Key Points
The VWFAg/ADAMTS13Ac ratio in acute IS is associated with clinical outcome, independent of age; a ratio >2.6 predicts mortality.
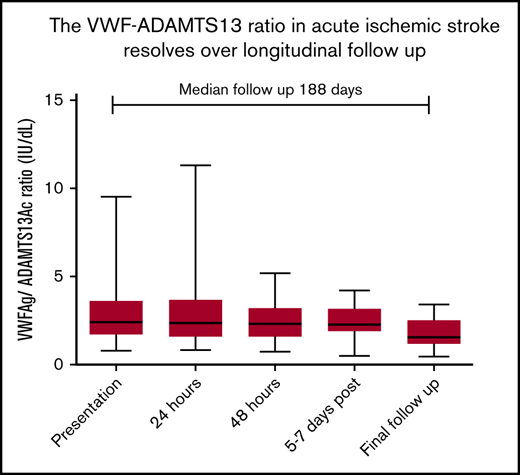
The VWFAg/ADAMTS13Ac ratio resolves over longitudinal follow-up; normalization is faster after thrombolysis.
Abstract
Acute ischemic stroke (IS) and transient ischemic attack (TIA) are associated with raised von Willebrand factor (VWF) and decreased ADAMTS13 activity (ADAMTS13Ac). Their impact on mortality and morbidity is unclear. We conducted a prospective investigation of the VWF-ADAMTS13 axis in 292 adults (acute IS, n = 103; TIA, n = 80; controls, n = 109) serially from presentation until >6 weeks. The National Institutes of Health Stroke Score (NIHSS) and modified Rankin scale (mRS) were used to assess stroke severity. Presenting median VWF antigen (VWF:Ag)/ADAMTS13Ac ratios were: IS, 2.42 (range, 0.78-9.53); TIA, 1.89 (range, 0.41-8.14); and controls, 1.69 (range, 0.25-15.63). Longitudinally, the median VWF:Ag/ADAMTS13Ac ratio decreased (IS, 2.42 to 1.66; P = .0008; TIA, 1.89 to 0.65; P < .0001). The VWF:Ag/ADAMTS13Ac ratio was higher at presentation in IS patients who died (3.683 vs 2.014; P < .0001). A presenting VWF:Ag/ADAMTS13Ac ratio >2.6 predicted mortality (odds ratio, 6.33; range, 2.22-18.1). Those with a VWF:Ag/ADAMTS13Ac ratio in the highest quartile (>3.091) had 31% increased risk mortality. VWF:Ag/ADAMTS13Ac ratio at presentation of ischemic brain injury was associated with higher mRS (P = .021) and NIHSS scores (P = .029) at follow-up. Thrombolysis resulted in prompt reduction of the VWF:Ag/ADAMTS13Ac ratio and significant improvement in mRS on follow-up. A raised VWF:Ag/ADAMTS13Ac ratio at presentation of acute IS or TIA is associated with increased mortality and poorer functional outcome. A ratio of 2.6 seems to differentiate outcome. Prompt reduction in the ratio in thrombolysed patients was associated with decreased mortality and morbidity. The VWF:Ag/ADAMTS13Ac ratio is a biomarker for the acute impact of an ischemic event and longer-term outcome.
Introduction
von Willebrand factor (VWF) together with ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) plays a key role in arterial thrombosis. VWF is integral for platelet adhesion to collagen fibers and atherosclerotic plaques and platelet aggregation under high shear conditions.1,2 ADAMTS13 is responsible for the breakdown and ultimate control of ultralarge VWF multimers secreted from the endothelium.3
Prospective studies have identified that raised VWF and decreased ADAMTS13 predispose patients to increased risk of stroke, including the well-characterized Rotterdam cohort of >6000 patients stroke free at baseline.4-10 Animal models have shown that extent of thrombosis in stroke is influenced by VWF and ADAMTS13 in an interdependent manner in genetically manipulated mice.11-13 In these models, ADAMTS13 deficiency in a VWF-dependent manner was associated with increased infarct volume, with consequential functional outcome. More recent mouse work supports the role of ADAMTS13 in thrombus dissolution and restoration of vessel patency.14,15
In a smaller cohort of acute stroke and transient ischemic attack (TIA) patients, ADAMTS13 activity was reduced and VWF antigen (VWF:Ag) increased within 4 weeks of onset of ischemic stroke (IS) or TIA. Examining patients at >3 months postpresentation showed that VWF:Ag levels remained high. ADAMTS13, in contrast, showed significant reduction in IS compared with controls in the early phase, but not later, potentially suggesting recovery of ADAMTS13 after consumption in IS.16
Clinical case-control studies have suggested a link between increased VWF and decreased ADAMTS13 at presentation and later follow-up, with severity and recurrence risk.17-21 There is no clear consensus about whether any marker of hemostasis can be used to predict clinical outcome poststroke.22 Neither the functional outcome of patients with IS related to baseline ADAMTS13 nor the comparison of the VWF-ADAMTS13 axis in patients with TIA or a more stuttering type of stroke phenotype has been studied. We hypothesized that increased levels and activity of VWF, low levels of ADAMTS13 activity (ADAMTS13Ac), and the balance of these hemostatic markers in individual patients would be predictive of functional recovery after acute IS and TIA.
This unique prospective study investigated VWF antigen (VWF:Ag), VWF activity (VWF:Act), and ADAMTS13Ac in patients presenting with acute IS or TIA, compared with controls and examined sequentially. This is the largest cohort examined over the hyperacute and convalescent phases, with correlation of these hemostatic markers with clinical outcome, as measured by functional stroke scores and follow-up mortality.
Deepening our understanding of the role of the VWF-ADAMTS13 axis in ischemic brain injury could herald the use of novel therapies, such as recombinant ADAMTS13, to reduce infarct size in an acute cerebral ischemic presentation, prevent deterioration of cerebral perfusion in patients presenting with TIA, reduce the risk of recurrence, and improve clinical outcome.
