing over to the alternate CI were not censored for survival analyses and thus could have an event in both cohorts. Estimates of NRM and IR were calculated using cumulative incidence rates to accommodate competing risks and were compared by Gray’s test. Categorical variables were described by absolute and relative frequencies. Fisher’s exact test was used to compare categorical factors between groups of patients. For continuous variables, the Mann-Whitney U test was applied. Cox regression models were used for multivariate survival comparisons and estimation of hazard ratios (HRs). Calculations were performed using GraphPad Prism software (release 5.2; San Diego, CA) and R software (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria). Significance levels were set at .05. Data were analyzed as of 24 June 2020.
Results
Patient characteristics at baseline
Altogether, 41 and 60 patients with LBCL received a tumor board decision recommending SOC CAR T cells and alloHCT, respectively, during the index periods and were eligible for the study. Of note, in both groups, almost all patients had active disease at the time of tumor board decision (Table 1). The cohorts were also comparable with regard to sex, time from diagnosis, PS, HCT comorbidity index, secondary International Prognostic Index, and ZUMA-1 eligibility; however, patients with a CAR T-cell recommendation tended to be older and significantly more often had primary refractory disease and bulky disease, whereas the alloHCT group contained more patients with prior autoHCT and transformed disease (Table 1). Per eligibility criteria, 21 patients in the alloHCT group but no patients in the CAR T-cell group had experienced failure of only a single line at baseline. The alternate CI had previously failed for 4 and 3 patients from the CAR T-cell and alloHCT groups, respectively, but only 2 patients from each cohort had received all respective tumor board decisions in Heidelberg and thus appeared in both groups.
Patient characteristics at baseline (tumor board decision)
| . | CAR T cells, n (%) (n = 41) . | alloHCT, n (%) (n = 60) . | P . |
|---|---|---|---|
| Age, y | .093 | ||
| Median | 55 | 51 | |
| Range | 20-73 | 19-69 | |
| Male sex | 31 (76) | 43 (72) | .82 |
| Diagnosis | .099 (DLBCL vs other) | ||
| DLBCL | 30 (73) | 34 (57) | |
| tFL | 4 (10) | 18 (30) | |
| PMBCL | 4 (10) | 2 (3) | |
| HGBCL-DH | 2 (5) | 3 (5) | |
| Other | 1 (2)* | 3 (5)† | |
| Stage III/IV at diagnosis | 27 (66) | 47 (78) | .18 |
| Type of first-line failure | .0044 (primary refractory vs other) | ||
| Primary refractory | 25 (61) | 19 (32) | |
| Early relapse (7-12 mo from start of first line) | 10 (24) | 22 (37) | |
| Late relapse | 6 (15) | 19 (32) | |
| Time from diagnosis, mo | .54 | ||
| Median | 12 | 16 | |
| Range | 4-207 | 2-231 | |
| No. of failed lines | <.0001 (1 vs >1) | ||
| Median | 2 | 2 | |
| Range | 2-7 | 1-7 | |
| 1 | 0 | 21 | |
| 2 | 21 | 23 | |
| >2 | 20 | 16 | |
| AutoHCT failure | 9 (22) | 26 (43) | .034 |
| Alternate CI failure | 4 (10) | 3 (5) | |
| HCT comorbidity index | 1.0 (0 vs >0) | ||
| 0 | 24 (59) | 34 (57) | |
| 1-2 | 8 (20) | 14 (23) | |
| >2 | 9 (22) | 12 (20) | |
| PS ≥2 | 8 (20) | 6 (10) | .24 |
| sIPI high intermediate/high | 23 (56) | 25/57 (44) | .31 |
| Bulk >10 cm | 8/40 (20) | 3/56 (5) | .047 |
| ZUMA-1 ineligible‡ | 30 (73) | 34/57 (60) | .20 |
| Rapid progression | 20 | 13 | |
| PS >1 | 7 | 5 | |
| Prior alloHCT/CAR T cells | 4 | 3 | |
| 2nd neoplasm | 1 | 4 | |
| CNS involvement | 1 | 8 | |
| Other | 4 | 5 | |
| Disease status | .39 | ||
| PD | 38 (93) | 58 (97) | |
| SD | 3 (7) | 0 | |
| CR/PR | 0 | 2 (3) | |
| Not proceeding to CI | 11 (27) | 21 (35) | .51 |
| PD | 7 | 14 | |
| Fatal infection | 2 | 1 | |
| No product/donor | 1 | 3 | |
| Refused | 1 | 1 | |
| AutoHCT | 0 | 2 | |
| Calendar year of study entry | |||
| Median | 2019 | 2014 | |
| Range | 2018-2020 | 2004-2019 | |
| Follow-up, mo | |||
| Median | 10 | 64 | |
| Range | 5-19 | 10-181 |
| . | CAR T cells, n (%) (n = 41) . | alloHCT, n (%) (n = 60) . | P . |
