Key Points
The unintentional and intentional nonadherence framework can provide a better understanding of hydroxyurea adherence among persons with SCD.
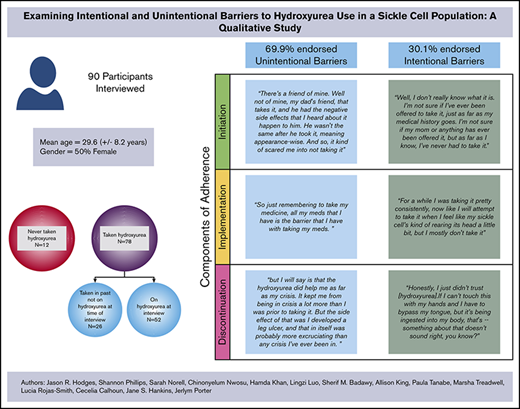
A majority of participants with SCD (70%) endorsed unintentional barriers to hydroxyurea adherence.
Abstract
Hydroxyurea is an efficacious treatment for sickle cell disease (SCD), but adoption is low among individuals with SCD. The objective of this study was to examine barriers to patients’ adherence to hydroxyurea use regimens by using the intentional and unintentional medication nonadherence framework. We interviewed individuals with SCD age 15 to 49.9 years who were participants in the Sickle Cell Disease Implementation Consortium (SCDIC) Needs Assessment. The intentional and unintentional medication nonadherence framework explains barriers to using hydroxyurea and adds granularity to the understanding of medication adherence barriers unique to the SCD population. In total, 90 semi-structured interviews were completed across 5 of the 8 SCDIC sites. Among interviewed participants, 57.8% (n = 52) were currently taking hydroxyurea, 28.9% (n = 26) were former hydroxyurea users at the time of the interview, and 13.3% (n = 12) had never used hydroxyurea but were familiar with the medication. Using a constructivist grounded theory approach, we discovered important themes that contributed to nonadherence to hydroxyurea, which were categorized under unintentional (eg, Forgetfulness, External Influencers) and intentional (Negative Perceptions of Hydroxyurea, Aversion to Taking Any Medications) nonadherence types. Participants more frequently endorsed adherence barriers that fell into the unintentional nonadherence type (70%) vs intentional nonadherence type (30%). Results from this study will help SCD health care providers understand patient choices and decisions as being either unintentional or intentional, guide tailored clinical discussions regarding hydroxyurea therapy, and develop specific, more nuanced interventions to address nonadherence factors.
Introduction
Sickle cell disease (SCD) is the most prevalent genetic condition worldwide with estimates of around 300 000 births of patients with SCD each year.1,2 In the United States, ∼100 000 people are living with the disease.3 The majority of cases in the United States are among African Americans and Hispanic/Latinx Americans, which impacts 1 of 365 births to African American parents and 1 of 16 300 births to Hispanic-American parents.1,3 In 1998, the US Food and Drug Administration approved hydroxyurea for use in SCD after a pivotal clinical trial which showed that hydroxyurea reduced the frequency of pain events by >40%.4,5 Subsequent trials continued to demonstrate the role of hydroxyurea in the reduction of pain crises6 and mortality rates and the improvement in quality of life for people living with SCD.7 Despite these positive outcomes, hydroxyurea remains underused by patients8,9 and providers.10 Poor adherence to hydroxyurea regimens contributes to greater use of health care, lower self-reported quality of life, and higher health care costs.11-13 Studies that seek to explain and intervene upon this underuse of hydroxyurea among patients are increasing; however, compared with studies of treatments used in other chronic diseases, studies that use various theories of adherence to treatments in SCD are underrepresented.14 It is important to test and investigate theoretical frameworks for understanding and intervening upon adherence to treatment in SCD because of the complexity of the disease’s impact over the lifespan, the sociodemographic characteristics of those living with SCD, and how these factors are intertwined in adherence behaviors.15
Understanding the complexity of adherence and developing interventions that aim to have an impact on adherence calls for a framework that considers the intentionality of nonadherence. Intentional nonadherence and unintentional nonadherence expand on the concept of adherence, moving from a simplistic binary construct (adherent vs nonadherent), by which the patient exerts little agency or control in medical decision-making and behavior, to a richer and more nuanced view of adherence, acknowledging the patient’s (and medical team’s) active and passive roles throughout the medical care continuum.16 Intentional nonadherence positions patients as actors who have agency in making rational decisions to adhere, modify, or disregard treatment advice from medical professionals.16 Factors that underlying their decisions vary from experiencing adverse effects17 to their perceptions of the need for the prescribed treatment.18 The concept of intentionality has been used to examine treatment nonadherence among individuals living with a variety of chronic conditions, including coronary heart disease,19-21 glaucoma,22 kidney disease,23 hypertension,20,24 epilepsy,25 and diabetes.21,24 This research has led to tailored interventions, with mixed results, and recommendations that focus on intentional and unintentional nonadherence.24,26-30 In addition to understanding the nuances of adherence types, it is important to understand the phases within the adherence process provided by the Components of Adherence concept. The Components of Adherence concept assigns barriers to adherence to specific phases of an individual’s adherence decision-making process: Initiation, Discontinuation, and Implementation.24 Initiation pertains to the processes involved in an individual’s decision to start taking hydroxyurea. Discontinuation is the phase in which an individual decides to discontinue taking hydroxyurea. Implementation encompasses those activities that concern everyday adherence to taking hydroxyurea. Adherence in this sense refers to taking hydroxyurea as prescribed and directed by the individual’s medical team.
