Key Points
In relapsed/refractory AML, CR and MRD negativity are associated with lower risk of relapse and better relapse-free survival.
Patients who underwent HSCT in second remission had the best outcomes, irrespective of hematologic or MRD response.
Abstract
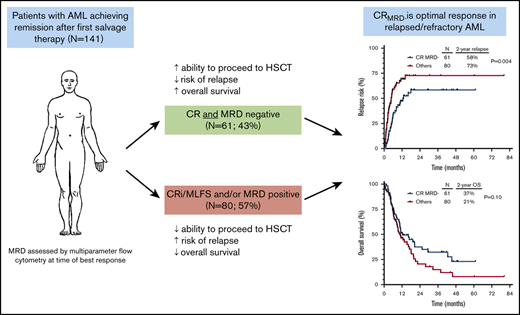
In relapsed/refractory acute myeloid leukemia (AML), the prognostic impact of complete remission (CR) and measurable residual disease (MRD) negativity is not well established. We retrospectively analyzed 141 patients with relapsed/refractory AML who received first salvage therapy and had MRD assessed by multiparameter flow cytometry at the time of response. Patients who achieved CR with full hematologic recovery as best response vs those with incomplete hematology recovery had lower cumulative incidence of relapse (P = .01) and better relapse-free survival (P = .004) but not overall survival (P = .15); a similar trend was observed in patients who achieved MRD negativity vs those who were MRD positive (P = .01, P = .05, and P = .21, respectively). By multivariate analysis, CR and MRD negativity were each independently associated with lower cumulative incidence of relapse (P = .001 and P = .003, respectively) and better relapse-free survival (P < .001 and P = .02) but not overall survival. Patients who achieved CR with MRD negativity had the lowest rates of relapse and best survival (2-year overall survival rate, 37%), which was driven largely by lower rates of early relapse and an increased ability in this group to undergo hematopoietic stem cell transplantation (HSCT); however, post-HSCT outcomes were similar regardless of response to salvage chemotherapy. Overall, in patients with relapsed/refractory AML, CR with MRD negativity was associated with the best outcomes, supporting it as the optimal response in this setting.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease, with widely variable disease biology and response to conventional therapies.1 Although cytogenetics and gene mutations are among the primary disease-related factors that influence prognosis,2 how well the disease responds to initial therapy is also a vital determinant of long-term outcomes and provides useful information about the chemosensitivity of an individual’s leukemia that cannot necessarily be predicted from pretreatment characteristics.3 In the frontline setting, the achievement of complete remission (CR) with full hematologic recovery has been shown to confer better long-term outcomes than morphologic remission (ie, <5% bone marrow blasts) with incomplete peripheral blood count recovery.4,5 Among patients who achieve morphologic remission, assessment of measurable (or “minimal”) residual disease (MRD) also provides important prognostic information, and multiple studies have shown that the achievement of MRD negativity is a strong predictor of better long-term outcomes in patients with AML undergoing frontline therapy.6-15 Because achievement of CR and MRD negativity are both independently associated with lower rates of relapse and superior survival in the frontline setting,5 recent consensus guidelines have supported the use of a new response criterion: “CR without MRD” (CRMRD–).16 CRMRD– is now considered the optimal response in AML, although, notably, the data supporting this recommendation are based almost exclusively on studies in the frontline setting, and its applicability to patients with relapsed/refractory disease is largely unknown.
For patients with AML in first relapse, established prognostic factors include: cytogenetics at diagnosis, prior allogeneic hematopoietic stem transplantation (HSCT), age at relapse, and length of relapse-free interval after first relapse.17 Together, these factors can stratify patients into widely disparate risk groups, with 5-year overall survival (OS) rates ranging from 4% to 46%. Although it may be reasonably assumed that the posttreatment factors which influence prognosis in the frontline setting would translate to patients with relapsed/refractory AML, to our knowledge, neither the role of hematologic recovery or achievement of MRD negativity at time of response has been systematically evaluated in the salvage setting, with the exception of a few small studies of specific novel agents.18-21 To evaluate the relevance of the response criteria of CRMRD– in patients with relapsed/refractory AML, we performed a retrospective study evaluating the impact of hematologic recovery and achievement of MRD negativity in patients receiving first salvage therapy with an intensive chemotherapy regimen. We also sought to determine how receiving subsequent HSCT might influence the prognostic impact of these responses.
Methods
Study design and participants
This retrospective study evaluated the prognostic impact of hematologic recovery and MRD status in adults with relapsed or refractory AML receiving first salvage therapy. Refractoriness to first-line therapy was defined as lack of response to at least 1 cycle of intensive induction chemotherapy or at least 2 cycles of lower intensity therapy (unless clear evidence of disease progression after 1 cycle). To be eligible for this analysis, patients were required to have achieved CR, CR with incomplete hematologic recovery (CRi), or morphologic leukemia-free status (MLFS) within 1 to 2 cycles of first salvage regimen and have an evaluable MRD measurement at the time of best response. To reduce heterogeneity among treatment regimens, only patients who received an intermediate- or high-dose cytarabine-based salvage regimen (defined as a cumulative dose of cytarabine ≥700 mg/m2 with re-induction) were included in the analysis. The various salvage regimens used are shown in supplemental Table 1. Patients with core-binding factor AML were excluded, as these patients are commonly evaluated with polymerase chain reaction–based MRD assays as standard of care.22
This study was conducted at a single academic center (The University of Texas MD Anderson Cancer Center), and it was approved by the institutional review board of the center. All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki.