|---|---|---|---|
| Age, y | .093 | ||
| Median | 55 | 51 | |
| Range | 20-73 | 19-69 | |
| Male sex | 31 (76) | 43 (72) | .82 |
| Diagnosis | .099 (DLBCL vs other) | ||
| DLBCL | 30 (73) | 34 (57) | |
| tFL | 4 (10) | 18 (30) | |
| PMBCL | 4 (10) | 2 (3) | |
| HGBCL-DH | 2 (5) | 3 (5) | |
| Other | 1 (2)* | 3 (5)† | |
| Stage III/IV at diagnosis | 27 (66) | 47 (78) | .18 |
| Type of first-line failure | .0044 (primary refractory vs other) | ||
| Primary refractory | 25 (61) | 19 (32) | |
| Early relapse (7-12 mo from start of first line) | 10 (24) | 22 (37) | |
| Late relapse | 6 (15) | 19 (32) | |
| Time from diagnosis, mo | .54 | ||
| Median | 12 | 16 | |
| Range | 4-207 | 2-231 | |
| No. of failed lines | <.0001 (1 vs >1) | ||
| Median | 2 | 2 | |
| Range | 2-7 | 1-7 | |
| 1 | 0 | 21 | |
| 2 | 21 | 23 | |
| >2 | 20 | 16 | |
| AutoHCT failure | 9 (22) | 26 (43) | .034 |
| Alternate CI failure | 4 (10) | 3 (5) | |
| HCT comorbidity index | 1.0 (0 vs >0) | ||
| 0 | 24 (59) | 34 (57) | |
| 1-2 | 8 (20) | 14 (23) | |
| >2 | 9 (22) | 12 (20) | |
| PS ≥2 | 8 (20) | 6 (10) | .24 |
| sIPI high intermediate/high | 23 (56) | 25/57 (44) | .31 |
| Bulk >10 cm | 8/40 (20) | 3/56 (5) | .047 |
| ZUMA-1 ineligible‡ | 30 (73) | 34/57 (60) | .20 |
| Rapid progression | 20 | 13 | |
| PS >1 | 7 | 5 | |
| Prior alloHCT/CAR T cells | 4 | 3 | |
| 2nd neoplasm | 1 | 4 | |
| CNS involvement | 1 | 8 | |
| Other | 4 | 5 | |
| Disease status | .39 | ||
| PD | 38 (93) | 58 (97) | |
| SD | 3 (7) | 0 | |
| CR/PR | 0 | 2 (3) | |
| Not proceeding to CI | 11 (27) | 21 (35) | .51 |
| PD | 7 | 14 | |
| Fatal infection | 2 | 1 | |
| No product/donor | 1 | 3 | |
| Refused | 1 | 1 | |
| AutoHCT | 0 | 2 | |
| Calendar year of study entry | |||
| Median | 2019 | 2014 | |
| Range | 2018-2020 | 2004-2019 | |
| Follow-up, mo | |||
| Median | 10 | 64 | |
| Range | 5-19 | 10-181 |
CNS, central nervous system; HGBCL-DH, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements/double-hit lymphoma; PD, progressive disease; SD, stable disease; sIPI, secondary International Prognostic Index; tFL, transformed follicular lymphoma.
DLBCL: leg type.
DLBCL: transformed from marginal zone lymphoma (n = 2), Gray zone lymphoma (n = 1).
Disregarding n of failed lines.
Survival from indication
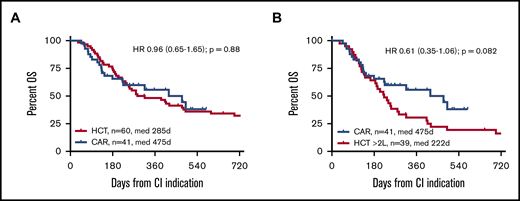
With median survival times of 475 and 285 days for patients for whom CAR T cells and alloHCT, respectively, were intended, OS from tumor board decision was not significantly different between the 2 cohorts (HR, 0.96; 95% confidence interval, 0.65-1.65; P = .88; Figure 1). HRs were similar when the second CIs of the crossovers were not included in the comparison (supplemental Figure 1). In univariate analysis, patients with a CAR T-cell recommendation had a significantly inferior OS if they had a PS >1 at baseline, and they tended to live shorter if they were ZUMA-1 ineligible (Table 2; supplemental Figures 2 and 3). ZUMA-1 ineligibility was also a predictor of inferior OS in the ITT alloHCT group, along with type of underlying LBCL, primary refractoriness, and >1 treatment line failure at baseline (Table 2). Explorative multivariable analyses of the main effect (OS by type of CI) accounting for sex, bridging, lines failed, ZUMA-1 ineligibility, PS, primary refractoriness, and interaction between PS and primary refractoriness, respectively, and the main effect confirmed noninferiority of ITT CAR T-cell therapy compared with alloHCT (HR, 0.53; 95% confidence interval, 0.20-1.47; supplemental Table 1). Median follow-up of survivors was 10 months (range, 5-19 months) for the CAR T-cell group and 64 months (range, 10-181 months) for the alloHCT group.
OS from CI indication by CI intended. (A) CAR T-cell candidates vs all alloHCT candidates. (B) CAR T-cell candidates vs alloHCT candidates for whom ≥2 lines had failed (≥2L) at indication.
OS from CI indication by CI intended. (A) CAR T-cell candidates vs all alloHCT candidates. (B) CAR T-cell candidates vs alloHCT candidates for whom ≥2 lines had failed (≥2L) at indication.