The concepts of intentional nonadherence and unintentional nonadherence are not currently found in the SCD adherence literature. This gap contributes to an understatement in the literature regarding the complexity of making decisions regarding adhering or not adhering to a hydroxyurea regimen by focusing primarily on individual-level factors and neglecting external influencers. Factors related to the unintentional nonadherence category can be interpreted as those that are not as closely linked to an individual’s attitudes or beliefs.16 Underlying contributors to unintentional nonadherence include misunderstanding of medical instructions, the burden of complex treatment regimens, never being offered the drug, being removed from a regimen because of the medical team’s decision, and the quality and strength of the patient-provider relationship.16 These categories of nonadherence could play an important role in helping care providers more fully understand adherence behaviors among people with SCD, especially given the demonstrated mistrust of medical providers and the medical establishment overall, including the perceived lack of agency in this power dynamic,31,32 evidence of low health literacy among people living with SCD,33 and beliefs about the efficacy and safety of hydroxyurea.34,35
The purpose of this article is to add to the literature on hydroxyurea adherence by exploring, via qualitative methodology, the unintentional and intentional nonadherence factors that influence adherence decisions among individuals with SCD who participated in a multicenter study and who represented a diverse geographic distribution in the United States.
Methods
Participant recruitment
Participants for in-depth interviews were recruited at 5 academic centers that participated in the qualitative portion of the Sickle Cell Disease Implementation Consortium (SCDIC) Needs Assessment. The SCDIC represents academic and medical centers across the United States that use implementation science to improve the health and well-being of individuals living with SCD.15 The purpose of the SCDIC Needs Assessment study was to identify barriers to hydroxyurea adherence and use, transitioning to adult care, emergency department care, and engagement in routine care.15,21 All individuals with a diagnosis of SCD between age 15 and 49.9 years were eligible to participate. Purposive sampling was used to identify potential participants at each site and obtain a sample that varied in terms of age, sex, disease complexity, history of SCD, current affiliation with a sickle cell provider, and rural vs urban residence.
Ethical considerations
Study sites obtained institutional review board approval before study startup. Trained study staff reviewed the overall study with participants; for participants age 18 years or older, they obtained signed written informed consent. Assent was obtained from participants age 15 to 17 years, with consent obtained from the parent or primary caregiver.
Data collection
Data from the SCDIC Needs Assessment were collected from 2017 to 2018.36 An interview guide was developed by an SCDIC subcommittee of consortium members who had qualitative research expertise and extensive experience working with individuals with SCD (supplemental Data). Subcommittee members met virtually twice per month to discuss and develop open-ended questions and probes to be included on the interview guides. Draft interview guides were circulated and revised by subcommittee members until consensus was reached on the draft of the interview guide. The interview guide was then voted on and approved by the SCDIC Steering Committee before being used. Data in this study are from the hydroxyurea use section of the interview guide. Participants were recruited by trained research staff during clinic visits and SCD-related community events. Interviews were conducted by trained qualitative researchers, and they lasted 45 to 75 minutes. Interviews were audio recorded and transcribed verbatim. Interview participants also completed a demographic survey. Participants were compensated for participating in the interview.
Data analysis
Interview transcripts were redacted for identifiable information, reviewed for accuracy, and uploaded into Dedoose Version 7.0.23 (SocioCultural Research Consultants, Los Angeles, CA) for qualitative analysis. This platform allowed the authors to collaborate in real-time during coding and analysis. Using real-time software solved the problem of authors not being physically located in the same space and allowed for constant collaboration throughout the data analysis process. Twice-per-month meetings occurred via WebEx (Cisco) to discuss study topics, address challenges with using the qualitative analysis software, and facilitate cross-site collaboration.
