Abstract
Systems-based hematology is dedicated to improving care delivery for patients with blood disorders. First defined by the American Society of Hematology in 2015, the idea of a systems-based hematologist arose from evolving pressures in the health care system and increasing recognition of opportunities to optimize the quality and cost effectiveness of hematologic care. In this review, we begin with a proposed framework to formalize the discussion of the range of initiatives within systems-based hematology. Classification by 2 criteria, project scope and method of intervention, facilitates comparison between initiatives and supports dialogue for future efforts. Next, we present published examples of successful systems-based initiatives in the field of hematology, including efforts to improve stewardship in the diagnosis and management of complex hematologic disorders (eg, heparin-induced thrombocytopenia and thrombophilias), the development of programs to promote appropriate use of hematologic therapies (eg, blood products, inferior vena cava filters, and anticoagulation), changes in care delivery infrastructure to improve access to hematologic expertise (eg, electronic consultation and disorder-specific care pathways), and others. The range of projects illustrates the broad potential for interventions and highlights different metrics used to quantify improvements in care delivery. We conclude with a discussion about future directions for the field of systems-based hematology, including extension to malignant disorders and the need to define, expand, and support career pathways.
Introduction
In 2015, the American Society of Hematology published “The role of hematologists in a changing United States health care system,”1 which outlined how hematologists could “optimally contribute in the emerging 21st century health ecosystem,”1(p2467) and in doing so identified a new role: the systems-based hematologist. The article accompanied a report from The Lewin Group published on the American Society of Hematology Web site explaining that although the specific responsibilities of systems-based hematologists may vary, the role is unified by a common intention of developing pathways that deliver high-quality, cost-effective, evidence-based care to patients with blood disorders across health care systems.2
The necessity for such a field within hematology arises from the reality that providing care for many hematologic disorders often transcends medical specialties. Hematology practice encompasses patients with primary hematologic disorders, such as sickle cell disease, who require complex, multidisciplinary care. It also includes patients with secondary hematologic complications, such as disseminated intravascular coagulation, who are cared for primarily by nonhematology providers. The field of systems-based hematology works to equip the health care system with the tools to provide consistent and coordinated care across care settings.
Given the recent development of systems-based hematology and the variability in institutional needs, uncertainty remains about the feasibility of the creation and implementation of the systems-based hematologist as a unique and supportable career path within the field of hematology. Challenges include fostering trainee and junior faculty interest, developing collaborative relationships across specialties, and cultivating institutional investment, including financial support.
Here, we first create a framework for discussion and classification of different initiatives within systems-based hematology. Second, we highlight published successes that illustrate how such initiatives can improve hematologic care delivery. Lastly, we discuss future directions in developing systems-based hematology as a necessary and sustainable field of practice.
What is a systems-based initiative?
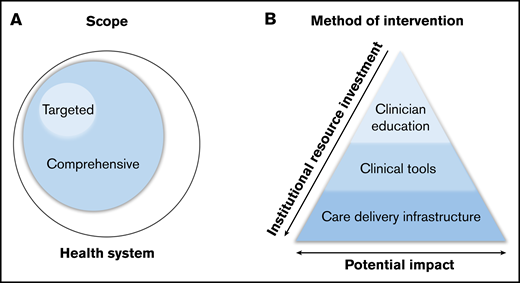
A systems-based initiative in hematology aims to ensure the delivery of high-quality, cost-effective, evidence-based care to patients with hematologic disorders. Given the broad range of disorders within hematology, the overlap with other specialties, and the diversity of health care delivery systems, the details of such initiatives vary significantly. Systems-based initiatives can be classified based on 2 characteristics: scope and method of intervention (Figure 1).
Classification of systems-based initiatives. Classification by scope (A) and method of intervention (B).
Classification of systems-based initiatives. Classification by scope (A) and method of intervention (B).
Scope
Scope refers to the intended reach of a given initiative, which can be either targeted or comprehensive (Figure 1A). Targeted initiatives focus on a specific hematologic disorder or care scenario. Such initiatives are commonly implemented in disorders for which existing evidence guides practice; these targeted initiatives aim to disseminate this evidence to ensure validated, reliable care throughout the health care system. In clinical scenarios where clear guidance does not exist, initiatives may focus on standardizing practice based on best available evidence, with opportunities to continually monitor and improve care recommendations. In contrast, comprehensive initiatives have a broader scope, focusing on the optimization of care delivery pathways across a health care system, to address the care of patients with a range of hematologic disorders. These initiatives often hinge on innovative methods of care delivery (rather than on dictating care itself), including finding new ways to facilitate access to high-quality hematologic care and expertise.
