Key Points
NK-cell levels and activity are impaired in severe COVID-19 patients.
Reduced NK-cell activity, elevated sCD25, and hyperferritinemia are indicative of immune dysregulation in severe COVID-19.
Abstract
The global pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–driven coronavirus disease 2019 (COVID-19) has caused unprecedented human death and has seriously threatened the global economy. Early data suggest a surge in proinflammatory cytokines in patients with severe COVID-19, which has been associated with poor outcomes. We recently postulated that the inflammatory response in patients with severe COVID-19 disease is not inhibited by natural killer (NK) cells, resulting in a “cytokine storm.” Here, we assessed the NK-cell functional activity and the associated cytokines and soluble mediators in hospitalized COVID-19 patients. Significantly impaired NK-cell counts and cytolytic activity were observed in COVID-19 patients when compared with healthy controls. Also, cytokines like interleukin 12 (IL12), IL15, and IL21 that are important for NK-cell activity were not detected systematically. Serum concentrations of soluble CD25 (sCD25)/soluble IL2 receptor α (sIL2-Rα) were significantly elevated and were inversely correlated with the percentage of NK cells. Impaired NK-cell cytolytic activity together with other laboratory trends including elevated sCD25 were consistent with a hyperinflammatory state in keeping with macrophage-activation syndrome. Our findings suggest that impaired counts and cytolytic activity of NK cells are important characteristics of severe COVID-19 and can potentially facilitate strategies for immunomodulatory therapies.
Introduction
First reported in late 2019,1,2 the coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),3 continues to spread across the globe. As of 16 August 2020, 21 756 357 cases have been confirmed with at least 771 635 deaths in over 180 countries worldwide.4 In addition to fever, nonproductive cough, dyspnea, fatigue, and lymphopenia, severe COVID-19 cases present with pneumonia and acute respiratory distress syndrome (ARDS), multiorgan failure, and even death.5 To date, there are no preventative vaccines, nor proven therapeutics that have been approved for this disease, though efforts worldwide have shown some promising results.6,7
Rapid activation of the innate immune system accompanied by an aggressive inflammatory response, associated with a surge in many proinflammatory cytokines, has been reported, especially in patients developing severe manifestations of COVID-19.5,8 This “hypercytokinemia” or “cytokine storm” is thought to be driving the COVID-19–related ARDS,5,8 and associated hyperferritinemia is reminiscent of macrophage-activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (HLH; sHLH). Timely and effective management of severe COVID-19 persists as one of the major unmet needs and can be facilitated by an understanding of the associated hyperinflammation.9,10
Although HLH-related laboratory trends may have limited relevance in severe COVID-19, natural killer (NK)–cell functionality could be critical in the evolving COVID-19–MAS/sHLH milieu.11 NK cells constitute the first line of defense against viral infections and are functionally impaired in MAS/sHLH as an outcome of an intrinsic functional deficiency, lymphopenia, or both.12 Moreover, NK cells regulate the macrophage turnover and provide a negative feedback mechanism to macrophage overabundance and a more tissue-aggressive inflammatory response to infection.13 The absence of NK-cell–mediated frontline defense is associated with an aggressive secondary hyperinflammatory response driving ARDS in severe COVID-19 patients and forms the hypothetical framework of this study. Here, we evaluated NK-cell counts and activity as well as serum concentrations of associated cytokines and soluble receptors as characteristics of hyperinflammation associated with severe COVID-19.
Study design
Seventy-eight healthy controls (42 men, 36 women; median age, 32 years; range, 18-76 years) and 10 hospitalized COVID-19 patients (7 men, 3 women; median age, 67 years; range, 39-87 years), positive for SARS-CoV-2 were included in the study. The patients were SARS-CoV-2+ by quantitative reverse transcription–polymerase chain reaction assay specific for envelope transcripts. Peripheral blood was collected within 24 to 48 hours of confirmatory polymerase chain reaction testing (5-8 days from the onset of symptoms). All patients were febrile, hypoxic, and were free from any coinfection. Of these, 4 patients required mechanical ventilation. The samples were obtained in the absence of any antiviral or immunomodulation therapies. The University of Alberta Health Research Ethics Board approved this study (Pro00099910).