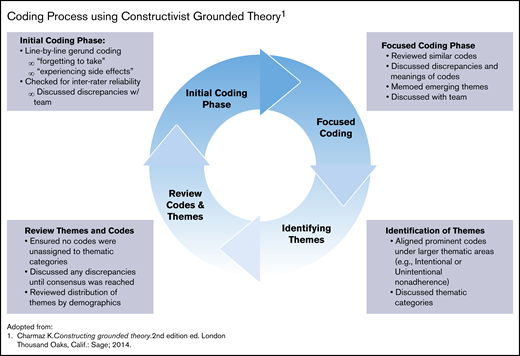
Charmaz’s constructivist grounded theory (CGT) guided the analytic approach. This analytic approach was ideal for this study because it focused on being immersed in the data and at the same time acknowledging the role of the researchers as coproducers in the analytic process.37 The authors followed a 2-phase, cyclical coding process that included initial (or open coding) and focused coding (Figure 1).38 Larger thematic concepts were then developed by holistically combining codes to understand how they contributed to the larger meaning making of the participants.
Coding process using constructivist grounded theory. Adapted from Charmaz.38
A subset of transcripts was coded by 2 independent coders using the initial code to examine agreement of codes using interrater reliability scores in Dedoose via Cohen’s κ coefficient with a score of 0.877. In addition, all coders met twice per month during each phase of the coding process to refine the codebook and resolve any discrepancies until a consensus was reached among the team members. Once thematic saturation was reached (ie, no new codes or themes were being discovered), themes (as defined by clustering of similar codes) were developed, categorized, and assigned to their respective cell within the Adherence Type (ie, Intentional and Unintentional) and Components of Adherence matrix (Table 1). 12,24,39 The text and codes were queried to explore the frequency and distribution of thematic codes by participants’ demographic characteristics. Examples include the frequency of codes for adherence barriers among male and female participants, age groups, or those exposed to hydroxyurea in the past, as well as how larger themes were distributed within smaller groups such as former and current hydroxyurea users. This checking provided assurance that the theories derived from the data properly identified divergences among subgroups when or if they existed.
Themes by adherence category
| Components of adherence . | Nonadherence themes . | ||||
|---|---|---|---|---|---|
| Intentional . | No. of participants . | Unintentional . | No. of participants . | ||
| Total | 31 | 69 | |||
| Initiation | Decided not to start | Negative attitudes or perceptions of hydroxyurea | 5 | External influencers | 11 |
| Fear of adverse effects | 4 | Doctor did not prescribe hydroxyurea | 7 | ||
| Does not like taking medication in general | 1 | Witnessed hydroxyurea not helping others | 3 | ||
| Uncertainty of potential effectiveness | 2 | Caregiver made decision to not start | 1 | ||
| Implementation | Adherence while taking | Does not like taking medicines in general | 6 | Forgetfulness | 22 |
| Did not perceive any benefits, contributing to lack of motivation to take regularly | 2 | Competing life demands | 3 | ||
| Complexity of treatment regimen (eg, multiple medications, different dosages, multiple pills) | 6 | ||||
| Taking hydroxyurea with food; forgetting or not getting a chance to eat so misses dose | 1 | ||||
| Physical and emotional state on any day | 1 | ||||
| Logistics of obtaining hydroxyurea (laboratory visits, pharmacy trips) | 1 | ||||
| Discontinuation | Decision to discontinue taking | Grew concerned about hydroxyurea because of potential adverse effects, history of hydroxyurea as a cancer drug, preference for treatments other than hydroxyurea (eg, chronic transfusions), did not perceive hydroxyurea as being effective | 8 | Experienced adverse effects | 16 |
| 3 | Hydroxyurea was not effective per medical team report | 10 | |||
| 5 | Taken off hydroxyurea because of poor adherence | 1 | |||
| 5 | Pregnancy | 3 | |||
| 4 | Medical team decision | 7 | |||
| Components of adherence . | Nonadherence themes . | ||||
|---|---|---|---|---|---|
| Intentional . | No. of participants . | Unintentional . | No. of participants . | ||
| Total | 31 | 69 | |||
| Initiation | Decided not to start | Negative attitudes or perceptions of hydroxyurea | 5 | External influencers | 11 |
| Fear of adverse effects | 4 | Doctor did not prescribe hydroxyurea | 7 | ||
| Does not like taking medication in general | 1 | Witnessed hydroxyurea not helping others | 3 | ||
| Uncertainty of potential effectiveness | 2 | Caregiver made decision to not start | 1 | ||