Methods
We prospectively investigated the VWF-ADAMTS 13 axis in acute ischemic brain injury over a 30-month period. The National Stroke Strategy has revolutionized stroke services in the United Kingdom, with 8 hyperacute stroke units commissioned in London.23 Each services a population of ∼1 million, with streamlining of services allowing for emergency brain scans and improved delivery of thrombolysis.24 In line with this, our institution has a daily TIA clinic for assessment of symptoms suggestive of TIA in patients well enough for outpatient scans and investigations.
Patients age ≥18 years presenting with symptoms suggestive of stroke with onset <48 hours were eligible and recruited from our institution, a regional hyperacute stroke unit and daily TIA clinic. Patients routinely underwent a full investigative pathway of clinical, laboratory, and imaging assessments. Imaging consisted of either computed tomography or magnetic resonance imaging of the head, reported by a neuroradiologist. Senior stroke physicians categorized patients (ie, stroke or TIA) following this clinical diagnostic pathway. Control patients were those who presented with symptoms suggestive of stroke or TIA, but for whom the diagnostic pathway found no evidence of acute ischemic brain injury, such as those with a subsequent diagnosis of migraine or seizure.
Exclusion criteria included secondary precipitating causes, such as known active malignancy or autoimmune disease. Written consent was obtained from patients or from their nearest relative, in the event of a lack of capacity or stroke-specific disabilities. The study was approved by an institutional review board (research ethics committee reference 14/EE/0169). Patients were categorized as IS, TIA, or control after neuroimaging and clinical assessment by senior stroke physicians. Control cases were patients referred to the stroke service with acute symptoms suggestive of stroke or TIA, but in whom subsequent investigations identified other medical causes for presentation, such as seizure or migraine. Clinical data were collected concerning vascular risk factors, admission medications, and IS subtype as defined by the TOAST classification.25 Functional and neurological impairments were documented at each time point using the modified Rankin scale (mRS), the National Institutes of Health Stroke Score (NIHSS), and the Glasgow Coma Score (GCS; information on TOAST classification and mRS and NIHSS scoring is provided in supplemental Tables 1-3).26-29
Blood was sampled at presentation, sequentially during admission at 24 and 48 hours and at 7 to 14 days postpresentation if feasible, and at the final follow-up a minimum 6 weeks postpresentation. Measurements at each time point were not achieved for every patient, such as those who attended the TIA clinic as outpatients, but particular focus was given to measuring blood at follow-up.
At each time point, blood collection consisted of 2 citrated plasma samples (each ×4.5 mL). Venepuncture was performed via standard procedures. Samples were double centrifuged at 1500 g for 10 minutes for each run. Samples were then stored at −80°C until further processing.
Laboratory processing
Laboratory measurements of VWF:Ag, VWF:Act, factor VIII (FVIII), ADAMTS13Ac and thrombin generation were conducted. VWF:Ag and VWF:Act were measured using a standard automated immunoturbimetric assay in a Sysmex CS-2000i analyzer with a Siemens kit (VWF:Ag and INNOVANCE VWF:Act, Siemens Healthcare Diagnostics, Marburg, Germany). FVIII analysis was also performed using a Sysmex CS-2000i analyzer with a Siemens kit (coagulation FVIII-deficient plasma; reference OTXW). ADAMTS13Ac measurement was performed using the FRETS-ADAMTS13 activity assay (Fluorescence Resonance Energy Transfer), based on previously published work.30,31
Statistics
Statistical packages used were STATA, for multiple regression and receiver operator curve (ROC) analysis, and GraphPad Prism, for correlation, Student t, Mann-Whitney U, Kruskal-Wallis, and χ2 testing. The VWF:Ag/ADAMTS13Ac ratio was calculated for each set of results for each patient. Comparison of presenting hemostatic markers between 2 groups was performed using the Mann-Whitney U (if nonparametric) or unpaired Student t test (if parametric) for unmatched data sets, and the Wilcoxon matched pairs signed rank test was used for nonparametric matched data sets. Kruskal-Wallis testing was used to compare the medians of ≥3 groups (nonparametric; ie, whether VWF:Ag varied significantly at each time point from t0 to t4). Multiple linear regression and ROC analysis were used to determine the independent influence of VWF and ADAMTS13 on stroke scores and mortality.
Results
Patient characteristics
A total of 292 patients were recruited, with 141 men and 151 women, age from 23 to 100 years (median, 71.5 years). Patients were categorized into subgroups according to discharge diagnosis: IS (n = 103), TIA (n = 80), or control (n = 109); 31% of the whole cohort had a history of IS or TIA. Cardiovascular risk factors were documented (additional demographic information, including comorbidities, ABO blood group, and anticoagulation, is provided in supplemental Tables 4 and 5).
Differences among IS, TIA, and control groups at presentation
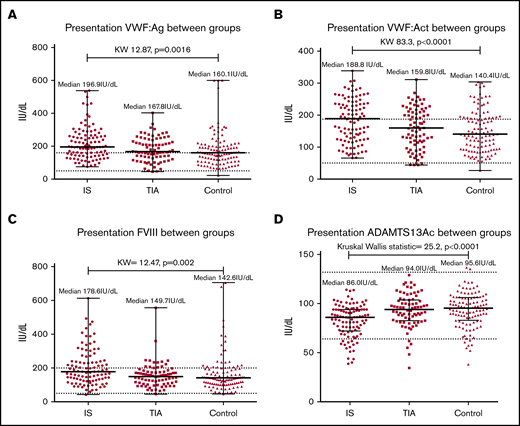
Comparing VWF:Ag, VWF:Act, and FVIII among groups showed significant differences, with the highest levels seen in IS. Conversely, ADAMTS13Ac was significantly lower in the IS group compared with the other groups (Figure 1).
Comparison of hemostatic markers among groups at presentation demonstrated significant differences between groups. (A) VWF:Ag. (B) VWF:Ac. (C) FVIII. (D) ADAMTS13Ac. IS demonstrates the highest median VWF:Ag (196.9IU/dl), VWF:Ac (188.8IU/dL) and FVIII (178.6IU/dL), followed by TIA (VWF:Ag 167.8, VWF:Ac 159.8, FVIII 149.7 IU/dL) and controls (VWF:Ag 160.1, VWF:Ac 140.4, FVIII 142.6IU/dL). The reverse trend was seen for ADAMTS13Ac, as illustrated in panel D (IS median 86.0, TIA 94.0, controls 95.6IU/dL). KW, Kruskal-Wallis.