MRD assessment
MRD was assessed by 8-color multiparameter flow cytometry (MFC) as previously described.6 Briefly, MRD assessment by MFC was performed on whole bone marrow specimens. Data were acquired for at least 2 × 105 cells when permitted by the specimen’s quality. MRD was identified compared with the known patterns of antigen expression by normal maturing myeloid precursors and monocytes as previously described.23,24 When available, the phenotypic profiles of pretreatment blasts were compared with those of specimens submitted for MRD testing. A distinct cluster of at least 20 cells showing altered expression of ≥2 antigens was regarded as an aberrant population. The sensitivity of this assay is 0.1% or higher. All specimens with unequivocally positive results were included in this analysis. However, specimens with indeterminate MRD assessment or with negative results but suboptimal cell counts were excluded.
Response and outcome definitions
CR, CRi, and MLFS were defined according to European LeukemiaNet consensus guidelines.16 The best response achieved within 1 to 2 cycles of first salvage chemotherapy was used for this analysis. Relapse was defined as recurrence of bone marrow blasts >5% or extramedullary AML. Cumulative incidence of relapse (CIR) was calculated from the time of best response until relapse, censored for death in morphologic remission, or if the patient was alive at last follow-up. Relapse-free survival (RFS) was calculated from the time of best response until relapse or death from any cause, censored if the patient was alive at last follow-up. OS was calculated from the time of treatment initiation until death from any cause, censored if the patient was alive at last follow-up. Survival estimates were not censored at the time of HSCT.
Statistical methods
Patient characteristics were summarized by using median (range) for continuous variables and frequencies (percentages) for categorical variables. To compare 2 groups, Fisher’s exact test was performed for categorical variables, and the Wilcoxon rank sum test was performed for continuous variables. Univariate Cox proportional hazards models were used to evaluate the risk factors associated with survival outcomes. A multivariate proportional hazards model was obtained by first including the factors with P < .20 on univariate analysis and then finalizing via backward elimination until all remaining factors had P < .05. Subgroup analysis was performed for transplanted patients and nontransplanted patients; the survival outcomes for transplanted patients were redefined from the time of HSCT. Landmark analysis was conducted for nontransplant patients using landmark time of 1.4 months, which was the median time to HSCT among patients undergoing transplant. Statistical analyses were conducted in R version 3.5.1.
Results
Patient characteristics and study cohort
Between August 2011 and July 2018, we identified 192 patients with relapsed/refractory AML who achieved CR/CRi/MLFS after first salvage therapy. Fifty-one patients were excluded due to no available MRD information (n = 30), equivocal MRD assessment (n = 20), and extramedullary disease only (n = 1). Overall, 141 patients with relapsed or refractory AML met inclusion criteria and were included in this analysis. Baseline characteristics of the study population are shown in Table 1. The median age was 58 years (range, 17-84 years). For first-line therapy, 90 patients (64%) received intensive cytotoxic chemotherapy, and 51 patients (36%) received lower intensity therapy, primarily with a hypomethylating agent. Eighty-eight patients (62%) were refractory to induction therapy or had a first remission duration <1 year. Among 80 patients who had responded to frontline therapy and in whom the duration of first response was known, 42 (53%) had a first remission duration <1 year, and 38 (48%) had a first remission duration ≥1 year. Thirteen patients (9%) had undergone prior HSCT. The majority of patients (87%) achieved best response after 1 cycle of salvage therapy.