| Implementation | Adherence while taking | Does not like taking medicines in general | 6 | Forgetfulness | 22 |
| Did not perceive any benefits, contributing to lack of motivation to take regularly | 2 | Competing life demands | 3 | ||
| Complexity of treatment regimen (eg, multiple medications, different dosages, multiple pills) | 6 | ||||
| Taking hydroxyurea with food; forgetting or not getting a chance to eat so misses dose | 1 | ||||
| Physical and emotional state on any day | 1 | ||||
| Logistics of obtaining hydroxyurea (laboratory visits, pharmacy trips) | 1 | ||||
| Discontinuation | Decision to discontinue taking | Grew concerned about hydroxyurea because of potential adverse effects, history of hydroxyurea as a cancer drug, preference for treatments other than hydroxyurea (eg, chronic transfusions), did not perceive hydroxyurea as being effective | 8 | Experienced adverse effects | 16 |
| 3 | Hydroxyurea was not effective per medical team report | 10 | |||
| 5 | Taken off hydroxyurea because of poor adherence | 1 | |||
| 5 | Pregnancy | 3 | |||
| 4 | Medical team decision | 7 | |||
Results
Ninety participants completed in-depth interviews from SCDIC sites located in Illinois, North Carolina, South Carolina, Tennessee, and California. Females represented 57% of participants with a mean age of 30.5 years (standard deviation, 8.7 years). Table 2 provides the distribution of demographic characteristics of interview participants. Most participants (87%) had taken hydroxyurea at some point in their lives, and 67% reported that they were taking hydroxyurea at the time of the interview. Of the participants who had taken hydroxyurea but were not currently taking it at the time of the interview, 14 types of barriers were identified; of those, 64.3% of the barriers (9) fell into the unintentional nonadherence category. Among those currently taking hydroxyurea, 75.7% of the barriers were considered unintentional nonadherence barriers. For the 87% of participants with any history of hydroxyurea use, approximately 69.9% endorsed unintentional barriers (Table 1). Five participants endorsed barriers that fell into both unintentional and intentional categories; in these cases, the coded barrier was counted for each category.
Demographic characteristics for interview participants according to hydroxyurea use
| . | Current users (n = 52 [57.8%]) . | Never users (n = 12 [13.3%]) . | Former users (n = 26 [28.9%]) . | All (N = 90 [100%]) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age group, y | ||||||||
| 15-24.9 | 19 | 3.8 | 4 | 16.6 | 3 | 3.8 | 26 | 28.9 |
| 25-34.9 | 17 | 32.7 | 4 | 33.3 | 12 | 46.1 | 33 | 36.7 |
| 35-49.9 | 16 | 30.7 | 4 | 33.3 | 11 | 42.3 | 31 | 34.4 |
| Sex | ||||||||
| Female | 26 | 50.0 | 8 | 66.6 | 17 | 65.4 | 51 | 56.7 |
| Male | 26 | 50.0 | 4 | 33.3 | 9 | 34.6 | 39 | 43.3 |
| Enrollment site | ||||||||
| Chicago | 6 | 11.5 | 1 | 8.3 | 3 | 11.5 | 10 | 11.1 |
| Duke University | 7 | 13.5 | — | — | 3 | 11.5 | 10 | 11.1 |
| Medical University of South Carolina | 9 | 17.3 | 3 | 25.0 | 3 | 11.5 | 15 | 16.7 |
| St. Jude Children’s Research Hospital | 13 | 25.0 | 5 | 41.7 | 2 | 7.7 | 20 | 22.2 |
| University of California San Francisco | 17 | 32.7 | 3 | 25.0 | 15 | 57.7 | 35 | 38.9 |
| Mean age (SD), y | 29.61 (8.2) | 28.96 (11.2) | 33.07 (8.25) | 30.5 (8.7) | ||||
| . | Current users (n = 52 [57.8%]) . | Never users (n = 12 [13.3%]) . | Former users (n = 26 [28.9%]) . | All (N = 90 [100%]) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age group, y | ||||||||
| 15-24.9 | 19 | 3.8 | 4 | 16.6 | 3 | 3.8 | 26 | 28.9 |
| 25-34.9 | 17 | 32.7 | 4 | 33.3 | 12 | 46.1 | 33 | 36.7 |
| 35-49.9 | 16 | 30.7 | 4 | 33.3 | 11 | 42.3 | 31 | 34.4 |
| Sex | ||||||||
| Female | 26 | 50.0 | 8 | 66.6 | 17 | 65.4 | 51 | 56.7 |
| Male | 26 | 50.0 | 4 | 33.3 | 9 | 34.6 | 39 | 43.3 |
| Enrollment site | ||||||||
| Chicago | 6 | 11.5 | 1 | 8.3 | 3 | 11.5 | 10 | 11.1 |
| Duke University | 7 | 13.5 | — | — | 3 | 11.5 | 10 | 11.1 |
| Medical University of South Carolina | 9 | 17.3 | 3 | 25.0 | 3 | 11.5 | 15 | 16.7 |
| St. Jude Children’s Research Hospital | 13 | 25.0 | 5 | 41.7 | 2 | 7.7 | 20 | 22.2 |
| University of California San Francisco | 17 | 32.7 | 3 | 25.0 | 15 | 57.7 | 35 | 38.9 |
| Mean age (SD), y | 29.61 (8.2) | 28.96 (11.2) | 33.07 (8.25) | 30.5 (8.7) | ||||
SD, standard deviation.