Comparison of hemostatic markers among groups at presentation demonstrated significant differences between groups. (A) VWF:Ag. (B) VWF:Ac. (C) FVIII. (D) ADAMTS13Ac. IS demonstrates the highest median VWF:Ag (196.9IU/dl), VWF:Ac (188.8IU/dL) and FVIII (178.6IU/dL), followed by TIA (VWF:Ag 167.8, VWF:Ac 159.8, FVIII 149.7 IU/dL) and controls (VWF:Ag 160.1, VWF:Ac 140.4, FVIII 142.6IU/dL). The reverse trend was seen for ADAMTS13Ac, as illustrated in panel D (IS median 86.0, TIA 94.0, controls 95.6IU/dL). KW, Kruskal-Wallis.
Calculation of the VWF:Ag/ADAMTS13Ac ratio for each patient was then performed to capture the intraindividual balance between these hemostatic markers and examine associations among the groups. A significant difference was seen among group medians as follows: IS, 2.42 (range, 0.78-9.53); TIA, 1.89 (range, 0.41-8.14); and control, 1.69 (range, 0.25-15.63; Kruskal-Wallis test, 24.7; P < .0001).
CRP and VWF:Ag/ADAMTS13Ac
There was a significant difference in median C-reactive protein (CRP; normal range, 0-5 mg/L) among groups at presentation: IS, 2.7 mg/L (range, 0.6-105 mg/L); TIA, 1.8 mg/L (range, 0-74.1 mg/L); and control, 1.5 mg/L (range, 0.6-120.9 mg/L; Kruskal-Wallis test, 11.34; P = .0035).
There was correlation between the control group CRP and VWF:Ag/ADAMTS13Ac ratio (r = 0.324; P = .0013), not seen in IS (r = 0.175; P = .107) or TIA (r = 0.175; P = .136). Multiple linear regression analysis identified CRP and bilirubin to be independent predictors of ADAMTS13 at presentation of IS or TIA, demonstrating a negative association (supplemental Table 6) not seen with VWF:Ag or VWF:Ag/ADAMTS13Ac.
Longitudinal follow-up
Follow-up blood samples from 6 weeks to >2 years postpresentation, with a median of 6 months, were obtained in 96 patients (IS, n = 34; TIA, n = 35; controls, n = 27; median follow-up, 188 days; range, 41-889 days).
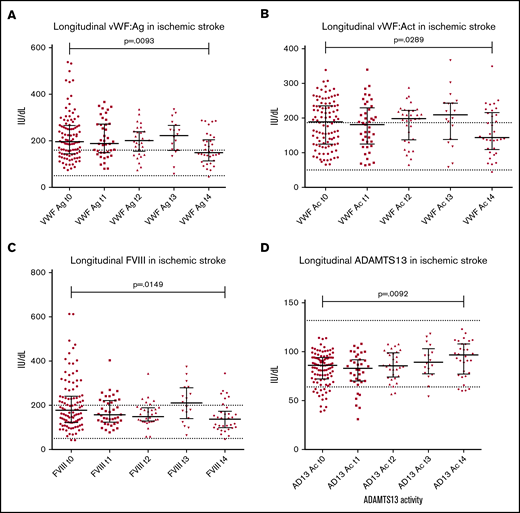
Both VWF:Ag and FVIII were significantly decreased in IS (Figure 2A,C), with the reverse seen with ADAMTS13Ac, increasing from presentation to final follow-up (Figure 2D), demonstrating normalization. The VWF:Ag/ADAMTS13Ac ratio correspondingly decreased over time (2.42 to 1.66; P = .0008; Wilcoxon matched pairs signed rank test median difference, 0.278; P = .005). Significant changes in IS were demonstrated for each marker in comparison of presentation vs final follow-up (Table 1). Paired testing of 36 TIA cases demonstrated increasing ADAMTS13Ac from presentation to final follow-up (by 3.6 IU/dL; P = .05), with a corresponding decrease in the VWF:Ag/ADAMTS13Ac ratio (P < .0001).
Longitudinal patterns in ischemic stroke. Longitudinal changes in all haemostatic markers were measured at presentation (t0), 24 hours later (t1), 48 hours post presentation (t2), 5-7 days post presentation (t3) and final follow up from 6 weeks post presentation (t4). Median follow up time for ischaemic stroke specifically was 257 days (range 48-889). (A) Decrease in VWFAg from presentation (median 196.9 IU/dL) to final follow up (median 157.7 IU/dL) was observed (P = .0093 on matched paired testing). The same trend was seen with VWFAc from presentation (median 188.7 IU/dL) to final follow up (median 143.7IU/dL; P = .0289) (B), and FVIII (presentation median 178.6 to final follow up 137.7 IU/dL; P = .0149) (C). (D) A clear reverse trend was seen with ADAMTS13Ac in ischaemic stroke, demonstrating a significant increase in ADAMTS13Ac from presentation (median 85.9 IU/dL) to final follow up (median 96.8IU/dL, P = .0092).
Longitudinal patterns in ischemic stroke. Longitudinal changes in all haemostatic markers were measured at presentation (t0), 24 hours later (t1), 48 hours post presentation (t2), 5-7 days post presentation (t3) and final follow up from 6 weeks post presentation (t4). Median follow up time for ischaemic stroke specifically was 257 days (range 48-889). (A) Decrease in VWFAg from presentation (median 196.9 IU/dL) to final follow up (median 157.7 IU/dL) was observed (P = .0093 on matched paired testing). The same trend was seen with VWFAc from presentation (median 188.7 IU/dL) to final follow up (median 143.7IU/dL; P = .0289) (B), and FVIII (presentation median 178.6 to final follow up 137.7 IU/dL; P = .0149) (C). (D) A clear reverse trend was seen with ADAMTS13Ac in ischaemic stroke, demonstrating a significant increase in ADAMTS13Ac from presentation (median 85.9 IU/dL) to final follow up (median 96.8IU/dL, P = .0092).