Method of intervention
In addition to variation in scope, initiatives can be further classified into 3 categories based on their method of intervention, each with varying degrees of resource investment and potential impact on clinical practice: clinician education, clinical tools, and care delivery infrastructure (Figure 1B).
Interventions in clinician education are developed to improve provider understanding of best practice in order to influence patient care. Provider knowledge is an essential part of improving clinical care, and such initiatives often require less institutional resource investment. However, when performed in isolation, provider education alone has limited clinical impact as a result of reliance on reaching individual providers, frequent provider turnover requiring repeated educational efforts, and challenges of knowledge retention and implementation in clinical practice.3
Clinical tools influence practice by guiding providers through clinical decision making. Examples range from the creation of institutional guidelines to the development of real-time decision support in the electronic medical record (EMR). This approach overcomes barriers of knowledge retention and implementation and therefore has potential for greater impact, with some increase in resource investment.
The redesign of the care delivery infrastructure facilitates the development of modified or novel systems to provide more efficient or effective patient care. Such efforts often include changing patient-provider interaction, adding hematologic expertise where it may have previously been absent, or rethinking existing interactions to optimize provider time and input. Changes in infrastructure have the potential for large impact but require significant resource investment to design, achieve, and maintain.
For most interventions to be both successful and sustainable, a combination of strategies, including improvements in provider education, clinical tools, and care delivery infrastructure, are often required.
Highlighting successful systems-based initiatives
To promote understanding of the potential for care improvement in systems-based hematology, we highlight successful initiatives that have promoted the delivery of evidence-based, high-value, cost-effective hematologic care. Initiatives are presented based on the scope of the project (targeted vs comprehensive); the methods of intervention used in each initiative are summarized in Table 1. Importantly, these initiatives are a result of efforts from hematologists and nonhematologists alike, often with multidisciplinary collaboration, but all highlight the principles of systems-based hematology. Furthermore, the initiatives often report immediate success after implementation, but an essential part of the role of a systems-based hematologist is to monitor and adapt an initiative to ensure the sustainability of care improvement.
Examples of systems-based initiatives, classified by scope and method of intervention
| Scope . | Method of intervention . | ||
|---|---|---|---|
| Provider education . | Clinical tools . | Care delivery infrastructure . | |
| Targeted initiatives | |||
| HIT | |||
| Smythe et al6 | Secondary | Primary | Secondary |
| Vaughn et al7 | Not used | Secondary | Primary |
| Tsui et al9 | Not used | Primary | Not used |
| McGowan et al10 | Not used | Secondary | Primary |
| Thrombophilia testing | |||
| Shen et al17 | Not used | Primary | Not used |
| Lim et al18 | Secondary | Primary | Not used |
| IVC filter use | |||
| Russo et al27 | Not used | Primary | Not used |
| Inagaki et al28 | Not used | Secondary | Primary |
| Disorder-specific care pathways | |||
| Condliffe et al35 | Not used | Secondary | Primary |
| Rabinovich et al45 | Not used | Not used | Primary |
| Holmes et al46 | Not used | Secondary | Primary |
| Zia et al49 | Not used | Secondary | Primary |
| Comprehensive initiatives | |||
| Blood product utilization | |||
| Hicks et al11 | Primary | Not used | Not used |
| Goodnough et al61 | Secondary | Primary | Not used |
| Guinn et al66 | Not used | Secondary | Primary |
| Abdulrehman et al69 | Secondary | Primary | Not used |
| Anticoagulation stewardship programs | |||
| Wychowski et al74 | Not used | Not used | Primary |
| Padron et al75 | Not used | Secondary | Primary |
| Reardon et al76 | Secondary | Secondary | Primary |
| Electronic consultation | |||
| Pai et al80 | Not used | Secondary | Primary |
| Cecchini et al81 | Not used | Not used | Primary |
| Scope . | Method of intervention . | ||
|---|---|---|---|
| Provider education . | Clinical tools . | Care delivery infrastructure . | |
| Targeted initiatives | |||
| HIT | |||
| Smythe et al6 | Secondary | Primary | Secondary |