Patient characteristics
| Characteristic . | Value (N = 141) . |
|---|---|
| Age, y | 58 [17-84] |
| WBC, ×109/L | 2.3 [0.3-207.0] |
| Hemoglobin, g/dL | 9.6 [3.8-14.3] |
| Platelets, ×109/L | 43 [3-464] |
| BM blasts, % | 32 [1-91] |
| Cytogenetics | |
| Diploid | 59 (42) |
| Poor risk | 38 (27) |
| Others | 42 (30) |
| Insufficient metaphases/not done | 2 (1) |
| CR1 response | |
| Refractory or CR1 duration <1 y | 88 (62) |
| CR1 duration ≥1 y | 38 (27) |
| Unknown | 15 (11) |
| Prior HSCT | 13 (9) |
| 2 cycles to best response | 19 (13) |
| Mutations* | |
| NPM1 | 22/57 (39) |
| NRAS/KRAS | 15/64 (23) |
| IDH2 | 16/79 (20) |
| IDH1 | 14/79 (18) |
| ASXL1 | 11/61 (18) |
| TET2 | 9/62 (15) |
| FLT3-ITD | 12/85 (14) |
| DNMT3A | 10/71 (14) |
| CEBPA | 9/69 (13) |
| RUNX1 | 8/62 (13) |
| WT1 | 6/61 (10) |
| PTPN11 | 6/68 (9) |
| FLT3-TKD | 7/85 (8) |
| EZH2 | 5/67 (7) |
| GATA2 | 4/62 (6) |
| Characteristic . | Value (N = 141) . |
|---|---|
| Age, y | 58 [17-84] |
| WBC, ×109/L | 2.3 [0.3-207.0] |
| Hemoglobin, g/dL | 9.6 [3.8-14.3] |
| Platelets, ×109/L | 43 [3-464] |
| BM blasts, % | 32 [1-91] |
| Cytogenetics | |
| Diploid | 59 (42) |
| Poor risk | 38 (27) |
| Others | 42 (30) |
| Insufficient metaphases/not done | 2 (1) |
| CR1 response | |
| Refractory or CR1 duration <1 y | 88 (62) |
| CR1 duration ≥1 y | 38 (27) |
| Unknown | 15 (11) |
| Prior HSCT | 13 (9) |
| 2 cycles to best response | 19 (13) |
| Mutations* | |
| NPM1 | 22/57 (39) |
| NRAS/KRAS | 15/64 (23) |
| IDH2 | 16/79 (20) |
| IDH1 | 14/79 (18) |
| ASXL1 | 11/61 (18) |
| TET2 | 9/62 (15) |
| FLT3-ITD | 12/85 (14) |
| DNMT3A | 10/71 (14) |
| CEBPA | 9/69 (13) |
| RUNX1 | 8/62 (13) |
| WT1 | 6/61 (10) |
| PTPN11 | 6/68 (9) |
| FLT3-TKD | 7/85 (8) |
| EZH2 | 5/67 (7) |
| GATA2 | 4/62 (6) |
Continuous variables are listed as median [range] and categorical variables as n (%) or n/N (%).
BM, bone marrow; CR1, first complete remission; WBC, white blood cell.
Mutations detected in ≥5% of tested patients are shown.
The median duration of follow-up of the entire cohort was 30.5 months (range, 0.3-80.3 months). The 1- and 2-year CIR rates were 59% and 67%, respectively. Median RFS was 5.6 months, with 1- and 2-year RFS rates of 34% and 23%. Median OS was 11.2 months, with 1- and 2-year OS rates of 48% and 29%.
Response rates
Ninety-five patients (67%) achieved CR, 26 (18%) achieved CRi, and 20 (14%) achieved MLFS as best response to salvage therapy. Eighty-six patients (61%) achieved MRD negativity at the time of best response. Best response was CRMRD– in 61 patients (43%), CR with MRD positivity in 34 patients (24%), CRi with MRD negativity in 12 patients (9%), CRi with MRD positivity in 14 patients (10%), MLFS with MRD negativity in 13 patients (9%), and MLFS with MRD positivity in 7 patients (5%). Notably, the rates of MRD negativity among patients achieving CR, CRi, and MLFS were not significantly different (64%, 46%, and 65%, respectively; P = .22), suggesting a lack of association between hematologic recovery and MRD response. Among MRD-positive patients, 53 had a quantifiable MRD value. MRD levels at best response were <0.1% in 4 patients (8%), 0.1% to 0.99% in 27 patients (51%), and ≥1% in 22 patients (42%). There was no difference in median level of MRD for patients who achieved CR, CRi, or MLFS (0.66%, 0.70%, and 1.50%; P = .68).
Factors associated with hematologic recovery and MRD status
Predictors for achievement of CR (vs CRi/MLFS) included diploid karyotype (78% vs 59% for patients with non-diploid karyotype; P = .02) and first remission duration ≥1 year (87% vs 57% for patients who were refractory to frontline therapy or with first remission duration <1 year; P = .001) (supplemental Table 2). Higher pretreatment bone marrow percentage was associated with a significantly increased rate of MRD negativity (median bone marrow blast percentage for MRD-negative vs MRD-positive cases, 35% vs 26%; P = .03) (supplemental Table 3). However, achievement of MRD negativity was not associated with any other pretreatment parameter.
Outcomes by hematologic recovery and MRD status for the entire cohort
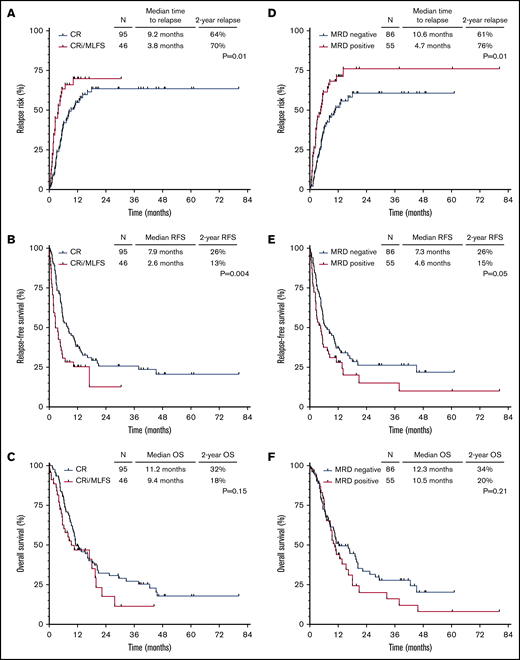
Given the similar outcomes for patients with CRi and MLFS in this cohort, these patients were combined for survival analyses. Patients who achieved CR vs CRi/MLFS had significantly lower CIR (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.34-0.88; P = .01) (Figure 1A) and better RFS (HR, 0.54; 95% CI, 0.35-0.82; P = .004) (Figure 1B) but not OS (HR, 0.72; 95% CI, 0.46-1.12; P = .15) (Figure 1C). A similar trend was observed in patients who achieved MRD negativity vs those who were MRD positive (CIR HR, 0.55 [95% CI, 0.35-0.85; P = .01]; RFS HR, 0.67 [95% CI, 0.45-0.99; P = .05]; OS HR, 0.76 [95% CI, 0.50-1.16; P = .21]) (Figure 1D-F). Notably, among MRD-positive patients, level of MRD (ie, <0.1% vs 0.1%-0.99% vs ≥1%) did not affect CIR (P = .88), RFS (P = .85), or OS (P = .91).