Univariable prognostic factor analyses for OS by ITT (log rank; n = 41/60)
| Variable . | CAR T cells . | alloHCT . | ||||||
|---|---|---|---|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | P . | HR . | Lower CL . | Upper CL . | |
| Age (<60 vs ≥60 y) | .27 | 1.67 | 0.68 | 4.04 | .12 | 0.56 | 0.27 | 1.17 |
| Male sex | .85 | 0.90 | 0.31 | 2.58 | .18 | 1.56 | 0.82 | 2.95 |
| Diagnosis (DLBCL vs other) | .25 | 0.55 | 0.20 | 1.53 | .016 | 0.47 | 0.25 | 0.87 |
| PIF (vs relapse) | .72 | 0.84 | 0.34 | 2.12 | .039 | 2.11 | 1.04 | 4.27 |
| Time from diagnosis (≤12 vs >12 mo) | .71 | 1.18 | 0.49 | 2.85 | .70 | 1.13 | 0.61 | 2.11 |
| No. of failed lines (CAR, 2 vs >2; HCT, 1 vs >1) | .69 | 1.20 | 0.50 | 2.90 | <.001 | 3.12 | 1.69 | 5.75 |
| AutoHCT failed (yes vs no) | .94 | 0.96 | 0.32 | 2.84 | .81 | 1.08 | 0.59 | 1.97 |
| HCT comorbidity index (>0 vs 0) | .81 | 0.90 | 0.36 | 2.24 | .22 | 0.68 | 0.37 | 1.25 |
| PS (>1 vs 0-1) | .006 | 7.41 | 1.78 | 30.1 | .13 | 2.66 | 0.76 | 9.32 |
| sIPI (HI/H vs L/LI) | .39 | 1.48 | 0.61 | 3.63 | .35 | 1.35 | 0.72 | 2.53 |
| Bulk >10 cm (yes vs no) | .11 | 2.62 | 0.61 | 3.63 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .08 | 2.32 | 0.90 | 5.86 | .015 | 2.19 | 1.17 | 4.12 |
| Need for bridging (yes vs no) | .0011 | 4.92 | 1.89 | 12.9 | — | — | — | — |
| Variable . | CAR T cells . | alloHCT . | ||||||
|---|---|---|---|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | P . | HR . | Lower CL . | Upper CL . | |
| Age (<60 vs ≥60 y) | .27 | 1.67 | 0.68 | 4.04 | .12 | 0.56 | 0.27 | 1.17 |
| Male sex | .85 | 0.90 | 0.31 | 2.58 | .18 | 1.56 | 0.82 | 2.95 |
| Diagnosis (DLBCL vs other) | .25 | 0.55 | 0.20 | 1.53 | .016 | 0.47 | 0.25 | 0.87 |
| PIF (vs relapse) | .72 | 0.84 | 0.34 | 2.12 | .039 | 2.11 | 1.04 | 4.27 |
| Time from diagnosis (≤12 vs >12 mo) | .71 | 1.18 | 0.49 | 2.85 | .70 | 1.13 | 0.61 | 2.11 |
| No. of failed lines (CAR, 2 vs >2; HCT, 1 vs >1) | .69 | 1.20 | 0.50 | 2.90 | <.001 | 3.12 | 1.69 | 5.75 |
| AutoHCT failed (yes vs no) | .94 | 0.96 | 0.32 | 2.84 | .81 | 1.08 | 0.59 | 1.97 |
| HCT comorbidity index (>0 vs 0) | .81 | 0.90 | 0.36 | 2.24 | .22 | 0.68 | 0.37 | 1.25 |
| PS (>1 vs 0-1) | .006 | 7.41 | 1.78 | 30.1 | .13 | 2.66 | 0.76 | 9.32 |
| sIPI (HI/H vs L/LI) | .39 | 1.48 | 0.61 | 3.63 | .35 | 1.35 | 0.72 | 2.53 |
| Bulk >10 cm (yes vs no) | .11 | 2.62 | 0.61 | 3.63 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .08 | 2.32 | 0.90 | 5.86 | .015 | 2.19 | 1.17 | 4.12 |
| Need for bridging (yes vs no) | .0011 | 4.92 | 1.89 | 12.9 | — | — | — | — |
Bold font indicates significance.
CL, confidence limit of 95% confidence interval; HI/H, high intermediate/high; L/LI, low/low intermediate; PIF, primary induction failure; sIPI, secondary International Prognostic Index.
Pre-CI failures
Eleven (27%) of 41 and 21 (35%) of 60 patients for whom CAR T-cell therapy and alloHCT, respectively, were intended did not proceed to CI. Apart from becoming ineligible because of disease progression, reasons were fatal infection, unavailability of cellular product, patient preference, and autoHCT (Table 1). Outcomes of these patients were generally poor, with a significant OS disadvantage for the patients for whom CAR T-cell treatment was intended (median survival, 85 vs 137 days; HR, 4.01; 95% confidence interval, 1.5-10.7; P = .0056). Actually, 8 of the 11 patients not undergoing intended CAR T-cell treatment deteriorated during the time needed for securing treatment costs and preparation for cell collection and did not undergo leukapheresis. All patients who did not receive intended CAR T-cell treatment died rapidly; however, there were 3 long-term survivors among the patients not proceeding to alloHCT, 2 of whom surviving after switching to an autoHCT strategy.
Patient characteristics at conditioning for CI
Altogether, 30 (73%) and 39 patients (65%) for whom CI was intended actually underwent CAR T-cell treatment and alloHCT, respectively, with a significantly shorter interval from indication to CI in the CAR T-cell group (72 vs 114 days for alloHCT; P < .001). Details on CI procedures and patient characteristics at conditioning are summarized in Table 3. Similar to the situation at indication, the CAR T-cell group was older and contained more patients who had been primary refractory and who had bulky disease. Bridging therapy had been administered to 95% of alloHCT patients but only to 67% of patients undergoing CAR T-cell therapy (P = .0032), resulting in a significant imbalance in terms of preconditioning disease status, which was responsive in 66% of patients in the alloHCT group vs 13% in the CAR T-cell group (P < .001; Table 3).