Comparison of longitudinal changes in IS
| Median, IU/dL . | Baseline (t0) . | Final follow-up (t4)* . | Difference in medians (matched pairs, n = 34)† . | Difference in medians (all data)‡ . | KW testing§ . |
|---|---|---|---|---|---|
| FVIII | 178.6 | 137.7 | −21.2 (P = .0149) | −40.9 (P = .0038) | 12.58 (P = .0135) |
| VWF:Ag | 196.9 | 157.7 | −18.24 (P = .0093) | −47.5 (P = .0046) | 9.568 (P = .0484) |
| VWF:Act | 188.7 | 143.7 | −11.6 (P = .0289) | −45.05 (P = .0783) | 5.21 (P = .2665) |
| ADAMTS13Ac | 85.9 | 96.8 | 4.9 (P = .0092) | 10.85 (P = .0043) | 11.87 (P = .0184) |
| VWF:Ag/ADAMTS13Ac ratio | 2.42 | 1.66 | −0.2775 (P = .0007) | −0.869 (P = .0008) | 12.42 (P = .0145) |
| Median, IU/dL . | Baseline (t0) . | Final follow-up (t4)* . | Difference in medians (matched pairs, n = 34)† . | Difference in medians (all data)‡ . | KW testing§ . |
|---|---|---|---|---|---|
| FVIII | 178.6 | 137.7 | −21.2 (P = .0149) | −40.9 (P = .0038) | 12.58 (P = .0135) |
| VWF:Ag | 196.9 | 157.7 | −18.24 (P = .0093) | −47.5 (P = .0046) | 9.568 (P = .0484) |
| VWF:Act | 188.7 | 143.7 | −11.6 (P = .0289) | −45.05 (P = .0783) | 5.21 (P = .2665) |
| ADAMTS13Ac | 85.9 | 96.8 | 4.9 (P = .0092) | 10.85 (P = .0043) | 11.87 (P = .0184) |
| VWF:Ag/ADAMTS13Ac ratio | 2.42 | 1.66 | −0.2775 (P = .0007) | −0.869 (P = .0008) | 12.42 (P = .0145) |
Minimum 6 weeks from presentation.
Wilcoxon paired Student t test.
Unpaired Student t test.
Is there a significant difference in medians across all time points?
Follow-up blood samples in 27 control patients (median follow-up, 204 days; range, 44-566) demonstrated a downward trend in VWF:Ag, VWF:Act, and FVIII that did not reach significance, consistent with resolution of an acute-phase response. There was no notable change in ADAMTS13 (95.6 to 98.9 IU/dL; P = .144) over follow-up. There was consequently no significant longitudinal change seen in the VWF:Ag/ADAMTS13 ratio in the control group (1.69 to 1.21; P = .074; Wilcoxon matched pairs signed rank test median difference, 0.0362; P = .562), suggesting that acute-phase elevation of the VWF:Ag/ADAMTS13 ratio with normalization over time is specific to ischemic brain injury.
Recurrence and mortality
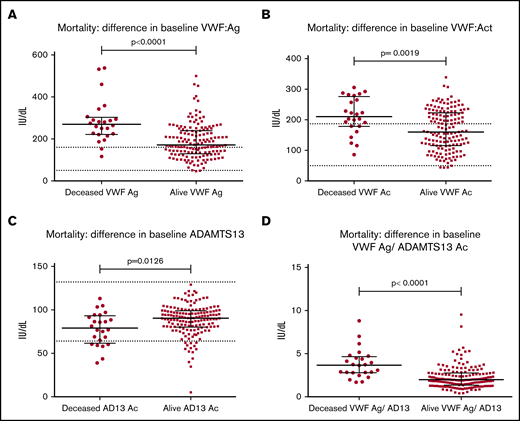
There were 5 patients with recurrent IS (all occurring in patients who had initially presented with IS) and 3 patients with recurrent TIA (all occurring in patients who had initially presented with TIA). Numbers were too small for meaningful interpretation, so we cannot comment further on whether there was a difference in the VWF:Ag/ADAMTS13Ac ratio in these patients at presentation or follow-up compared with the larger stroke/TIA cohort. Within IS and TIA, the mortality rate was 13% (IS, n = 21; TIA, n = 3), with a median time of 152 days from recruitment until death. Median age at presentation of those patients who survived was 75 years (range, 25-99 years); it was 84 years in those who died (range, 61-97 years). Patients who had died (n = 24) vs patients alive at final follow-up (n = 156) showed significant differences in all presentation hemostatic markers (Figure 3). The VWF:Ag/ADAMTS13Ac ratio was significantly higher in those who died (median, 3.68; range, 1.70-8.81) compared with those who survived (median, 2.01; range, 0.41-9.53).
Mortality outcome: difference in baseline hemostatic markers in IS and TIA groups combined. Significant differences were seen in all haemostatic markers at baseline between those patients whom had subsequently died at final follow up (n =2 4) vs those whom survived (n = 156) at a median follow up time of 152 days post initial presentation (minimum 6 weeks from first presentation). Differences were as follows (died vs survived): VWF:Ag (269.1 vs 171.2IU/dL, P < .0001) (A), VWF:Ac (210.5 vs 159.8IU/dL, P = .0019) (B), ADAMTS13 activity (79.1 vs 90.3IU/dL, P = .0126) (C), and mean VWF:Ag/ ADAMTS13Ac ratio (3.683 vs 1.988, P < .0001) (D).
Mortality outcome: difference in baseline hemostatic markers in IS and TIA groups combined. Significant differences were seen in all haemostatic markers at baseline between those patients whom had subsequently died at final follow up (n =2 4) vs those whom survived (n = 156) at a median follow up time of 152 days post initial presentation (minimum 6 weeks from first presentation). Differences were as follows (died vs survived): VWF:Ag (269.1 vs 171.2IU/dL, P < .0001) (A), VWF:Ac (210.5 vs 159.8IU/dL, P = .0019) (B), ADAMTS13 activity (79.1 vs 90.3IU/dL, P = .0126) (C), and mean VWF:Ag/ ADAMTS13Ac ratio (3.683 vs 1.988, P < .0001) (D).