Outcomes of patients according to hematologic recovery and MRD status. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to hematologic response to salvage chemotherapy. CIR (D), RFS (E), and OS (F) for the entire cohort, stratified according to MRD response to salvage chemotherapy.
Outcomes of patients according to hematologic recovery and MRD status. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to hematologic response to salvage chemotherapy. CIR (D), RFS (E), and OS (F) for the entire cohort, stratified according to MRD response to salvage chemotherapy.
Impact of HSCT on outcomes
Sixty-two patients (44%) underwent allogeneic HSCT after first salvage therapy, with a median time of 1.4 months between achievement of second remission and HSCT. HSCT after first salvage therapy was the strongest prognostic factor identified for CIR, RFS, and OS (P < .001 for all). HSCT rate was higher in those who achieved CR vs CRi/MLFS (52% vs 28%, respectively; P = .008) and in those who were MRD negative vs MRD positive (52% vs 31%; P = .01).
Overall, 15 patients relapsed within 1.4 months after achievement of second remission. The relapse rate in this period was significantly higher in patients who achieved only CRi/MLFS and/or were MRD positive compared with those who achieved CRMRD–. The relapse rates within 1.4 months of second remission for patients who achieved CRMRD–, CR with MRD positivity, CRi/MLFS with MRD negativity, and CRi/MLFS with MRD positivity were 3%, 15%, 8%, and 29%, respectively. Overall, 2 (3%) of 61 patients with CRMRD– relapsed in this period, compared with 13 (16%) of 80 patients with CRi/MLFS and/or MRD positivity (P = .01). This increased rate of very early relapse was a major driver of the inferior outcomes observed in this latter group.
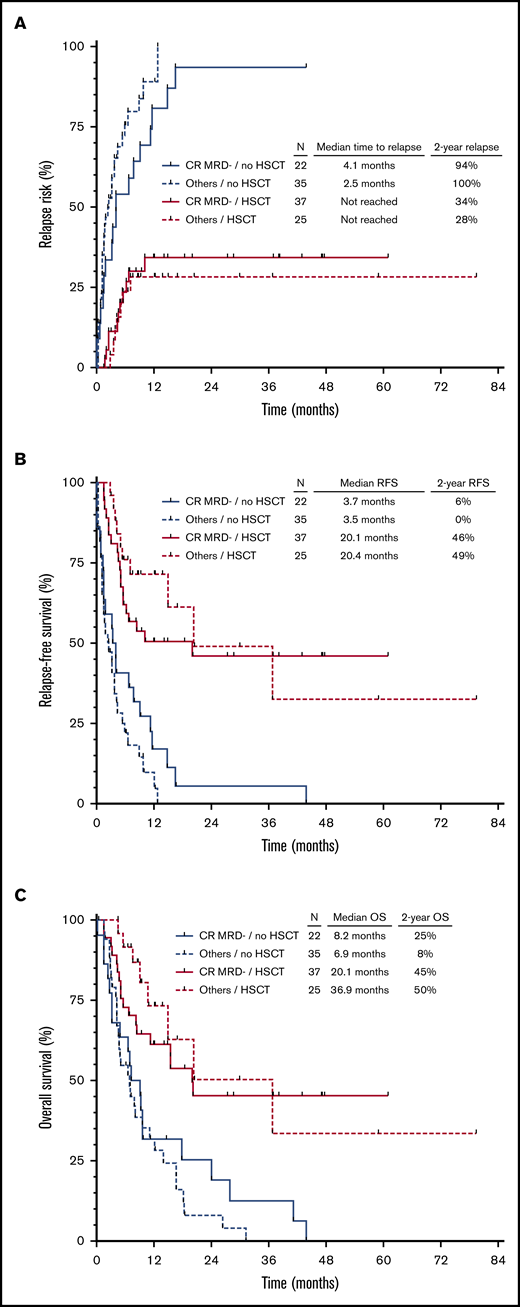
In a landmark HSCT analysis, 22 patients who relapsed (n = 15), died in remission (n = 4), or were lost to follow-up (n = 3) within 1.4 months of second remission were excluded. Among the remaining 57 patients who did not undergo HSCT in second remission, stratification of patients into 4 groups according to hematologic response (CR vs CRi/MLFS) and MRD response (positive vs negative) was associated with significant differences in CIR (P = .004) and RFS (P = .008) but not OS (P = .8) (supplemental Figure 1A-C). As expected, outcomes in patients who did not undergo HSCT in second remission were very poor, regardless of their response to salvage chemotherapy, with only 1 patient alive without relapse or death at 2 years.