Patient characteristics at conditioning for CI
| . | CAR T-cells, n (%) (n = 30) . | alloHCT, n (%) (n = 39) . | P* . |
|---|---|---|---|
| Age, y | .049 | ||
| Median | 55 | 45 | |
| Range | 20-73 | 23-67 | |
| Male sex | 22 (73) | 26 (67) | .61 |
| Diagnosis | .20 | ||
| DLBCL | 23 (77) | 23 (59) | |
| tFL | 2 (7) | 9 (23) | |
| PMBCL | 3 (10) | 1 (3) | |
| HGBCL-DH | 1 (3) | 3 (8) | |
| Other | 1(3)† | 3 (8)‡§ | |
| Type of first-line failure | .0038 (primary refractory vs other) | ||
| Primary refractory | 20 (67) | 12 (31) | |
| Early relapse (7-12 mo from start of first line) | 5 (17) | 14 (36) | |
| Late relapse | 5 (17) | 13 (33) | |
| Prior no. of lines | .19 (.076 [2 vs >2 lines]) | ||
| Median | 3.5 | 3 | |
| Range | 2-7 | 2-8 | |
| Prior autoHCT | 5 (17) | 17 (44) | .021 |
| Prior alternative CI | 4 (13) | 4 (18)‖ | |
| No. of bridging lines | .0032 (0 vs >0) | ||
| 0 | 10 (33) | 2 (5) | |
| 1 | 15 (50) | 24 (62) | |
| >1 | 5 (17) | 13 (33) | |
| Bridging regimens | .27 (immunotoxins and/or ibrutinib yes/no) | ||
| CIT | 7 (23) | 30 (77) | |
| Dexamethasone ± rituximab | 6 (20) | 4 (10) | |
| Immunotoxins (CD30, CD79) | 7 (23) | 2 (5) | |
| Ibrutinib | 4 (13) | 5 (13) | |
| Radiotherapy | 3 (10) | 5 (13) | |
| Other | 1 (3) | 1 (3) | |
| Time from indication to CI, d | .0003 | ||
| Median | 72 | 114 | |
| Range | 35-285 | 36-451 | |
| HCT comorbidity index | .81 (0 vs >0) | ||
| 0 | 18 (60) | 21/38 (55) | |
| 1-2 | 5 (17) | 11/38 (30) | |
| >2 | 7 (23) | 6/38 (16) | |
| PS ≥2 | 3 (10) | 4 (10) | 1.0 |
| sIPI high intermediate/ high | 16 (53) | 15/37 (41) | .33 |
| Bulk >10 cm | 7 (20) | 1 (3) | .018 |
| ZUMA-1 ineligible¶ | 19 (63) | 19/37 (51) | .81 |
| Disease status | .0008 (PD/SD vs CR/PR) | ||
| PD | 21 (70) | 7/38 (18) | |
| SD | 5 (17) | 6/38 (16) | |
| PR | 3 (10) | 14/38 (37) | |
| CR | 1 (3) | 11/38 (29) | |
| LDH elevated | 15 (50) | 14 (36) | .33 |
| Conditioning | — | ||
| NMA | 30 (100) | ||
| RIC | — | 9 (23) | |
| MAC | — | 30 (77) | |
| CAR/donor type | — | ||
| Tisa-cel 4 (13) | MRD 5 (13) | ||
| Axi-cel 26 (87) | MUD 15 (38) | ||
| MMUD 10 (26) | |||
| MMRD 9 (23) | |||
| Calendar year of CI | — | ||
| Median | 2019 | 2014 | |
| Range | 2018-2020 | 2005-2019 |
| . | CAR T-cells, n (%) (n = 30) . | alloHCT, n (%) (n = 39) . | P* . |
|---|---|---|---|
| Age, y | .049 | ||
| Median | 55 | 45 | |
| Range | 20-73 | 23-67 | |
| Male sex | 22 (73) | 26 (67) | .61 |
| Diagnosis | .20 | ||
| DLBCL | 23 (77) | 23 (59) | |
| tFL | 2 (7) | 9 (23) | |
| PMBCL | 3 (10) | 1 (3) | |
| HGBCL-DH | 1 (3) | 3 (8) | |
| Other | 1(3)† | 3 (8)‡§ | |
| Type of first-line failure | .0038 (primary refractory vs other) | ||
| Primary refractory | 20 (67) | 12 (31) | |
| Early relapse (7-12 mo from start of first line) | 5 (17) | 14 (36) | |
| Late relapse | 5 (17) | 13 (33) | |
| Prior no. of lines | .19 (.076 [2 vs >2 lines]) | ||
| Median | 3.5 | 3 | |
| Range | 2-7 | 2-8 | |
| Prior autoHCT | 5 (17) | 17 (44) | .021 |
| Prior alternative CI | 4 (13) | 4 (18)‖ | |
| No. of bridging lines | .0032 (0 vs >0) | ||
| 0 | 10 (33) | 2 (5) | |
| 1 | 15 (50) | 24 (62) | |
| >1 | 5 (17) | 13 (33) | |
| Bridging regimens | .27 (immunotoxins and/or ibrutinib yes/no) | ||
| CIT | 7 (23) | 30 (77) | |
| Dexamethasone ± rituximab | 6 (20) | 4 (10) | |
| Immunotoxins (CD30, CD79) | 7 (23) | 2 (5) | |
| Ibrutinib | 4 (13) | 5 (13) | |
| Radiotherapy | 3 (10) | 5 (13) | |
| Other | 1 (3) | 1 (3) | |
| Time from indication to CI, d | .0003 | ||
| Median | 72 | 114 | |
| Range | 35-285 | 36-451 | |
| HCT comorbidity index | .81 (0 vs >0) | ||
| 0 | 18 (60) | 21/38 (55) | |
| 1-2 | 5 (17) | 11/38 (30) | |
| >2 | 7 (23) | 6/38 (16) | |
| PS ≥2 | 3 (10) | 4 (10) | 1.0 |
| sIPI high intermediate/ high | 16 (53) | 15/37 (41) | .33 |
| Bulk >10 cm | 7 (20) | 1 (3) | .018 |
| ZUMA-1 ineligible¶ | 19 (63) | 19/37 (51) | .81 |
| Disease status | .0008 (PD/SD vs CR/PR) | ||
| PD | 21 (70) | 7/38 (18) | |
| SD | 5 (17) | 6/38 (16) | |
| PR | 3 (10) | 14/38 (37) | |
| CR | 1 (3) | 11/38 (29) | |
| LDH elevated | 15 (50) | 14 (36) | .33 |
| Conditioning | — | ||
| NMA | 30 (100) | ||
| RIC | — | 9 (23) | |
| MAC | — | 30 (77) | |
| CAR/donor type | — | ||
| Tisa-cel 4 (13) | MRD 5 (13) | ||
| Axi-cel 26 (87) | MUD 15 (38) | ||
| MMUD 10 (26) | |||
| MMRD 9 (23) | |||
| Calendar year of CI | — | ||
| Median | 2019 | 2014 | |
| Range | 2018-2020 | 2005-2019 |
CIT, chemoimmunotherapy; MAC, myeloablative conditioning; MMRD, mismatched related donor (haploidentical); MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; NMA, nonmyeloablative; RIC, reduced-intensity conditioning.
For comparison CAR T cells vs alloHCT >2L.
DLBCL: leg type.
DLBCL: transformed from marginal zone lymphoma.
Gray zone lymphoma.