ROC analysis was used to determine sensitivity of the presenting VWF:Ag/ADAMTS13Ac ratio for mortality, demonstrating clear predictive value (area under curve, 0.765; 95% confidence interval [CI], 0.664- 0.866; Figure 1; supplemental Data). In comparison, in the ROC analysis for sensitivity of presenting age for mortality, the predictive value was slightly lower (area under curve, 0.75; 95% CI, 0.665- 0.845).
Using ROC analysis to determine sensitivity and specificity of the VWF:Ag/ADAMTS13Ac ratio for mortality, a ratio of 2.6 was considered a suitable cutoff, because it provided a good balance of sensitivity (79.2%) and specificity (62.3%). This cutoff ratio was then applied to χ2 testing of the IS and TIA groups split according to presentation VWF:Ag/ADAMTS13Ac ratio. Those patients with a VWF:Ag/ADAMTS13Ac ratio >2.6 were significantly more likely to die (χ2 test, 14.2; P = .002; odds ratio [OR], 6.33; range, 2.22-18.1; supplemental Table 7).
Of patients presenting with a VWF:Ag/ADAMTS13Ac ratio <2.6, 80 were alive and 5 dead at follow-up (mortality, 6%). Of those presenting with a VWF:Ag/ADAMTS13Ac ratio ≥2.6, 48 were alive at follow-up, with 19 dead (mortality, 28%).
Logistic regression demonstrated that the association of the presenting VWF:Ag/ADAMTS13Ac ratio with mortality was independent of age. In an unadjusted logistic regression model, the ratio was associated with mortality (OR, 1.67; 95% CI, 1.25-2.22; P = .00). After adjusting for age, there was still an association between the ratio and mortality, although slightly reduced in magnitude (OR, 1.50; 95% CI, 1.11-2.02; P = .008).
Difference in mortality was also illustrated by splitting the IS and TIA groups into quartiles according to presentation VWF:Ag/ADAMTS13 ratio. Those patients in in the highest quartile (VWF:Ag/ADAMTS13Ac ratio, 3.09- 9.53) had a 31% mortality rate (n = 14) compared with those in the lowest quartile (VWF:Ag/ADAMTS13Ac ratio, 0.41-1.55), where no deaths occurred (P < .0001; Table 2).
Mortality according to VWF:Ag/ADAMTS13Ac ratio quartiles in IS and TIA
| Quartile . | VWF:Ag/ADAMTS13Ac ratio, range . | No. of surviving patients . | No. of patients who died . | Total no. of patients . | Mortality rate, % . |
|---|---|---|---|---|---|
| 1 | 0.41-1.55 | 45 | 0 | 45 | 0 |
| 2 | 1.551-2.11 | 42 | 3 | 45 | 6.7 |
| 3 | 2.111-3.09 | 37 | 7 | 44 | 15.9 |
| 4 | 3.091-9.53 | 31 | 14 | 45 | 31.1 |
| Quartile . | VWF:Ag/ADAMTS13Ac ratio, range . | No. of surviving patients . | No. of patients who died . | Total no. of patients . | Mortality rate, % . |
|---|---|---|---|---|---|
| 1 | 0.41-1.55 | 45 | 0 | 45 | 0 |
| 2 | 1.551-2.11 | 42 | 3 | 45 | 6.7 |
| 3 | 2.111-3.09 | 37 | 7 | 44 | 15.9 |
| 4 | 3.091-9.53 | 31 | 14 | 45 | 31.1 |
VWF:Ag/ADAMTS13Ac ratio is associated with functional scores
At presentation, those in the IS group demonstrated median higher mRS and NIHSS scores compared with TIA and control groups (Table 3). Spearman rank testing showed correlation of the VWF:Ag/ADAMTS13Ac ratio with age (0.577; P < .0001), mRS score (0.477; P < .0001), and NIHSS score (0.337; P < .0001). A negative correlation was seen with GCS (−0.255; P < .0001), corresponding with disability (supplemental Table 8).
Baseline demographic and clinical characteristics at presentation
| . | IS (n = 103) . | TIA (n = 80) . | Controls (n = 109) . | Total (N = 292) . |
|---|---|---|---|---|
| No. of patients (% of total cohort) | 103 (35.3) | 80 (27.4) | 109 (37.3) | 292 (100) |
| Age, y | 77 (42-97) | 75.5 (25-99) | 55 (23-100) | 71 (23-100) |
| Sex, n (% of total cohort) | ||||
| Male | 56 | 40 | 45 | 141 (48) |
| Female | 47 | 40 | 64 | 151 (52) |
| Preadmission mRS score | 1 (0-5) | 0 (0-4) | 0 (0-5) | — |
| Baseline mRS score | 3 (0-5) | 0 (0-4) | 0 (0-5) | — |
| Baseline NIHSS score | 4.5 (0-28) | 0 (0-16) | 0 (0-15) | — |
| Baseline GCS score | 15 (10-15) | 15 (14-15) | 15 (9-15) | — |
| Baseline FVIII, IU/dL | 178.6 (43.1- 612.7) | 149.7 (46.6-557.1) | 142.6 (46.3-705.5) | KW, 12.47 (P = .002) |
| Baseline VWF:Ag, IU/dL | 196.9 (75.4-538.1) | 167.8 (46.5-403.4) | 160.1 (22.6- 600) | KW, 12.87 (P = .0016) |
| Baseline VWF:Ac, IU/dL | 188.8 (65.5-338.4) | 159.8 (43.9-310.8) | 140.4 (26.7-304.2) | KW, 83.3 (P < .0001) |
| Baseline ADAMTS13Ac, IU/dL | 85.9 (38.8-114.2) | 94.0 (34.5-129) | 95.6 (38.4-137) | KW, 25.2 (P < .0001) |
| VWF:Ag/ADAMTS13Ac ratio | 2.42 (0.79-9.53) | 1.89 (0.41-8.14) | 1.69 (0.25-15.63) | KW, 24.65 (P < .0001) |
| Blood group, n (%) | χ2, 3.292 (P = .1928) | |||
| O | 52 (50) | 42 (53) | 45 (42) | Total n = 139; 47.6% of total cohort |
| Non-O | 51 (50) | 36 (45) | 64 (48) | Total n = 151; 51.7% of overall cohort |