Overall, 62 patients underwent HSCT in first remission. The conditioning regimen was myeloablative in 37 of these patients (60%) and reduced-intensity in 25 patients (40%). Twelve patients (19%) received post-HSCT maintenance therapy (azacitidine, n = 6; sorafenib, n = 3; crenolanib, n = 2; SGI-110, n = 1). Outcomes were significantly better for patients who underwent HSCT, regardless of their response to first salvage therapy (Figure 2). Interestingly, among patients who underwent HSCT, neither hematologic nor MRD response after salvage therapy was associated with CIR (P = .94), RFS (P = .77), or OS (P = .54) (supplemental Figure 2A-C). Fifty-nine of these patients also had pre-HSCT MRD information available (defined as MRD assessment within 6 weeks before HSCT), which was analyzed for impact on clinical outcomes. Forty-two of the 59 patients with pre-HSCT MRD information had at least one additional MRD assessment performed before HSCT. Thirty patients who were MRD negative remained MRD negative before HSCT, 1 patient converted from MRD negative to MRD positive, 7 patients converted from MRD positive to MRD negative, and 4 patients who were MRD positive remained MRD positive before HSCT. Overall, 50 patients (85%) were MRD negative and 9 patients (15%) were MRD positive before HSCT. Among MRD-positive patients, the median level of pre-HSCT MRD was 0.5% (range, 0.04%-2%). MRD status before HSCT was not associated with differential outcomes with respect to either CIR (P = .70), RFS (P = .46), or OS (P = .48) (supplemental Figure 3A-C).
Outcomes of patients according to hematologic recovery, MRD status, and subsequent HSCT. CIR (A), RFS (B), and OS (C) stratified according to CRMRD– vs lesser responses and HSCT vs no HSCT. Landmark analysis excluded patients who relapsed or died within 1.4 months from the time of response to salvage chemotherapy.
Outcomes of patients according to hematologic recovery, MRD status, and subsequent HSCT. CIR (A), RFS (B), and OS (C) stratified according to CRMRD– vs lesser responses and HSCT vs no HSCT. Landmark analysis excluded patients who relapsed or died within 1.4 months from the time of response to salvage chemotherapy.
Multivariate analysis and integration of hematologic recovery and MRD response
According to multivariate analysis, including established predictors for outcomes in relapsed/refractory AML (modified from Breems et al17 ) and using HSCT as a time-dependent variable, both CR and MRD negativity were independently associated with lower CIR (HR of 0.45 [95% CI, 0.28-0.73; P = .001] and HR of 0.50 [95% CI, 0.32-0.79; P = .003], respectively) and better RFS (HR of 0.46 [95% CI, 0.30-0.71; P < .001] and HR of 0.62 [95% CI, 0.41-0.93; P = .02]) but not with OS (Table 2; supplemental Tables 4-6).
Multivariate analysis for CIR, RFS, and OS
| Characteristic . | HR (95% CI) . | P . |
|---|---|---|
| CIR | ||
| MRD status (negative vs positive) | 0.50 (0.32-0.79) | .003 |
| Response (CR vs CRi/MLFS) | 0.45 (0.28-0.73) | .001 |
| Log of platelets | 0.74 (0.59-0.94) | .01 |
| HSCT after salvage therapy (time-dependent) | 0.20 (0.11-0.35) | <.001 |
| RFS | ||
| MRD status (negative vs positive) | 0.62 (0.41-0.93) | .02 |
| Response (CR vs CRi/MLFS) | 0.46 (0.30-0.71) | <.001 |
| Log of platelets | 0.77 (0.63-0.96) | .02 |
| HSCT after salvage therapy (time-dependent) | 0.25 (0.15-0.41) | .02 |
| OS | ||
| Cytogenetics (diploid vs others) | 0.58 (0.38-0.88) | .01 |
| HSCT after salvage therapy (time-dependent) | 0.28 (0.18-0.46) | <.001 |
| Characteristic . | HR (95% CI) . | P . |
|---|---|---|
| CIR | ||
| MRD status (negative vs positive) | 0.50 (0.32-0.79) | .003 |
| Response (CR vs CRi/MLFS) | 0.45 (0.28-0.73) | .001 |
| Log of platelets | 0.74 (0.59-0.94) | .01 |
| HSCT after salvage therapy (time-dependent) | 0.20 (0.11-0.35) | <.001 |
| RFS | ||
| MRD status (negative vs positive) | 0.62 (0.41-0.93) | .02 |
| Response (CR vs CRi/MLFS) | 0.46 (0.30-0.71) | <.001 |
| Log of platelets | 0.77 (0.63-0.96) | .02 |
| HSCT after salvage therapy (time-dependent) | 0.25 (0.15-0.41) | .02 |
| OS | ||
| Cytogenetics (diploid vs others) | 0.58 (0.38-0.88) | .01 |
| HSCT after salvage therapy (time-dependent) | 0.28 (0.18-0.46) | <.001 |
Variables included in multivariate analysis were: age, white blood cell count, platelet count, hemoglobin, bone marrow blast percentage, cytogenetics (diploid vs others), response to first induction (relapsed with first remission duration ≥1 year vs relapsed with first remission duration <1 year or refractory), prior HSCT, HSCT after salvage therapy (as time-dependent variable), hematologic response (CR vs CRi/MLFS), and MRD response (negative vs positive).