One patient received tisagenlecleucel (without durable response) in a clinical trial in a different center after an initially unsuccessful donor search in our institution.
Disregarding n of failed lines.
CI outcomes
Among the 29 CAR T-cell recipients evaluable for response, best response was complete response (CR) in 38% and partial response (PR) in 41% of patients (overall response rate [ORR], 79%), whereas best response after alloHCT was CR in 50% and PR in 21% of 34 evaluable patients (ORR, 71%; supplemental Figure 4). Actual response improvement by alloHCT, however, was only observed in 12 (52%) of 23 patients not in CR at conditioning.
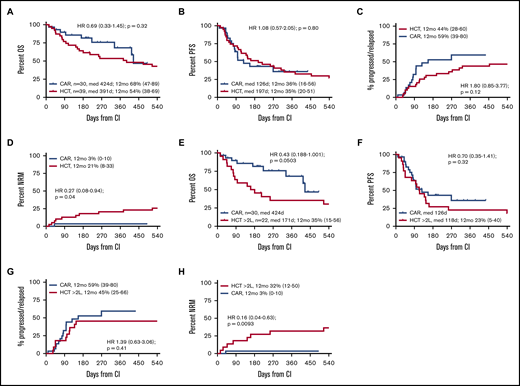
Within the first year after CI, there was 1 nonrelapse death in the CAR T-cell group compared with 8 nonrelapse deaths in the alloHCT group, translating into a significantly lower 12-month NRM risk for CAR T-cell recipients (3% vs 21%; HR, 0.27; 95% confidence interval, 0.08-0.94; P = .04; Figure 2). In contrast, 12-month IR tended to be higher after CAR T-cell therapy, although this difference did not reach statistical significance (59% vs 44%; HR, 1.8; 95% confidence interval, 0.85-3.77; P = .12). Similarly, there was no significant difference for PFS and OS at 12 months after CI administration between CAR T-cell and alloHCT groups (PFS, 36% vs 35%; HR, 1.08; 95% confidence interval, 0.57-2.05; P = .80; OS, 68% vs 54%; HR, 0.69; 95% confidence interval, 0.33-1.45; P = .32; Figure 2). HRs for OS were similar when the second CIs of the crossovers were not included in the comparison (supplemental Figure 1).
Outcomes from CI administration by CI actually received. (A-D) CAR T-cell recipients vs all alloHCT patients. (E-H) CAR T-cell recipients vs alloHCT patients for whom alloHCT was intended after failure of ≥2L. (A,E) OS. (B,F) PFS. (C,G) Incidence of relapse/progression. (D,H) NRM. Note that all CAR T-cell applications were intended after failure of ≥2L.
Outcomes from CI administration by CI actually received. (A-D) CAR T-cell recipients vs all alloHCT patients. (E-H) CAR T-cell recipients vs alloHCT patients for whom alloHCT was intended after failure of ≥2L. (A,E) OS. (B,F) PFS. (C,G) Incidence of relapse/progression. (D,H) NRM. Note that all CAR T-cell applications were intended after failure of ≥2L.
In univariate analysis, predictors of adverse OS and PFS after CAR T-cell treatment were bulky disease and elevated lactate dehydrogenase (LDH) at conditioning (supplemental Figure 5), whereas after alloHCT, diagnosis other than DLBCL, primary refractoriness, and >3 lines of pretreatment at conditioning were associated with inferior PFS and OS (Table 4). Again, explorative multivariable analyses of the main effect (OS by type of CI) accounting for relevant confounders confirmed noninferiority of CAR T-cell therapy compared with alloHCT (HR, 0.45; 95% confidence interval, 0.17-1.22; supplemental Table 1).
Univariable prognostic factor analyses for post-CI outcomes (log rank; n=30/39)
| Variable . | CAR T cells . | alloHCT . | ||||||
|---|---|---|---|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | P . | HR . | Lower CL . | Upper CL . | |
| OS | ||||||||
| Diagnosis (DLBCL vs other) | .59 | 0.66 | 0.15 | 2.97 | .0062 | 0.31 | 0.14 | 0.72 |
| PIF (vs relapse) | .37 | 1.88 | 0.47 | 7.55 | .036 | 2.92 | 1.08 | 7.94 |
| Prior lines (>3 vs 2-3) | .62 | 1.02 | 0.75 | 1.29 | .019 | 2.91 | 1.19 | 7.09 |
| Prior autoHCT (yes vs no) | .17 | 0.30 | 0.05 | 1.71 | .44 | 0.73 | 0.33 | 1.61 |
| Bridging (yes vs no) | .029 | 4.5 | 1.16 | 17.4 | — | — | — | — |
| Time from indication to CI (> vs < median) | .22 | 2.31 | 0.61 | 8.81 | .50 | 1.32 | 0.60 | 2.88 |
| HCT comorbidity index (>0 vs 0) | .72 | 0.76 | 0.17 | 3.39 | .62 | 0.82 | 0.37 | 1.80 |
| sIPI (HI/H vs L/LI) | .45 | 1.68 | 0.44 | 6.47 | .43 | 1.40 | 0.65 | 3.61 |
| Bulk >10 cm (yes vs no) | .0008 | 23.7 | 3.73 | 151 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .58 | 1.46 | 0.39 | 5.55 | .19 | 1.75 | 0.76 | 4.05 |