| Unknown | 0 (0) | 2 (3) | 0 (0) | Total n = 2; 0.7% of overall cohort |
| . | IS (n = 103) . | TIA (n = 80) . | Controls (n = 109) . | Total (N = 292) . |
|---|---|---|---|---|
| No. of patients (% of total cohort) | 103 (35.3) | 80 (27.4) | 109 (37.3) | 292 (100) |
| Age, y | 77 (42-97) | 75.5 (25-99) | 55 (23-100) | 71 (23-100) |
| Sex, n (% of total cohort) | ||||
| Male | 56 | 40 | 45 | 141 (48) |
| Female | 47 | 40 | 64 | 151 (52) |
| Preadmission mRS score | 1 (0-5) | 0 (0-4) | 0 (0-5) | — |
| Baseline mRS score | 3 (0-5) | 0 (0-4) | 0 (0-5) | — |
| Baseline NIHSS score | 4.5 (0-28) | 0 (0-16) | 0 (0-15) | — |
| Baseline GCS score | 15 (10-15) | 15 (14-15) | 15 (9-15) | — |
| Baseline FVIII, IU/dL | 178.6 (43.1- 612.7) | 149.7 (46.6-557.1) | 142.6 (46.3-705.5) | KW, 12.47 (P = .002) |
| Baseline VWF:Ag, IU/dL | 196.9 (75.4-538.1) | 167.8 (46.5-403.4) | 160.1 (22.6- 600) | KW, 12.87 (P = .0016) |
| Baseline VWF:Ac, IU/dL | 188.8 (65.5-338.4) | 159.8 (43.9-310.8) | 140.4 (26.7-304.2) | KW, 83.3 (P < .0001) |
| Baseline ADAMTS13Ac, IU/dL | 85.9 (38.8-114.2) | 94.0 (34.5-129) | 95.6 (38.4-137) | KW, 25.2 (P < .0001) |
| VWF:Ag/ADAMTS13Ac ratio | 2.42 (0.79-9.53) | 1.89 (0.41-8.14) | 1.69 (0.25-15.63) | KW, 24.65 (P < .0001) |
| Blood group, n (%) | χ2, 3.292 (P = .1928) | |||
| O | 52 (50) | 42 (53) | 45 (42) | Total n = 139; 47.6% of total cohort |
| Non-O | 51 (50) | 36 (45) | 64 (48) | Total n = 151; 51.7% of overall cohort |
| Unknown | 0 (0) | 2 (3) | 0 (0) | Total n = 2; 0.7% of overall cohort |
Patients presenting with symptoms similar to those of IS and TIA but subsequently found to be negative for ischemic brain injury were included as controls. This group was subsequently not age or sex matched for the ischemic brain injury groups, which should be taken into account. Unless otherwise indicated, data are median (range).
KW, Kruskal-Wallis test (nonparametric testing to compare medians of >2 groups).
Division of the IS and TIA groups according to mRS score at both presentation and final follow-up showed prognostic differences in the VWF:Ag/ADAMTS13 ratio. Patients presenting with an mRS score of 3 to 5 (n = 78) compared with patients with an mRS score of 0 to 2 (n = 98) demonstrated a higher median VWF:Ag/ADAMTS13Ac ratio (mRS score, 3-5: median ratio, 2.722; range, 0.85-8.81 vs mRS score, 0-2: median ratio, 1.858; range, 0.415-9.53; P < .0001). Despite an overall longitudinal reduction of the VWF:Ag/ADAMTS13Ac ratio, this significant difference persisted at final follow-up. Patients with more functional impairment at follow-up (n = 18), as reflected by an mRS score of 3 to 5, maintained a higher VWF:Ag/ADAMTS13Ac ratio of 1.521 (range, 0.32-3.42) compared with patients at follow-up with an mRS score of 0 to 2 (n = 48; median ratio, 0.845; range, 0.42-3.29; P = .0102). Although the ranges overlapped, this higher VWF:Ag/ADAMTS13Ac ratio was closely related to increased morbidity.
Defining the VWF:Ag/ADAMTS13Ac ratio of 2.6 as predictive of mortality was then extrapolated to morbidity. Patients with a presenting ratio <2.6 (n = 109) had a significantly lower mRS score (median, 1; range, 0-5) compared with those with a presenting ratio ≥2.6 (n = 65; median mRS score, 3; range, 0-5; P < .0001). This was also reproduced with NIHSS scoring. Patients presenting with a ratio <2.6 (n = 111) had a significantly lower NIHSS score (median, 1; range, 0-28) compared with those with a presenting ratio ≥2.6 (n = 66; median NIHSS score, 4; range, 0-22; P < .0001).
Regression modeling similarly reflected higher mRS and NIHSS scores, reflective of more disability, to be positively associated with presentation VWF:Ag and negatively associated with ADAMTS13Ac in combined modeling (supplemental Tables 9-11).
During follow-up, presentation VWF:Ag/ADAMTS13Ac ratio was associated with NIHSS score at 7 to 14 days postpresentation (P = .029) and with mRS score at >6 weeks postpresentation (P = .021) on regression (supplemental Table 12). GCS at 7 to 14 days postpresentation was significantly negatively associated with the VWF:Ag/ADAMTS13Ac ratio (coefficient, −4.425; P = .011), in keeping with the hypothesis (ie, as VWF increases and ADAMTS13 decreases, GCS decreases, synonymous with a worsening clinical state). Multiple linear regression associations of presentation VWF:Ag-ADAMTS13Ac axis scores with final follow-up functional scores were specific to ischemic brain injury. Controls demonstrated no significant change in median functional scores from presentation to final follow-up, in contrast to the improved functional status seen in the IS and TIA groups.