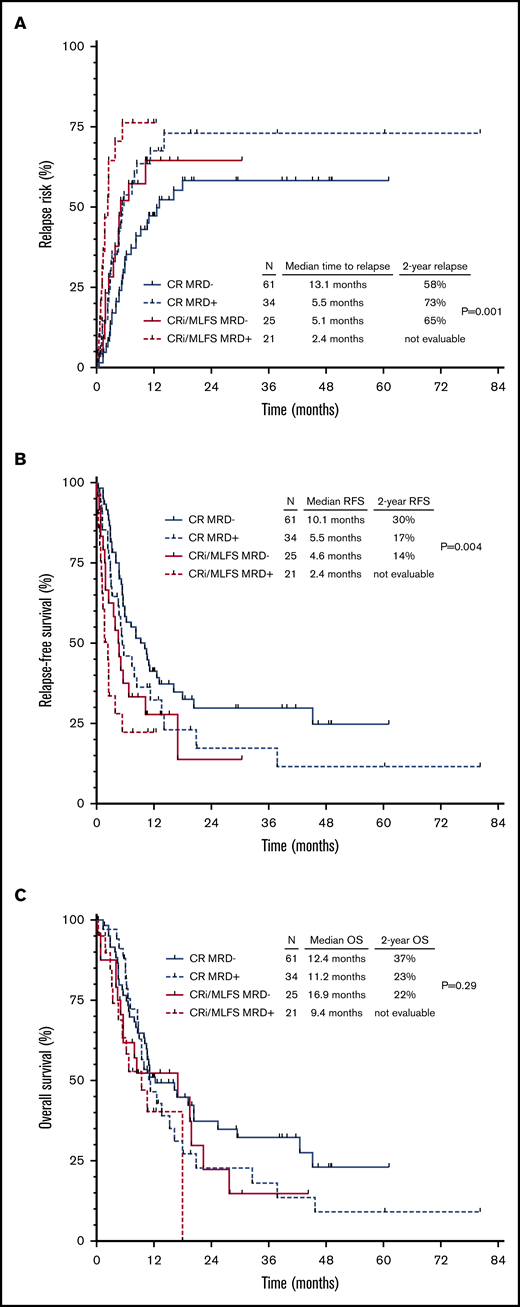
In light of the favorable and independent prognostic impact of achieving CR and of achieving MRD negativity, as well as to assess whether CRMRD– might be the optimal response for patients with relapsed/refractory AML, these variables were combined for analyses of relapse and survival outcomes. Integration of hematologic recovery and MRD information appeared to stratify patients into 3 groups according to CIR and RFS, ranging from most favorable outcomes to poorest outcomes: (1) CRMRD–; (2) CR with MRD positivity or CRi/MLFS with MRD negativity; and (3) CRi/MLFS with MRD positivity (Figure 3A-B). The 1-year CIR rates for these groups were 47%, 67%, and 76%, respectively (P = .001), and the median RFS were 10.1 months, 5.1 months, and 2.4 months (P = .004). However, OS was not significantly different between groups when stratified according to hematologic recovery and MRD status (P = .29) (Figure 3C).
Outcomes of patients according to integrated hematologic and MRD response. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to hematologic and MRD responses to salvage chemotherapy.
Outcomes of patients according to integrated hematologic and MRD response. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to hematologic and MRD responses to salvage chemotherapy.