| Disease status at conditioning (PD vs CR/PR/SD) | .26 | 2.35 | 0.54 | 10.3 | .57 | 1.37 | 0.46 | 4.04 |
| LDH (> normal vs normal) | .022 | 5.18 | 1.27 | 21.2 | .67 | 0.84 | 0.36 | 1.92 |
| Calendar year of HCT (<2014 vs ≥2014) | — | — | — | — | .71 | 0.81 | 0.39 | 1.90 |
| Donor (related vs unrelated) | — | — | — | — | .099 | 2.08 | 0.87 | 4.98 |
| PFS | ||||||||
| Diagnosis (DLBCL vs other) | .55 | 0.70 | 0.22 | 2.24 | .014 | 0.37 | 0.17 | 0.81 |
| PIF (vs relapse) | .26 | 1.84 | 0.64 | 5.25 | .0078 | 3.59 | 1.40 | 9.22 |
| Prior lines (>3 vs 2-3) | .57 | 1.34 | 0.50 | 3.59 | .023 | 2.63 | 1.14 | 6.01 |
| Prior autoHCT (yes vs no) | .95 | 1.05 | 0.29 | 3.80 | .57 | 0.81 | 0.39 | 1.69 |
| Bridging (yes vs no) | .24 | 1.84 | 0.67 | 5.11 | — | — | — | — |
| Time from indication to CI (> vs < median) | .26 | 1.78 | 0.65 | 4.85 | .88 | 0.95 | 0.45 | 1.97 |
| HCT comorbidity index (>0 vs 0) | .56 | 0.74 | 0.27 | 2.04 | .73 | 1.14 | 0.54 | 2.40 |
| sIPI (HI/H vs L/LI) | .53 | 1.37 | 0.51 | 3.72 | .56 | 1.25 | 0.59 | 2.64 |
| Bulk >10 cm (yes vs no) | .077 | 3.50 | 0.88 | 14.0 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .67 | 0.81 | 0.44 | 1.18 | .14 | 1.82 | 0.83 | 4.00 |
| Disease status at conditioning (PD vs CR/PR/SD) | .064 | 2.68 | 0.95 | 7.60 | .31 | 1.72 | 0.60 | 4.95 |
| LDH at conditioning (> normal vs normal) | .012 | 3.68 | 1.34 | 10.1 | .49 | 0.76 | 0.35 | 1.66 |
| Donor (related vs unrelated) | — | — | — | — | .086 | 2.03 | 0.91 | 4.53 |
| Variable . | CAR T cells . | alloHCT . | ||||||
|---|---|---|---|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | P . | HR . | Lower CL . | Upper CL . | |
| OS | ||||||||
| Diagnosis (DLBCL vs other) | .59 | 0.66 | 0.15 | 2.97 | .0062 | 0.31 | 0.14 | 0.72 |
| PIF (vs relapse) | .37 | 1.88 | 0.47 | 7.55 | .036 | 2.92 | 1.08 | 7.94 |
| Prior lines (>3 vs 2-3) | .62 | 1.02 | 0.75 | 1.29 | .019 | 2.91 | 1.19 | 7.09 |
| Prior autoHCT (yes vs no) | .17 | 0.30 | 0.05 | 1.71 | .44 | 0.73 | 0.33 | 1.61 |
| Bridging (yes vs no) | .029 | 4.5 | 1.16 | 17.4 | — | — | — | — |
| Time from indication to CI (> vs < median) | .22 | 2.31 | 0.61 | 8.81 | .50 | 1.32 | 0.60 | 2.88 |
| HCT comorbidity index (>0 vs 0) | .72 | 0.76 | 0.17 | 3.39 | .62 | 0.82 | 0.37 | 1.80 |
| sIPI (HI/H vs L/LI) | .45 | 1.68 | 0.44 | 6.47 | .43 | 1.40 | 0.65 | 3.61 |
| Bulk >10 cm (yes vs no) | .0008 | 23.7 | 3.73 | 151 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .58 | 1.46 | 0.39 | 5.55 | .19 | 1.75 | 0.76 | 4.05 |
| Disease status at conditioning (PD vs CR/PR/SD) | .26 | 2.35 | 0.54 | 10.3 | .57 | 1.37 | 0.46 | 4.04 |
| LDH (> normal vs normal) | .022 | 5.18 | 1.27 | 21.2 | .67 | 0.84 | 0.36 | 1.92 |
| Calendar year of HCT (<2014 vs ≥2014) | — | — | — | — | .71 | 0.81 | 0.39 | 1.90 |
| Donor (related vs unrelated) | — | — | — | — | .099 | 2.08 | 0.87 | 4.98 |
| PFS | ||||||||
| Diagnosis (DLBCL vs other) | .55 | 0.70 | 0.22 | 2.24 | .014 | 0.37 | 0.17 | 0.81 |
| PIF (vs relapse) | .26 | 1.84 | 0.64 | 5.25 | .0078 | 3.59 | 1.40 | 9.22 |
| Prior lines (>3 vs 2-3) | .57 | 1.34 | 0.50 | 3.59 | .023 | 2.63 | 1.14 | 6.01 |
| Prior autoHCT (yes vs no) | .95 | 1.05 | 0.29 | 3.80 | .57 | 0.81 | 0.39 | 1.69 |
| Bridging (yes vs no) | .24 | 1.84 | 0.67 | 5.11 | — | — | — | — |
| Time from indication to CI (> vs < median) | .26 | 1.78 | 0.65 | 4.85 | .88 | 0.95 | 0.45 | 1.97 |
| HCT comorbidity index (>0 vs 0) | .56 | 0.74 | 0.27 | 2.04 | .73 | 1.14 | 0.54 | 2.40 |
| sIPI (HI/H vs L/LI) | .53 | 1.37 | 0.51 | 3.72 | .56 | 1.25 | 0.59 | 2.64 |
| Bulk >10 cm (yes vs no) | .077 | 3.50 | 0.88 | 14.0 | — | — | — | — |
| ZUMA-1 ineligible (yes vs no) | .67 | 0.81 | 0.44 | 1.18 | .14 | 1.82 | 0.83 | 4.00 |
| Disease status at conditioning (PD vs CR/PR/SD) | .064 | 2.68 | 0.95 | 7.60 | .31 | 1.72 | 0.60 | 4.95 |
| LDH at conditioning (> normal vs normal) | .012 | 3.68 | 1.34 | 10.1 | .49 | 0.76 | 0.35 | 1.66 |
| Donor (related vs unrelated) | — | — | — | — | .086 | 2.03 | 0.91 | 4.53 |
Bold font indicates significance.
.