| Vaughn et al7 | Not used | Secondary | Primary |
| Tsui et al9 | Not used | Primary | Not used |
| McGowan et al10 | Not used | Secondary | Primary |
| Thrombophilia testing | |||
| Shen et al17 | Not used | Primary | Not used |
| Lim et al18 | Secondary | Primary | Not used |
| IVC filter use | |||
| Russo et al27 | Not used | Primary | Not used |
| Inagaki et al28 | Not used | Secondary | Primary |
| Disorder-specific care pathways | |||
| Condliffe et al35 | Not used | Secondary | Primary |
| Rabinovich et al45 | Not used | Not used | Primary |
| Holmes et al46 | Not used | Secondary | Primary |
| Zia et al49 | Not used | Secondary | Primary |
| Comprehensive initiatives | |||
| Blood product utilization | |||
| Hicks et al11 | Primary | Not used | Not used |
| Goodnough et al61 | Secondary | Primary | Not used |
| Guinn et al66 | Not used | Secondary | Primary |
| Abdulrehman et al69 | Secondary | Primary | Not used |
| Anticoagulation stewardship programs | |||
| Wychowski et al74 | Not used | Not used | Primary |
| Padron et al75 | Not used | Secondary | Primary |
| Reardon et al76 | Secondary | Secondary | Primary |
| Electronic consultation | |||
| Pai et al80 | Not used | Secondary | Primary |
| Cecchini et al81 | Not used | Not used | Primary |
Table indicates whether method was primary or secondary method of intervention, or whether method was not used.
HIT, Heparin-induced thrombocytopenia; IVC, inferior vena cava.
Targeted initiatives
HIT
HIT is an example of a disorder well suited to systems-based interventions for several reasons: strong evidence and clinical practice guidelines exist establishing best practice,4 the diagnosis is commonly considered by clinical providers outside the field of hematology who have varying expertise in this content area, and deviation from best practice is common and associated with excess cost (eg, unnecessary laboratory testing and inappropriate use of direct thrombin inhibitors) as well as increased risk for patient harm (eg, resulting from either thrombosis or bleeding).5 Despite the universal need for initiatives to improve diagnosis and management of HIT, the methods of intervention of published initiatives vary, reflecting how each stewardship strategy has been adapted to a given institution or health care system to address a shared clinical problem.
Authors from Beaumont Hospital (Royal Oak, MI) reported the creation of a multipronged approach to HIT care optimization.6 After an initial needs assessment, they developed a protocol for the diagnosis and management of HIT, including guidance for the correct use of direct thrombin inhibitors (DTIs). In addition, they improved relevant EMR documentation and developed both initial and ongoing clinician education programs. These efforts improved adherence to the DTI protocol from 31% to 100%, and appropriate EMR documentation increased to 100%.
Authors from the Medical University of South Carolina (Charleston, SC) similarly developed guidelines for testing and DTI use but also created the Anticoagulation and Bleeding Management Service, which reviewed all cases with positive screening assays to provide management guidance.7 With this intervention, use of screening immunoassays decreased by 20%, use of confirmatory assays decreased by 70%, and DTI use decreased by 78% to 1.2 times per month. Importantly, changes in the EMR in subsequent years reversed some of these successes, but continual monitoring allowed for identification of these setbacks and intervention to regain progress.8
Authors from Dartmouth-Hitchcock Medical Center (Lebanon, NH) developed an EMR order set for use when HIT was suspected, which required 4T score calculation and provided recommendations based on the result, specifically recommending against testing and heparin discontinuation in patients with a low likelihood of HIT.9 Before the intervention, patients with low pretest probability accounted for 50% of tested patients, which decreased to 30% after implementation of the order set (P = .001). Furthermore, appropriate discontinuation of heparin at the time of HIT testing increased from 66% to 74% (P < .001).
Authors from Sunnybrook Health Sciences Centre (Toronto, ON, Canada) took a novel approach; rather than solely focusing on recognition and management of HIT, they also attempted to decrease its incidence.10 With the Avoid-Heparin Initiative, unfractionated heparin was replaced with low molecular weight heparin for prophylactic and therapeutic indications, because the latter has a five- to 10-fold lower risk of HIT. As a result, the annual rate of suspected HIT decreased from 85.5 to 49.0 per 10 000 admissions (P < .001), and the incidence of HIT with thrombosis decreased from 4.6 to 0.4 per 10 000 admissions (P < .001).