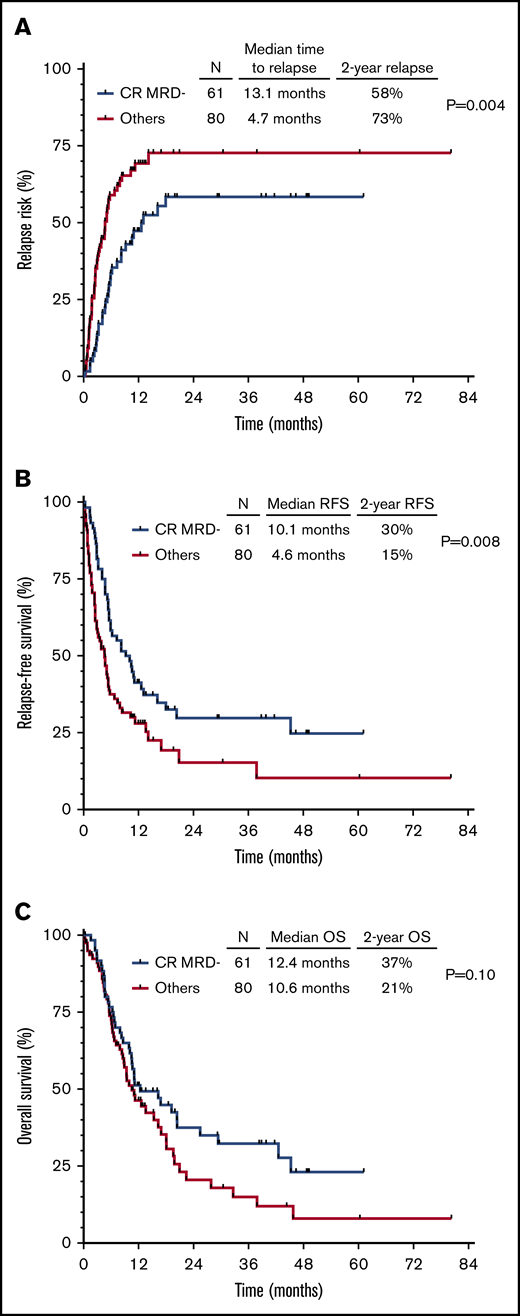
Patients who achieved CRMRD– as best response (n = 61 [43% of the entire cohort]) had better outcomes than those who achieved CR/MLFS and/or MRD positivity. Patients who achieved CRMRD– had significantly lower CIR (2-year CIR rate, 58% vs 73% [HR, 0.52; 95% CI, 0.33-0.82; P = .004]) (Figure 4A) and better RFS (2-year RFS rate, 30% vs 15% [HR, 0.58; 95% CI, 0.39-0.87; P = .008]) (Figure 4B). A trend for better OS was also observed in patients who achieved CRMRD– (2-year OS rate, 37% vs 21% [HR, 0.70; 95% CI, 0.46-1.07; P = .10]) (Figure 4C).
Outcomes of patients achieving CRMRD– vs lesser responses. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to CRMRD– vs lesser responses.
Outcomes of patients achieving CRMRD– vs lesser responses. CIR (A), RFS (B), and OS (C) for the entire cohort, stratified according to CRMRD– vs lesser responses.
The impact of achieving CRMRD– vs lesser responses was similar in both younger and older populations. Achievement of CRMRD– was associated with better RFS in patients aged <60 years (HR, 0.60; 95% CI, 0.35-1.05; P = .07) and in patients aged ≥60 years (HR, 0.57; 95% CI, 0.33-1.01; P = .05). Patients with diploid cytogenetics who achieved CRMRD– had a trend toward better RFS compared with those with lesser responses (HR, 0.66; 95% CI, 0.35-1.25; P = .20); this benefit of CRMRD– was also observed in patients with non-diploid cytogenetics (HR, 0.60; 95% CI, 0.36-0.99; P = .047).
Discussion
CR with full hematologic recovery and achievement of MRD negativity have both been shown in several studies to be associated with superior outcomes in patients with AML undergoing frontline therapy.4-15 In contrast, established prognostic factors in the relapsed/refractory setting have historically been limited to pretreatment clinical variables.17 In this study of patients with relapsed/refractory AML treated with first salvage chemotherapy, we showed that achievement of CR and MRD negativity by MFC are both independently associated with lower rates of relapse and superior RFS, even when accounting for subsequent HSCT. When hematologic recovery and MRD status were integrated, the best outcomes were observed in patients who achieved CRMRD– (43% of patients in the cohort), and the superior outcomes in these patients were driven, at least in part, by their ability to be bridged to allogeneic HSCT. Together, these data support the European LeukemiaNet consensus guidelines defining CRMRD– as the optimal response in AML16 and provide evidence that this response definition is also applicable to patients with relapsed/refractory disease.
In nearly all fit patients with relapsed/refractory AML (with the possible exception of select patients with core binding factor AML25 ), the goal of salvage therapy is to induce a response and bridge to potentially curative allogeneic HSCT. Because coordination of HSCT may take ≥6 weeks, transient responses may not provide adequate disease control to proceed with HSCT and thus may require additional lines of treatment before HSCT can be performed. We found that the rate of very early relapse (ie, within 1.4 months, which was the median time to HSCT in our study) was lower in patients who achieved CRMRD– vs those who achieved lesser responses (relapse rate within 1.4 months, 3% and 16%, respectively), which increased the ability of patients with CRMRD– to undergo HSCT after first salvage. In contrast, the suboptimal disease control associated with achieving only CRi/MLFS or MRD positivity contributed to the poorer outcomes of these patients, largely because fewer patients with these responses were able to undergo potentially curative HSCT. Not surprisingly, the outcomes of patients who did not undergo subsequent HSCT were poor, regardless of initial response to salvage chemotherapy. Although risk of relapse was lower, and RFS was superior for patients who achieved CR and/or MRD negativity, outcomes were still universally poor in all groups who did not undergo HSCT, with only 1 patient being alive without relapse 2 years after first salvage.