Survival stratified by treatment line
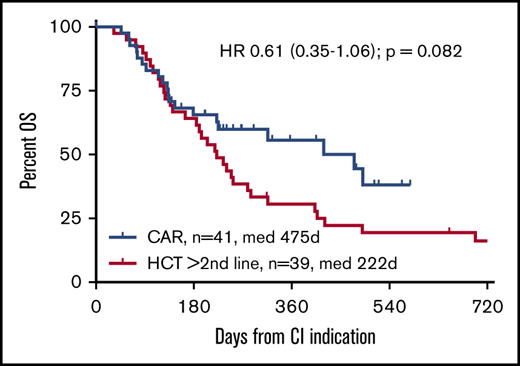
To focus the comparison on the CAR T-cell label, stratified comparisons were performed after segregating the alloHCT group into patients receiving a transplantation recommendation after first-line failure (HCT 2L; n = 21) and those considered for alloHCT only after failure ≥2L (corresponding to the SOC CAR T-cell indication window; n = 39). Stratified patient characteristics are listed in supplemental Tables 2 and 3. Although not reaching statistical significance, the CAR T-cell cohort tended to show superior OS compared with the HCT ≥2L patients, both when analyzed by ITT (HR, 0.61; 95% confidence interval, 0.35-1.06; P = .082) or from CI (HR, 0.43; 95% confidence interval, 0.18-1.001; P = .0503). OS and PFS at 12 months from CI for the HCT ≥2L group were 35% and 23%, respectively (Figures 1 and 2). In contrast, OS from indication of the HCT 2L group was significantly better than that of patients for whom CAR T cells were intended, whereas OS from CI was comparable between these 2 cohorts (supplemental Figure 6).
Outcomes after post-CI relapse/progression
Fifteen patients experienced relapse/progression after CAR T-cell treatment at a median time of 82 days (range, 9-269 days) after dosing, and 18 patients did so at a median time of 114 days (range, 33-453 days) after alloHCT. Overall, 19 salvage lines were administered to the 15 patients with disease recurrence after CAR T-cell therapy and 26 lines to the 18 patients for whom alloHCT failed. Salvage therapy for CAR T-cell failures consisted predominantly of targeted agents, such as immunotoxins, pathway inhibitors, and checkpoint inhibitors, whereas post-alloHCT relapses were mainly treated with chemotherapy or immune modulation (supplemental Table 4). Although response rates tended to be better in the CAR T-cell cohort (ORR, 42% vs 8%; P = .01), survival after LBCL relapse/progression post-CI was poor for both cohorts, with median survival times of 276 days (range, 4-349+ days) and 71 days (range, 1-1524 days) for CAR T-cell and alloHCT failures, respectively (P = .13; supplemental Figure 6).
Alternate CI for first CI failure
When also considering CI procedures decided and performed externally, overall, 5 patients in our series underwent alloHCT after CAR T-cell failure, 4 of whom with unresponsive disease at conditioning. All 4 of these patients rapidly (in 36-115 days) progressed after transplantation, whereas the fifth patient who entered alloHCT in PR died early as a result of toxicity. In contrast, 2 of 4 patients who underwent salvage axi-cel for posttransplantation relapse were alive and disease free 297 and 347 days after dosing. These 4 patients have been described in detail elsewhere.29
Discussion
This is the first comparative analysis between CAR T-cell therapy and alloHCT as the former standard for eligible patients in the setting of R/R LBCL, showing that outcomes with CAR T-cell treatment might be at least noninferior to those with alloHCT, whether measured by ITT or from CI administration.
However, a number of limitations of the present study must be taken into account. Apart from the relatively small sample size and retrospective character, the observation time of the CAR T-cell arm was clearly immature, with a 10-month median follow-up, even though it is known that a majority of relapse/progression events as the predominant causes of failure after CD19 CAR T-cell treatment occur within the first 6 months postdosing.14-16,23,30 In contrast, it might be argued that the outcomes of our alloHCT cohort were relatively poor, with 12-month PFS at the lower end of the published range of 35% to 50%.6,9-11 Reasons for this could lie in the relatively high proportion of patients undergoing transplantation with active disease in our series and the predominant use of myeloablative conditioning, which was associated with poorer outcomes at least in 1 large registry study.6 In contrast, at 68% at 12 months after dosing, OS of our CAR T-cell group seems to be comparable to that in published axi-cel data, although our 12-month PFS of 36% tends to be lower than those in the large axi-cel studies (ie, >40%).15,16,23 Apart from the fact that confidence intervals are still wide, an explanation for that might be the relatively high proportion of patients in our series with features known to be associated with unfavorable outcomes after CAR T-cell therapy, such as need for bridging, elevated LDH, and ZUMA-1 ineligibility.15,16,31
Finally, the comparison might have been confounded by the fact that the alloHCT group was treated over a wide timespan of >15 years, whereas CAR T-cell patients were exclusively treated during the past 2 years. As shown in a recent Center for International Blood & Marrow Transplant Research analysis, alloHCT results for lymphoma have indeed improved over time, but not particularly during the index period covered by our study, and the better survival since 2006 seems to be mainly due to improved outcomes after posttransplantation relapse.32 Accordingly, in the present series, improved outcomes in the more recent allogeneic transplantations did not become evident (Table 4).
However, because the main driver of lower performance of alloHCT in our study was inferiority in terms of NRM but not in terms of disease control, strategies for reducing NRM of alloHCT would help to make this tool more targeted. Using less aggressive reduced-intensity conditioning regimens might be helpful for this purpose.6
A unique strength of our study is the consistent ITT design, with comprehensive assessment of potential risk factors, thereby minimizing selection bias at study entry and permitting monitoring of selection mechanisms during the pre-CI phase. By fixing the study starting point as the indication for CI, it could be ensured that patients were captured at the time when the medical need for CI became evident and not only after they had been successfully prepared for CI. Only this approach allows an informative comparison of different treatment modalities and their potential impact on disease course in defined clinical settings, if a prospective trial is not available. As illustrated by the present study, it reflects the real world more accurately than ITT analyses using leukapheresis as a starting point, thereby dismissing preapheresis failures.15,31 Nevertheless, accepted indications may vary over time and also depend on the efficacy and toxicity of the treatment modality intended. This effect may have contributed to the fact that the overall risk profile of our CAR T-cell group seemed more unfavorable than that of the transplantation group, both at baseline and at conditioning for CI. In fact, the alloHCT cohort had a higher proportion of patients with transformed disease, but CAR T-cell patients were older, more heavily pretreated, and more often had bulky disease and/or a history of primary refractoriness. This notion is supported by the significantly poorer outcomes of patients with pre-CI failures in the CAR T-cell arm despite access to more modern salvage therapies.