Importantly, optimal workflow for the most cost-effective diagnosis and management of HIT should remain an area of active research, especially given the increasing empiric use of direct oral anticoagulant therapy for this condition.
Thrombophilia testing
There is strong consensus in the hematology community that thrombophilia testing has a limited role in the management of patients with venous thromboembolism, especially in the inpatient setting,11,12 and multiple clinical practice guidelines exist to guide decision making.13-16 Nevertheless, inappropriate testing is common, in part because of a limited understanding of the indications for and pitfalls of testing by noncoagulation specialists. Efforts to better inform providers and patients about appropriate thrombophilia testing are needed, because indiscriminate use is associated with unnecessary costs and potential for patient harm (eg, inappropriate use of extended anticoagulation or false reassurance in the setting of negative testing).
Authors from the University of Texas Southwestern Medical Center (Dallas, TX) identified significant overuse and developed guidelines for appropriate thrombophilia testing in hospitalized patients.17 With these efforts, inpatient testing decreased from an average of 87 to 18 inpatients per month (79% reduction). To further ensure adherence to guideline-based care, the Transfusion Medicine Hemostasis Service would review all orders, which further decreased the testing average from 18 to 5 inpatients per month. Together, these efforts resulted in an estimated saving of approximately US$1.2 million per year.
Authors from the Medical University of South Carolina similarly attempted to decrease inpatient thrombophilia testing by developing an EMR ordering plan that included instructions to deter inpatient ordering.18 If testing was requested, a hematology consult was required for approval. Also, high-use services and internal medicine residents were targeted for education on appropriate testing. Combined, these efforts reduced testing by 79% (8 inpatients in 3 months) and resulted in an estimated US$334 100 per year in cost savings.
Inferior vena cava (IVC) filters
The use of IVC filters to prevent pulmonary embolism (PE) is controversial. Although guidelines for filter indications exist,19-21 recommendations are variable, compliance with recommendations is inconsistent,22 newer randomized trials continue to challenge their clinical benefit,23,24 and placement and removal are associated with significant cost.25 Furthermore, retrievable filters are frequently not removed, which can result in significant and potentially fatal mechanical and thrombotic complications.26 Collaboration among hematology, cardiovascular medicine, interventional radiology, and surgical services is paramount and provides an opportunity to improve collective practice.
Authors from Staten Island University Hospital (Staten Island, NY) focused on efforts to decrease filter placement, creating a strict indication policy for the health care system.27 After policy implementation, the average number of filters placed decreased by 41% (from 167 to 100 filters per year; P = .02). However, challenges remained, because use for relative indications and prophylactic placement continued, and only 8% of removable filters were retrieved.
To address the latter challenge of filter retrieval, authors from Boston Medical Center (Boston, MA) assembled a multidisciplinary task force that developed an IVC filter retrieval protocol, which included patient education, improved documentation, a centralized interdepartmental IVC filter registry, and a dedicated administrative coordinator.28 With these efforts, the rate of successful retrieval increased from 11% to 54% (P < .001).
Disorder-specific care pathways
One dominant example of a targeted initiative that uses infrastructural change is the development of disorder-specific care pathways for outpatient clinics. Comprehensive hemophilia and sickle cell treatment centers are well-known examples of targeted initiatives for hematologic disorders that have in turn benefitted from coordinated and consolidated care.29-31 Published successes in other common hematologic disorders have further demonstrated how the centralization and standardization of resources and expertise can improve patient and fiscal outcomes.
For example, institutions have developed care pathways for patients with low-risk PE. Previous studies have shown that a significant number of patients with acute PE do not require hospitalization and may be safely managed in the ambulatory setting,32,33 and outcomes for hemodynamically stable patients with acute PE may be better with outpatient treatment vs hospitalization.34 Authors from Sheffield Teaching Hospitals (Sheffield, United Kingdom) reported the successful development of a pathway for outpatient care of PE and deep vein thrombosis as an example of best practice,35 and other institutions, including Kaiser and the University of Wisconsin, have made institutional protocols available online.36,37
Treatment of cancer-associated thrombosis is complex and rapidly evolving, with emerging evidence supporting the use of direct oral anticoagulants for therapeutic and prophylactic indications.38-42 However, it is often not feasible for oncologists to integrate thrombosis care into already complex clinic visits, leading to variation in clinical practice and divergence from guidelines.43,44 In response to this need, authors from the Cleveland Clinic (Cleveland, OH) created a centralized care team for cancer-associated thrombosis, and initial results suggested improvement in both patient-related outcomes and institutional costs by decreasing treatment variation and avoiding unnecessary hospitalization.45 Authors from the University of Vermont (Burlington, VT) developed an EMR tool to calculate a thrombosis risk score and facilitate referral to discuss options for ambulatory thromboprophylaxis.46 Of 918 evaluated patients, 213 (23.2%) were identified as having a high risk of thrombosis; 93.4% of high-risk patients were referred and seen in the clinic, and 93.8% of those initiated prophylactic anticoagulation.