Previous reports, including a meta-analysis of 19 studies, have largely shown that achievement of MRD negativity before HSCT is associated with superior post-HSCT outcomes.10-13,26 In contrast, we found that MRD response after salvage chemotherapy or immediately before HSCT did not affect relapse rates or survival after HSCT. Our finding is consistent with another retrospective report that showed no difference in post-HSCT outcomes according to pre-HSCT MRD status (measured by MFC) in patients with relapsed or refractory AML.27 There are also emerging data that a myeloablative conditioning regimen may overcome the poor prognostic impact of pre-HSCT MRD when HSCT is performed in first remission, although similar analyses in patients in second remission or beyond are lacking.28,29 One limitation of our study is that the number of patients with pre-HSCT MRD information available was relatively small (n = 59), with only 9 patients who were MRD positive before HSCT; it was therefore not possible to perform meaningful subgroup analyses evaluating the interaction between conditioning regimen and pre-HSCT MRD status. One recent study has suggested that myeloablative and reduced-intensity conditioning regimens result in similar survival outcomes in patients who undergo transplant in second remission.30 Further analyses integrating both conditioning intensity and MRD status in the salvage setting are therefore warranted.
When comparing our results vs those of other reported studies on the impact of pre-HSCT MRD, it is important to note that most of these other studies were limited to patients who underwent transplant in first remission or combined patients who underwent transplant in first or later remissions in their analysis. However, AML disease biology is significantly different in patients who have not responded to or who relapsed after frontline therapy and is characterized by increased clonal complexity and chemoresistance.31 We therefore hypothesize that patients who achieve “MRD negativity” after frontline therapy and those who seemingly achieve the same response after salvage chemotherapy likely possess very different quantities of residual disease that are present below the level of detection of the MRD assay (in our study, sensitivity of at least 0.1%). This could similarly explain why patients in our study who were able to proceed to HSCT had similar post-HSCT outcomes, regardless of response to salvage chemotherapy. It is very possible that in the relapsed/refractory setting in which the disease is generally more chemoresistant, the difference in total quantity of residual disease between patients who are “MRD negative” and “MRD positive” is less pronounced than in the frontline setting, and these differences therefore may be more easily negated by HSCT. Additional studies using ultrasensitive MRD assays would be needed to better characterize and quantify the true level of residual disease that persists after chemotherapy, how these differ between frontline and relapsed/refractory patients, and how these very small amounts of residual disease affect outcomes after HSCT. Such an approach will be particularly important in the frontline setting in which deep MRD-negative responses identified by using highly sensitive assays may help to the inform decision for HSCT in first remission.
There are several important implications of our findings. First, the strong and independent impact of both CR and MRD negativity on risk of relapse and RFS support the use of CRMRD– as a valuable end point for clinical trials in patients with relapsed/refractory AML, which may allow for more rapid clinical evaluation and approval of novel agents. Although the impact of achieving CRMRD– was not statistically associated with OS (2-year OS rate, 37% vs 21%; P = .10), there was a strong trend toward better OS in patients who achieved CRMRD–, with these patients having nearly twice the 2-year OS rate as those with lesser responses. The lack of a significant difference may be in part due to the increasing availability of effective salvage regimens for these patients, including the development of novel FMS-like tyrosine kinase 3 inhibitors (eg, gilteritinib), IDH1 and IDH2 inhibitors (eg, ivosidenib and enasidenib), and venetoclax-based regimens, which became available in clinical trials and commercially over the study period.32 However, it remains possible that with a larger cohort of patients, an OS benefit might have been observed, which would further support the importance of CRMRD– in this context.
Our findings that hematologic recovery and MRD status do not significantly affect post-HSCT outcomes also inform the optimal timing of HSCT in the salvage setting. In contrast with the frontline setting, where outcomes after HSCT are significantly better in patients who achieve MRD negativity, we found no difference in post-HSCT outcomes according to response to salvage chemotherapy or pre-HSCT MRD. Our data therefore suggest that, whenever possible, immediate HSCT should be considered for any patient with relapsed/refractory AML who achieves a marrow remission, regardless of hematologic recovery or MRD response. In light of the high rates of early relapse in patients who achieve only CRi/MLFS and/or MRD positivity, our findings argue against a practice of attempting to administer additional cycles of chemotherapy in an effort to deepen a patient’s response before undergoing HSCT in the salvage setting; this may sometimes be necessary, however, when HSCT is not yet available.
In conclusion, among patients with relapsed/refractory AML receiving first salvage chemotherapy, both CR and MRD negativity were independently associated with a lower risk of relapse and longer RFS. Patients who achieved CRMRD– had the best outcomes, which were driven in part by an increased ability to undergo subsequent HSCT. Given the superior outcomes in patients who achieve CRMRD–, this response end point should be considered in clinical trials evaluating novel agents and combinations in relapsed/refractory AML.
Acknowledgments
This study was supported by an MD Anderson Cancer Center Support Grant (CA016672) and SPORE. N.J.S. is supported by the K12 Paul Calabresi Clinical Oncology Scholar Award and the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Authorship
Contribution: N.J.S., H.R., and F.R. designed the study, collected and analyzed the data, and wrote the manuscript; H.H. and J.N. performed statistical analyses; J.L.J. and S.A.W. performed MRD analyses; N.D., J.C., T.M.K., C.D.D., E.J., U.P., B.O., M.K., M.Y., G.C.I., and H.K. treated patients; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicholas J. Short, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: nshort@mdanderson.org.
References
Author notes
N.J.S. and H.R. contributed equally to this study.
Requests for data sharing may be submitted to the corresponding author (Nicholas J. Short; e-mail: nshort@mdanderson.org).
The full-text version of this article contains a data supplement.