Despite this disadvantage, survival of the patients for whom CAR T cells were intended was not inferior to that of the patients for whom alloHCT was intended and even tended to be superior in multivariate and stratified comparisons (excluding patients not meeting the approved CAR T-cell indication window). In contrast, patients for whom alloHCT as second-line treatment was intended had better outcomes than the CAR T-cell group, but in the complete absence of 2L CAR T-cell patients, our study was clearly not designed to investigate the second-line setting, and autoHCT would actually be the better comparator once CAR T cells become SOC in the second-line setting.5,11,33 Furthermore, it might be argued that the crossovers could have biased the comparison. However, because outcomes with secondary CAR T-cell treatments were much better than those with secondary transplantation, this should have confounded the results more in favor of the alloHCT group than vice versa.
Notably, the benefit seen in the CAR T-cell group in stratified comparisons relied on the coaction of different effects, namely fewer patients experiencing pre-CI failures (44% of the >2L alloHCT ITT patients did not undergo transplantation), better PFS because of less NRM, and longer survival after post-CI relapse. Although none of these factors was significant on its own, this suggests that a potential advantage of CAR T cells over alloHCT, if validated by follow-up studies, could be multifactorial (ie, losing fewer patients before CI, fewer nonrelapse deaths, and better survival after post-CAR relapse).
Although the first 2 of these factors are quite plausible, it is unclear if the better postrelapse outcomes in the CAR T-cell group resulted from better patient resilience compared with relapsed transplantation patients or from the better treatment and supportive care options of recent times. Compared with the literature, our relapsed CAR T-cell recipients had better survival and our relapsed alloHCT recipients had poorer survival than observed in the small series previously published.34-36
Nevertheless, patient outcomes after CAR T-cell therapy failure remain unsatisfying, and effective rescue strategies are urgently needed. To this end, alloHCT might warrant consideration,37 although our experience with allogeneic transplantation for disease progression after axi-cel in the present study was clearly not encouraging. However, in 4 of our 5 patients subsequently undergoing transplantation, response induction before salvage alloHCT was impossible. Therefore, it might be worthwhile to consider transplantation earlier after CAR T-cell treatment (eg, as consolidation in partial responders before further LBCL progression).19 However, CAR T cells are only at the beginning of their clinical development. Next-generation CARs comprising more than a single costimulatory molecule38,39 or targeting antigens other than CD19 or multiple antigens40 have the potential for a better safety profile and for greater and more enduring efficacy compared with CAR T-cell therapy.
The risk factors for adverse outcomes identified in this study were in line with published data for both CAR T cells (ie, poor PS, need for bridging, bulky disease, elevated LDH at lymphodepletion)15,31 and alloHCT (ie, primary refractory disease, multiple lines of pretreatment),10,41 but they were not congruent. One might speculate that patients with R/R LBCL might be assigned to the 2 modalities according to their individual risk profile (eg, patients with bulky disease and high LDH should rather undergo alloHCT). However, bulky disease could not be tested in our alloHCT group because too few patients were at risk, and LDH might be relevant only in the presence of active disease (eg, in a vast majority of CAR T-cell patients proceeding to lymphodepletion) and not as much in responding patients (eg, alloHCT candidates proceeding to conditioning). Therefore, this data set does not identify risk profiles favoring alloHCT instead of CAR T cells as first CI in R/R LBCL.
Another idea could be to assign type of CI according to disease status after bridging (ie, offering alloHCT to responders and CAR T cells to nonresponders). However, an exploratory analysis comparing outcomes with alloHCT vs CAR T-cell therapy in sensitive patients did not support such a strategy (supplemental Figure 8). This observation is in keeping with preliminary reports suggesting that CAR T-cell treatment in the absence of measurable disease results in good outcomes.42
In summary, although further validation is clearly needed, this study supports current algorithms recommending CAR T cells as first-choice CI in patients with multiply R/R LBCL.17-19 It also highlights that future retrospective comparisons of CAR T cells and alloHCT (eg, registry analyses) must employ matching strategies of high granularity to compensate for the considerable differences in baseline risk profiles and selection dynamics between these 2 CI modalities.
Send data sharing requests via e-mail to the corresponding author, Peter Dreger (peter.dreger@med.uni-heidelberg.de).
Authorship
Contribution: P.D., S.D., and M.S. designed the concept of the study, analyzed data, and wrote the manuscript; and all other authors contributed to data generation, helped in writing the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.D. reports consultancy for AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, and Roche; speakers’ bureau for AbbVie, Gilead, Novartis, Riemser, and Roche; and research support from Neovii and Riemser. C.K. reports advisory board membership for Gilead. M.S. reports research grants from Apogenix, Hexal, and Novartis; travel grants from Hexal and Kite; financial support for educational activities and conferences from bluebird bio, Kite, and Novartis; and advisory board membership for Merck Sharp & Dohme and is co–principal investigator of clinical trials for Merck Sharp & Dohme, GlaxoSmithKline, Kite, and Bristol-Myers Squibb and cofounder and shareholder of TolerogenixX Ltd. A.S. reports travel grants from Hexal and Jazz Pharmaceuticals and a research grant from Therakos/Mallinckrodt and is cofounder and part-time employee of TolerogenixX LtD. The remaining authors declare no competing financial interests.
Correspondence: Peter Dreger, Department of Medicine V, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: peter.dreger@med.uni-heidelberg.de.
References
Author notes
The full-text version of this article contains a data supplement.