There are many challenges associated with the identification, diagnosis, and management of blood disorders in women and girls, with guidelines and expert opinion suggesting that care be provided in multidisciplinary clinics (eg, joint hematology-gynecology clinic) whenever possible.47,48 Authors from University of Texas Southwestern Medical Center published the steps taken to create such a clinic, along with diagnosis and treatment protocols. Preliminary results from their center suggested that the multidisciplinary clinic improved time to diagnosis of blood disorders in women with heavy menstrual bleeding.49 Furthermore, patient satisfaction with coordinated care is high; 1 institution reported that 88% of patients valued the presence of hematology and gynecology in the same consultation.50
Comprehensive initiatives
Blood product utilization
Blood transfusion is a valuable clinical tool in the care of patients across health care systems, but overuse is common51 and associated with excess patient risk and cost.52 Blood product stewardship becomes increasingly important during times of increased demand coupled with fewer donations, such as those seen during summer months or public health emergencies. The transfusion medicine community has devoted significant effort to establishing best practices for patient blood management, and international professional societies53-56 and consensus conferences57,58 have published guidelines promoting blood product stewardship. There are a wide range of efforts published in the literature to translate guidelines into practice, often led by transfusion medicine clinicians, frequently in partnership with hematology.
At Johns Hopkins Hospital (Baltimore, MD), authors identified institutional overuse of blood products in surgical patients and developed an educational initiative targeting not only attending physicians but also clinical fellows, residents, and midlevel providers to decrease inappropriate use.11 With education and provider feedback via transfusion report cards, compliance with guidelines improved, and utilization of red blood cells and plasma decreased by 15% and 24%, respectively, resulting in a cost savings of US$125 558 over 9 months.
Clinical decision tools in the EMR have been consistently shown to increase compliance with appropriate use transfusion guidelines.59,60 Authors from Stanford University (Stanford, CA) first developed institutional guidelines and performed educational outreach to ordering providers, followed by the implementation of an interruptive alert when transfusion was ordered outside guideline thresholds.61 With this intervention, the percentage of blood ordered for patients with hemoglobin >8 g/dL without another clinical indication decreased from ∼57% to 66% to 35% (P = .02), with an estimated purchase cost savings of US$1 616 750 per year. Similar efforts have been successful in decreasing unnecessary use of blood plasma products62 in accordance with the American Society of Clinical Pathology Choosing Wisely recommendation to not transfuse plasma to correct a mildly prolonged international normalized ratio.63
Rather than focusing on guidance for appropriate use of blood products, other interventions have focused on optimizing erythropoiesis to decrease the need for transfusion. Perioperative anemia is prevalent and associated with increased blood product utilization and poor surgical outcomes,64 so efforts to address and standardize treatment of anemia in the preoperative setting have gained increasing attention.65 To achieve this goal, multiple institutions have developed preoperative anemia clinics along with institution-specific management algorithms that have successfully optimized patient hemoglobin and therefore decreased perioperative transfusion requirements as well as improved perioperative patient outcomes.66-68 Such efforts have also proven cost effective and self sustainable; for instance, the creation of a preoperative anemia clinic at Duke University Medical Center (Durham, NC) generated an estimated positive net value of US$2.5 million over 5 years.66 Similar efforts have been effective in the optimization of iron repletion in pregnant women. Authors from St. Michael’s Hospital (Toronto, ON, Canada) developed a comprehensive initiative (IRON MOM) including both provider and patient education as well as clinical pathways for the diagnosis and management of iron deficiency in pregnancy.69 These efforts increased the number of women screened for iron deficiency, decreased the number of women with antenatal anemia, and decreased red cell transfusion during pregnancy (from 1.2% to 0.8%; P = .0499) and immediately after delivery (from 2.3% to 1.6%; P = .214).