Impact of thrombolysis on VWF:Ag-ADAMTS13Ac parameters
Thirty-eight patients in the IS group received thrombolysis within 4 hours of onset of symptoms. Presentation ADAMTS13Ac was comparable between the groups, but the thrombolysed group showed increased ADAMTS13Ac at follow-up (84 to 100.8 IU/dL; P = .0025; median follow-up, 221 days), with no change in the nonthrombolysed group (86 to 90.9 IU/dL; P = .285; median follow-up, 286 days). Presentation VWF:Ag was lower in the thrombolysed group compared with the nonthrombolysed group at presentation (median, 187.9 vs 227.2 IU.dL; P = .0352), with no differences between the groups at any point in later follow-up. It is likely that VWF was higher in the group of patients not subsequently thrombolysed, because these patients had reached their ischemic peak, reflected in the VWF levels, but timing of symptom onset mean they were excluded from being safely thrombolysed (ie, >4.5 hours from symptom onset).
The thrombolysed group showed significantly reduced VWF:Ag at follow-up (187.9 to 141.1 IU/dL; P = .0187), which was not evident on follow-up in the nonthrombolysed group (227.2 to 190.9 IU/dL; P = .0944).
The difference in VWF:Ag/ADAMTS13Ac ratio from presentation to final follow-up was therefore far more marked in the thrombolysed group compared with the nonthrombolysed group (thrombolysed group: 2.024 to 1.355; P = .0052; nonthrombolysed group: 2.672 to 2.091; P = .0339). This suggests that normalization of the VWF:Ag-ADAMTS13Ac balance is better achieved with thrombolysis.
Similarly, there was a significant difference in mRS score from presentation to final follow-up in the thrombolysed IS group (3.5 to 0; P = .0020), not seen in the nonthrombolysed group (3 to 2; P = .0622; supplemental Figure 2).
Discussion
We have, for the first time, shown a strong association between the levels of VWF and ADAMTS13 on admission with respect to the severity, functional outcome, and mortality of acute IS. We have summarized the relationship by focusing on the VWF:Ag/ADAMTS13Ac ratio as a biological measure of the disturbed hemostatic balance after IS or TIA, incorporating both the elevation of VWF and reduction of ADAMTS13. Longitudinal changes in those presenting with acute cerebral ischemia supports incremental ADAMTS13Ac recovery in the aftermath of acute IS or TIA in the largest cohort of patients yet published, with no such longitudinal changes seen in controls. Our data suggest the corresponding VWF:Ag/ADAMTS13Ac ratio can be used as a biomarker for clinical outcome in acute IS and TIA. The presentation VWF:Ag/ADAMTS13Ac ratio was significantly higher in patients who subsequently died (3.683 vs 2.014, respectively). A ratio ≥2.6 at presentation of IS or TIA was associated with increased mortality (26%) vs those a ratio <2.6 (8%), based on ROC analysis accounting for sensitivity and specificity of the VWF:Ag/ADAMTS13Ac ratio as discriminatory for mortality (area under curve, 0.765; 95% CI, 0.65-0.87). This also fits with the median VWF:Ag/ADAMTS13Ac ratios of 1.69 at presentation and 1.21 in convalescence in the control group, which were significantly different to the IS ratio of 2.42 at presentation and 1.66 in convalescence. There was an escalating risk of death according to presentation VWF:Ag/ADAMTS13Ac ratio, as reflected by comparison of the highest with the lowest quartile, showing a 31% increase in mortality.
In addition to mortality, regression modeling showed an association of presentation VWF:Ag/ADAMTS13Ac ratio with later functional outcome, specific to ischemic brain injury. A higher ratio was associated with higher functional and neurological impairments, as defined by the mRS and NIHSS scoring systems, both of which have been extensively validated within stroke medicine. In the era of anti-VWF and recombinant ADAMTS13 treatments for thrombotic thrombocytopenic purpura, therapeutic implications of limiting raised VWF levels and normalizing ADAMTS13 activity could be critical in optimizing outcomes from IS or TIA.
Increased VWF and reduced ADAMTS13 in acute stroke have been previously described, and it is known that ischemic brain injury and the damaged vascular endothelium are associated with increased VWF.4,5,9,16,19,32,33 It is unclear whether reduced baseline ADAMTS13 predisposes to stroke or whether it is consumed as it meets the demand of the acute outpouring of ultralarge VWF multimers in ischemic insult. We found a significant reduction in ADAMTS13Ac in IS compared with both TIA and control groups. Conversely, VWF:Ag was significantly higher in IS compared with both TIA and control groups, with similar trends seen with VWFAc. Normalization of both markers was seen in convalescence, therefore consistent with consumption of ADAMTS13 in the ischemic insult.
Earlier work demonstrated reduction of ADAMS13Ac in the early phase poststroke compared with the later phase.16 However, an inverse relationship between VWFAg and ADAMTS13 levels was seen only in the early phase. This supports the hypothesis that ADAMTS13 is consumed in the action of degradation of ultralarge VWF multimers released in the ischemic insult. The Rotterdam prospective study suggests that reduced ADAMTS13 predisposes to stroke.6-8 The nature of our study design means we cannot comment further on whether reduction of ADAMTS13 in acute IS is causative or a consumptive phenomenon in the ischemic insult. We have determined that although presenting VWF:Ag-ADAMTS13Ac perturbations are witnessed in general sickness and linked with consequent disability, resolution of VWF:Ag-ADAMTS13Ac is specifically associated with convalescence after ischemic brain injury.
The overall trend indicated by regression modeling is an increased morbidity according to presentation VWF:Ag/ADAMTS13Ac ratio in both the acute phase and convalescence. Firstly, higher mRS and NIHSS scores at presentation, reflective of greater impairment, were associated with a higher VWF:Ag/ADAMTS13Ac ratio. Secondly, presentation VWF:Ag/ADAMTS13Ac ratio was associated with longitudinal clinical scoring in IS, as reflected by significant association with final mRS score. Altogether this supports the hypothesis that the acute-phase VWF:Ag-ADAMTS13Ac axis is predictive of poorer functional outcome in patients presenting with acute ischemic brain injury, extending to mortality.