Flow cytometry–based absolute counts of NK cells (CD45+CD3−CD56+CD16+/−) and their subset distribution (CD56dim and CD56bright) were determined as a routine laboratory investigation and were calculated using clinical hematology laboratory–determined absolute lymphocyte counts. NK-cell functional activity was assessed using a flow cytometry–based CD107a (degranulation) and interferon-γ (IFN-γ) production assay described earlier.14 Briefly, peripheral blood mononuclear cells were isolated using the Ficoll-gradient method. Mononuclear cells were cocultured with erytholeukemic K562 cells in a 2:1 effector-to-target ratio in complete RPMI 1640 medium and mouse anti-human CD107a–fluorescein isothiocyanate (FITC, clone eBioH4A3) for 4 hours at 37°C, 5% CO2. Golgi inhibitors (monensin and brefeldin) were added after 1 hour of culture to enable intracellular staining. Cells harvested after culture were stained with monoclonal mouse anti-human antibodies: CD56-allophycocyanin (APC, clone TULY56), CD3-eflour450 (clone 17A2), and IFN-γ–phycoerythrin (PE, clone 45-15) before flow cytometry–based enumeration of CD56+CD3− NK cells that were either degranulating (CD107a+) or producing cytokine (IFN-γ+). CD107a+ and IFN-γ+ NK cells were expressed as a percentage of parent (NK) cells.
Luminex-based multiplex immunoassays were used to determine the serum concentrations of interleukin 12 (IL12), IL15, and IL21 (ThermoFisher Scientific Inc), and soluble CD25 (sCD25)/soluble IL2 receptor α (sIL2-Rα) (R&D Systems Inc) as per the manufacturer’s instructions.
NK-cell counts, functional activities, and serum analytes obtained for COVID-19 patients were compared with those obtained for healthy controls using Mann-Whitney U statistics. Where applicable, correlation analyses were performed using Spearman rank correlation. Test performance characteristics of all of the assays were determined in the clinically accredited Hematology Translational Laboratory, University of Calgary.
Results and discussion
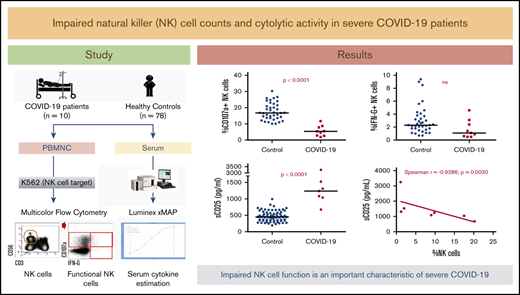
Absolute counts of total NK cells were significantly lower in COVID-19 patients (median, 1.1 × 108/L; range, 0.2 × 108/L to 2.9 × 108/L) than in controls (median, 2 × 108/L; range, 1.1 × 108/L to 8.3 × 108/L; Figure 1). Significantly higher percentages of CD56dim and lower percentages of CD56bright NK-cell fractions were observed in patients than in controls (Figure 1). Similarly, higher percent CD16+ and lower percent CD16− populations were observed when comparing the COVID-19 patients to controls (data not shown).
NK-cell quantity and function in severe COVID-19. Absolute NK-cell counts (A), percentage of CD56dim cytolytic NK cells (of total NK cells) (B), and percentage of CD56bright NK cells (of total NK cells) (C) of severe COVID-19 patients were compared with those obtained for healthy controls using Mann-Whitney U statistics. Upon stimulation with erythroleukemic K562 cells, the percentage of degranulating (CD107a+) NK cells (D), degranulation per NK cell expressed as CD107a mean fluorescence intensity (MFI; E), the percentage of IFN-γ (IFN-G)–producing NK cells (F) and IFN-G production per cell (IFN-G MFI; G) in severe COVID-19 patients was also compared. Two-tailed P < .05 was considered statistically significant. *P < .05; **P < .0001. ns, not significant.