Anticoagulation stewardship programs
The use of anticoagulation is a significant driver of drug-related adverse events across health care systems.70,71 On 1 July 2019, the Joint Commission implemented 6 new National Patient Safety Goals (NPSG.03.05.01) to guide improvement in the safety of anticoagulation prescribing.72 This initiative reflects growing national and international awareness of the potential for harm associated with anticoagulation and the need to develop programs to promote the delivery of safe, evidence-based, cost-effective anticoagulation care.
To address this need, the Anticoagulation Forum recently created a guide for the development of anticoagulation stewardship teams.73 Such teams, modeled after successful antimicrobial stewardship programs, are designed to oversee safe anticoagulation prescribing across health care systems. Published examples have variable structures with differences in scope (eg, anticoagulation, factor products, and/or reversal agents) and team members (eg, physicians, advanced practice providers, nurses, and/or pharmacists), but they have consistently shown increased adherence to established clinical practice guidelines and improved safe prescribing practices.74-79 Furthermore, they have demonstrated financial feasibility; authors from Brigham and Women’s Hospital (Boston, MA) estimated their program’s annual cost to be US$175 000, which was more than covered by an estimated annual cost savings of approximately US$1.5 million as a result of the team’s interventions.76
Electronic consultation
Unfortunately, access to hematology expertise is limited, because an imbalance between a decreasing pool of subspecialty providers and an increasing number of patients with hematologic disorders continues to grow.1 In response, electronic consultation (e-consultation) is expanding to optimize access to hematologic subspecialty care. By forgoing face-to-face encounters when clinical questions may be answered with a medical record review alone, hematology expertise may be extended farther across large health care systems and geographic regions.
The Kaiser Permanente health care system developed a hematology e-consultation system across 5 medical centers, along with ordering panels for the laboratory workup of common disorders and prepopulated templates for frequently asked questions.80 All nonemergent hematology consults were triaged by a hematologist, and 75% were successful managed electronically. The average time for a hematologist to complete a consult was 14.5 minutes (95% confidence interval, 14.0-14.9 minutes), with 90.3% of consults addressed within 24 hours.
However, questions remain about the potential drawbacks of e-consultation in hematology. In the Veterans Affairs health care system, implementation of e-consultation decreased face-to-face encounters by 18% but was accompanied by an increase in the total number of consults received, raising concern that the ease of e-consultation may increase consults that may not be appropriate or may not contribute to patient care.81 To this point, a recent study developed metrics to assess e-consultation appropriateness, and 73.3% of hematology consults within their e-consultation network met all 4 appropriateness metrics.82 Other concerns include effects of e-consultation on patient and provider satisfaction, potential missed information without a patient evaluation, and compensation structure in fee-for-service systems. Importantly, the COVID-19 pandemic has necessitated a dramatic increase in virtual care options across many subspecialties, including hematology, and future reports are likely to enrich our understanding of when and how e-consultation can be most effective.
Another proposed use of e-consultation is the targeted automatic e-consultation, where e-consultation is triggered by specific EMR parameters for disorders that commonly benefit from specialist intervention.83 HIT has been proposed as a potential target, because there is a need to improve the diagnosis and management of HIT (as described in “HIT” under “Targeted initiatives”), and a majority of clinical decisions in HIT management do not hinge on face-to-face interactions.
Future directions
Early work by systems-based hematologists has largely focused on nonmalignant hematologic disorders, but expansion into malignant hematology is needed. Malignant hematology relies heavily on molecular diagnostic testing, and many patients are admitted to the inpatient setting for at least part of their treatment course. Clear diagnostic and treatment pathways within health care systems to maximize outcomes and value have the potential to benefit both patients and the health care system.
Existing efforts have also been concentrated in health care systems in the United States and Canada, so expansion and adaptation to the needs of other countries are also needed. Previous research has identified and proposed solutions to overcome the challenges of implementing care initiatives in low-income countries,84 and a systems-based hematologist would be well suited to apply such principles to improve the delivery of hematologic care in those environments.