The impact of thrombolysis on the VWF:Ag-ADAMTS13Ac axis has not been previously investigated, and we have demonstrated normalization of the VWF:Ag-ADAMTS13Ac axis to be more effectively achieved with thrombolysis. This further reinforces a role for the VWF:Ag-ADAMTS13Ac axis in the pathophysiology of IS, with thrombolysis demonstrating a significant reduction in ratio and impact on clinical scores in follow-up. Animal model studies have shown recombinant ADAMTS13 treatment in cerebrovascular occlusion vs thrombolysis with tissue plasminogen activator reduces infarct volume and improves blood flow, without risk of massive intracerebral hemorrhage associated with tissue plasminogen activator.34 The postulated mechanism was the specificity of ADAMTS13 for ultralarge VWF in conditions of high shear stress and thus pathological thrombi, rather than targeting of the VWF platelet primary hemostatic thrombi and induction of hemorrhage after the dissolution of thrombi postischemia.34,35 More recent clinical work has suggested ADAMTS13 levels predict response to reperfusion attempts in patients with acute stroke, via endovascular treatment or thrombolysis, with higher levels associated with arterial recanalization and lower levels predicting an unfavorable clinical outcome postthrombolysis.36-38 Potential mechanisms include a role for ADAMTS13 in postischemic angiogenesis, with recombinant ADAMTS13 markedly boosting neovascularization, vascular repair, and functional recovery at 2 weeks after ischemic insult in recent murine data.39
Limitations of our study include, firstly, the 48-hour allowance of symptom onset before recruitment. This may have affected biomarkers in this period. Secondly, those patients who were thrombolysed would have presented within 4.5 hours of symptom onset, so would arguably have received more prompt medical attention in relation to their symptoms; thus, clinical recovery may have been independent of thrombolytic therapy. Thirdly, the cause of death in our cohort was not documented, so we cannot comment on how aberrations in the VWF:Ag-ADAMTS13Ac axis may have contributed, nor can we comment on whether death was related to stroke or other causes. Patients may have re-presented to other hospitals, so recurrence may not have been fully captured. Our recurrence data were limited, and further work is merited to investigate whether persistently depleted ADAMTS13Ac heightens recurrence risk of acute ischemic brain injury (eg, via increased thrombus propensity and increased cardiovascular mortality). We acknowledge that although we have demonstrated significance between the VWF-ADAMTS13 balance and mortality, there was overlapping among patient groups, and there was no defining VWF:Ag/ADAMTS13Ac ratio predictive of functional outcome. Finally, further work is warranted on how inflammation interacts with the VWF-ADAMTS13 axis. We have demonstrated a significant difference in CRP among groups at presentation and a negative association with presentation CRP and ADAMTS13 on multivariate analysis, as seen by other groups.10 This would be in keeping with an escalating CRP in conjunction with inflammation, with a correspondingly low ADAMTS13. This certainly supports the inflammatory stimulus of IS contributing to a disordered VWF:Ag- ADAMTS13Ac axis, but it does not clarify whether this is causative or reactive.
In conclusion, presentation VWF:Ag/ADAMTS13Ac ratio is a surrogate biomarker for extent of brain injury and consequential clinical recovery and may be used for future prognostication and guidance of therapy. The VWF:Ag/ADAMTS13Ac ratio in acute ischemic brain injury has potential as a hemostatic parameter for cerebral ischemia. The ratio reflects not just severity of functional impairment, but also eventual mortality, independent of age. A presenting ratio >2.6 defines a significantly poorer prognosis relating to functional outcome and mortality in patients with ischemic brain injury, with a mortality rate of 26%, compared with 8% in those presenting with a ratio ≤2.6. We have also demonstrated worsening impact as the ratio increases. Over a ratio of 2, the mortality rate is 21%, rising to 29% over a ratio of 3 and 43% over a ratio of 4.
Resolution of the VWF:Ag-ADAMTS13Ac axis over convalescence is linked with clinical recovery. Given the limited acute treatments available for IS and TIA, there may be potential for the VWF:Ag/ADAMTSA13Ac ratio beyond its role as a biomarker. Manipulation of the axis via reducing raised VWF levels and normalizing ADAMTS13Ac in acute IS and TIA offers important therapeutic potential.
Acknowledgments
The authors acknowledge the stroke research practitioner team at the Hyperacute Stroke Research Centre, University College London Hospitals NHS Foundation Trust, for patient recruitment, particularly Shez Feerick, Caroline Watchurst, and Renuka Erande. They also acknowledge Gareth Ambler, Associate Professor in Medical Statistics, Department of Statistical Science, University College London, for his invaluable help in reviewing and advising on the statistical analysis
A.T. and C.V. were both clinical fellows employed by University College London Hospitals NHS Foundation Trust. The clinical fellow posts were funded by a grant donated by Baxter (now Takeda). The Chair in Stroke Medicine (M.M.B.) is supported by the Reta Lila Weston Trust for Medical Research. Part of this work was undertaken at University College London, which received a proportion of funding from the UK Department of Health National Institute for Health Research Biomedical Research Centres funding scheme.
The funders of this study had no role in study design or final publication.
Authorship
Contribution: A.T. recruited patients, designed and performed research, performed laboratory analysis, analyzed data, and wrote the paper; C.V. and D.S. performed laboratory analysis; M.M.B. designed the research and reviewed the paper; and M.S. designed the research and reviewed and revised the paper.
Conflict-of-interest disclosure: M.S. has received funding from Baxalta/Takeda, including speakers’ fees and attendance of advisory boards. The remaining authors declare no competing financial interests.
Correspondence: Alice Taylor, Haemophilia Comprehensive Care Centre, Level 5, Royal London Hospital for Integrated Medicine (RLHIM), Great Ormond Street Hospital, Great Ormond St, London WC1N 3JH, United Kingdom; e-mail: alice.taylor@ucl.ac.uk.
References
Author notes
The full-text version of this article contains a data supplement.
Original data may be requested by e-mail to the corresponding author (alice.taylor@ucl.ac.uk).