NK-cell quantity and function in severe COVID-19. Absolute NK-cell counts (A), percentage of CD56dim cytolytic NK cells (of total NK cells) (B), and percentage of CD56bright NK cells (of total NK cells) (C) of severe COVID-19 patients were compared with those obtained for healthy controls using Mann-Whitney U statistics. Upon stimulation with erythroleukemic K562 cells, the percentage of degranulating (CD107a+) NK cells (D), degranulation per NK cell expressed as CD107a mean fluorescence intensity (MFI; E), the percentage of IFN-γ (IFN-G)–producing NK cells (F) and IFN-G production per cell (IFN-G MFI; G) in severe COVID-19 patients was also compared. Two-tailed P < .05 was considered statistically significant. *P < .05; **P < .0001. ns, not significant.
Upon stimulating with K562, the cytolytic responses expressed as the percentage of CD107a+ NK cells were remarkably impaired with significantly low cytolytic activity per cell in COVID-19 patients as compared with controls (Figure 1). Nine of 10 patients had <10% CD107a+ NK cells in response to K652 cells, which was below the healthy control reference range (10% to 38%) established as the 5th to 95th percentile using this assay. Intracellular IFN-γ production was not significantly different with 7 of 10 patients having >1% IFN-γ–producing NK cells (reference range, 1% to 7%). The observed low NK-cell counts and cytolytic activity is consistent with what is known in MAS/sHLH.15,16 Similar observations were recently reported in COVID-19, however, an NKG2A-mediated functional suppression was attributed.17
Serum concentrations of NK-cell–activating cytokines (IL12, IL15, and IL21) were significantly lower in patients than in controls (Figure 2). These cytokines are typically elevated in patients with MAS/sHLH, suggesting that an alternative mechanism of NK-cell suppression in COVID-19 is in play. Reduced expression of CD107a, Ksp37, granzyme B, and granulysin in addition to impaired production of chemokines, IFN-γ, and tumor necrosis factor α (TNF-α) was recently shown in ex vivo cells from peripheral blood of COVID-19 patients.17,18 Increased immune checkpoints through the upregulation of inhibitory receptors on NK cells contributing to viral escape have been suggested.18-20 Furthermore, other RNA viruses causing pulmonary infections such as influenza A have also been reported to reduce NK-cell activity and levels.21,22 In vitro stimulation by IL6 (and IL6R) has been shown to result in impaired NK-cell cytotoxicity, which was restored following IL6R blockade using tocilizumab.23 In the early studies, COVID-19 patients were shown to have higher plasma concentrations of IL6,5 which significantly correlated with lower NK-cell numbers.24,25 TNF-α, known to contribute to NK-cell differentiation,26 is also shown to be upregulated in the plasma of COVID-19 patients.5 These data suggest that NK-cell recognition and killing of SARS-CoV-2–infected cells may be impaired by the cross talk with monocytes, and antibodies targeting IL6 and TNF signaling may benefit enhanced NK-cell functions in COVID-19 patients.
NK-cell–related cytokines and sCD25 in severe COVID-19. Serum concentrations of cytokines involved in NK-cell activation, IL12 (A), IL15 (B), and IL21 (C), in addition to sCD25 (sIL2-Rα; D), in severe COVID-19 patients were compared with those obtained among healthy controls using Mann-Whitney U statistics. Two-tailed P < .05 was considered statistically significant. (E) Association between sCD25 serum concentrations and percentage of NK cells in COVID-19 patients was evaluated by Spearman rank correlation. **P < .0001. nd, not determined (no detectable levels obtained in COVID-19 patients).