The development of career pathways in systems-based hematology is also needed. Currently, a majority of systems-based hematologists have performed their efforts informally, and many of the examples in this review represent isolated quality improvement projects. To foster further expansion and sustainability in the field, institutions must be willing to invest in provider time devoted to these pursuits to allow for greater focus on multiple projects, leading to improved patient care and much greater cost savings to the institution. Support for the systems-based hematologist would likely vary between institutions but might come from the health care system or from the academic department/division in the form of salary support or as protected full-time equivalency.
Along with the creation and financial support of systems-based hematology careers, it is important to concurrently generate trainee interest. With increasing emphasis on quality improvement in medical training, systems-based hematology may be particularly appealing to trainees with such interests and serve as an avenue to encourage interest in nonmalignant hematology.85 Dedicated rotations with systems-based hematologists for medical students, residents, and hematology/oncology fellows might increase the number of future trainees interested in this career path and could help to improve the care of patients with hematologic disorders at health care systems where inpatient hematology expertise is not readily available.
In addition, the COVID-19 pandemic has introduced new urgency and need for the systems-based hematology skillset. As health care systems have been forced to rapidly adapt and provide care while maintaining social distancing, the use of telemedicine has rapidly expanded,86 providing the systems-based hematologist with the opportunity to guide its use to care for patients with hematologic disorders. Previous barriers to virtual care, including lower reimbursement and lack of technological infrastructure, have been expeditiously overcome, and therefore, there may be opportunity beyond the pandemic for continued expansion of these platforms within hematology. Furthermore, COVID-19 has posed unique clinical challenges to hematologists. Infected patients often develop significant coagulopathies87,88 ; caring for patients with malignant hematologic disorders has new complexities, given the unclear vulnerability of these patients to infection89,90 ; and there is a need for stewardship of limited health care resources, such as blood products.91,92 The systems-based hematologist is uniquely suited to rapidly synthesize emerging data, interpret conflicting consensus guidelines, and communicate a consistent management approach to providers across an institution or health care system to address these challenges.
There are numerous opportunities for stakeholders to support systems-based hematologists. Specifically, hospital administrators and clinical directors should consider global evaluation of budget models to ensure that savings recognized in 1 area of the hospital budget may be attributed to their systems-based hematologists working to improve care quality. Additionally, given the ever-changing practice environment, residency and fellowship training program directors should evaluate educational curricula to ensure trainees receive robust training in quality improvement frameworks and health outcomes research methods, while trainees should seek out educational opportunities and mentoring with current systems-based hematologists. Clinicians already practicing as systems-based hematologists should make themselves available for mentoring and networking outside their institutions to ensure sustained growth in the field. Finally, professional societies are uniquely positioned to advocate across the entire breadth of the field to facilitate networking, education opportunities, appropriate training resources, and curriculum development.
In summary, systems-based hematologists optimize care for both patients with blood disorders and the systems that deliver care to those patients. With initiatives of varying scope and methods of intervention, the work done by the systems-based hematologist improves patient outcomes, results in cost savings, and facilitates dissemination and implementation of evidence-based care. In this review, we have outlined successful case studies that highlight how systems-based hematology can improve patient care. We further emphasize the importance of continued investment in the expansion of this field to improve hematologic care delivery across health care systems.
Authorship
Contribution: J.E.M. and N.T.C. developed the concept and design; J.E.M. wrote the first draft of the manuscript; J.E.M., P.C.I., K.B., and N.T.C. contributed to, critically reviewed, revised, and contributed to the intellectual content of the first and subsequent drafts of the manuscript; E.M., S.F., D.A.G., L.K.H., J.L., M.Y.L., C.T.M., A.R., S.S., M.S.Z., and R.M.P. critically reviewed, revised, and contributed to the intellectual content of the manuscript; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: S.F. has served as medical director for HemeOnc Call, LLC, which has a section on telemedicine. A.R. has served on advisory boards for Alexion, Baxter, Bayer, and Kedrion Biopharma Octapharma Plasma, and her institution has received research support on her behalf from Alnylam (Sanofi Genzyme), Baxalta (Shire), Biomarin, Dimensions Therapeutics, Genetech, Janssen Pharmaceuticals, and Roche. M.S.Z. has served as a member of the American Board of Internal Medicine’s Hematology Board and provided consultancy for Novartis and legal case review. The remaining authors declare no competing financial interests.
Correspondence: Nathan T. Connell, Brigham and Women’s Hospital, Hematology Division, 75 Francis St, SR322, Boston, MA 02115; e-mail: ntconnell@bwh.harvard.edu.