NK-cell–related cytokines and sCD25 in severe COVID-19. Serum concentrations of cytokines involved in NK-cell activation, IL12 (A), IL15 (B), and IL21 (C), in addition to sCD25 (sIL2-Rα; D), in severe COVID-19 patients were compared with those obtained among healthy controls using Mann-Whitney U statistics. Two-tailed P < .05 was considered statistically significant. (E) Association between sCD25 serum concentrations and percentage of NK cells in COVID-19 patients was evaluated by Spearman rank correlation. **P < .0001. nd, not determined (no detectable levels obtained in COVID-19 patients).
Given the accumulating evidence of an overactive immune system driving severe COVID-19–related ARDS, widespread interest has developed both for anticytokine therapies27,28 and early identification of hyperinflammation.10,29 Indeed, we observed elevated serum concentrations of ferritin (median, 1124 [162-20 081] µg/mL) and C-reactive protein (median, 179.3 [20.7-337.9] mg/L), in addition to high fever and lymphopenia in the studied group of patients. Significantly elevated sCD25 was also observed in COVID-19 patients (median, 1239 [676-3248] pg/mL) as compared with controls (median, 450 [194-995] pg/mL) and was inversely correlated with the percentage of NK cells in the patients (Figure 2). However, consistent with earlier observations,30,31 these parameters, and especially those that weigh in heavy in the H-score (eg, hyperferritinemia, sCD25), do not reach the “HLH-high” thresholds. Furthermore, high fever (>39°C) is not exclusive to severe COVID-1932 and other H-score criteria such as hypertriglyceridemia, organomegaly, and bone marrow hemophagocytosis do not appear to apply to COVID-19.5,32 Here, we describe impaired NK-cell cytolytic activity associated with a loss of cytokines important in NK-cell stimulation in severe COVID-19 patients. This impairment and the resulting absence of a negative feedback mechanism for the secondary tissue-aggressive inflammatory reaction may explain the proinflammatory cytokine surge observed in severe COVID-19 patients, as postulated earlier.11
In its attempt to understand the pathophysiology of the COVID-19 pandemic during these unprecedented times, this study faces several challenges and limitations, a small sample size being one of them. The clinical presentations and laboratory data of only severe COVID-19 patients, representing only a subset of infected individuals, were studied. Finally, given the skewed demographics of disease severity, the healthy controls are not matched for age. Nevertheless, these findings are important as a substantial addition to the rapidly evolving understanding of the pathophysiology in COVID-19 and can be used as relevant biomarkers in patients receiving immunomodulating therapies.33
In conclusion, this study demonstrates that reduced NK-cell counts and impaired cytolytic activity are important characteristics associated with severe COVID-19–related hyperinflammation. Further prospective studies could address whether NK-cell characteristics, including a genetic predisposition for impaired NK-cell function, could facilitate early identification of severe COVID-19 and guide interventions such as targeted immunomodulation to reduce cytokine storm and hopefully reduce morbidity and mortality.
Data-sharing requests may be e-mailed to the corresponding author, Faisal M. Khan, at fkhan@ucalgary.ca.
Acknowledgments
The authors thank the Executive Leadership Team of Alberta Precision Laboratories for supporting this study. The authors also thank Stephanie Dookie for help in cytokine analyses.
Authorship
Contribution: M.O., F.M.K., M.-T.S.-R., W.S., and J.W.C.T. conceived the study and recruited the patients; R.M.F. performed NK-cell experiments, analyzed the data, and took the lead in writing the manuscript in consultation with F.M.K., P.D.-K., and M.O.; M.O. and F.M.K. edited the manuscript; P.D.-K., A.K., and M.B.T. performed cytokine profiling of patients and controls; M.-T.S.-R., J.S., and A.P. provided critical feedback; F.M.K. designed the study, planned the experiments, and provided all of the laboratory infrastructure in this project; and all of the authors provided critical feedback and helped shape the research, analysis, and manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Faisal M. Khan, Department of Pathology and Laboratory Medicine, University of Calgary, HMRB-320 Heritage Medical Research Building, 3330 Hospital Dr NW, Calgary, AB T2N 4N1, Canada; e-mail: fkhan@ucalgary.ca.
References
Author notes
M.O. and R.M.F. contributed equally to this work.