Initiation
A majority of the 12 participants who had never taken hydroxyurea (n = 7) endorsed initiation barriers categorized as unintentional nonadherence. These participants discussed never being offered hydroxyurea by their doctor as the primary reason for never taking hydroxyurea. This could be attributed to the participants’ perceptions that their genotype was less severe than other genotypes, thereby making it less likely that a physician would determine a need for a hydroxyurea regimen.40 Themes within the intentional nonadherence barriers categories were less frequently observed compared with unintentional nonadherence barrier themes.
Unintentional nonadherence.
Among participants who never started hydroxyurea, the primary codes in this category were a lack of discussion with health care providers about hydroxyurea and no prescription recommendation for hydroxyurea. These codes were somewhat varied across age groups with younger participants noting a lack of awareness or initial discussion about hydroxyurea from their experiences.
Well, I don’t really know what it is. I’m not sure if I’ve ever been offered to take it, just as far as my medical history goes. I’m not sure if my mom or anything has ever been offered it, but as far as I know, I’ve never had to take it. (18-year-old female)
Older participants were somewhat more familiar with hydroxyurea yet described no formal discussion with their providers about starting the treatment regimen. “I’ve heard some talks about it, but I haven’t heard about ‘are you interested in trying it as of yet’” (32-year-old female).
Intentional nonadherence.
Among participants endorsing an intentional barrier to starting hydroxyurea, most pointed to fears about potential adverse effects (including a fear of cancer), witnessing a friend or family member either not benefiting from hydroxyurea or experiencing unwanted adverse effects, and being generally against taking more medications.
There’s a friend of mine. Well not of mine, my dad’s friend, that takes it, and he had the negative side effects that I heard about it happen to him. He wasn’t the same after he took it, meaning appearance-wise. And so, it kind of scared me into not taking it. (17-year-old female)
[…] the side effects that I heard were cancer, that it can cause cancer, and let’s see, I heard about the falling out of the hair and the nail beds getting yellower or yellow or something. Those didn’t – the main thing that made me not heed [the doctor’s] recommendations of taking it or that previous physician’s recommendations of taking it was the cancer fear, the fear of like look, I already have sickle cell, I don’t need anything else. I just wasn’t convinced. That physician wasn’t convincing [me] that it would be something that would be worth the risk. (45-year-old male)
Implementation
Most participants identified implementation barriers that could be categorized as unintentional nonadherence. Fifty-two participants were taking hydroxyurea at the time of the interview; of these 52, only 15% (n = 8) mentioned intentional barriers to adherence. Interestingly, almost half this group (n = 25), stated they experienced no barriers to adherence with hydroxyurea maintenance (ie, implementation phase).
Uunintentional nonadherence.
The overwhelming majority of unintentional barriers within the implementation phase fell into the theme of Forgetting to Take Hydroxyurea. Codes grouped under this theme included Competing Life Demands and Forgetting to Take Hydroxyurea With Them When Not at Home.
The theme of Forgetting to Take Hydroxyurea seemed to be prevalent across all age groups; only the Competing Life Demands code differed by age group. For example, one 15-year-old male stated, “Sometimes I forget or we’re out of it. That’s the only time. But, it’s not like I don’t feel like it or anything. Sometimes I just forget, if I’m watching TV or playing a game.” Others pointed to the complexity of a multiple-drug regimen as a factor contributing to Forgetting to Take Hydroxyurea:
I have so many meds that I have to take. Like I have I want to say 14 to 18 meds that I have to take a day, and Hydroxyurea’s the one that I be like okay I’m taking this at night, set schedule, take it. I even have like the little planner with the days on it, I have that. So just remembering to take my medicine, all my meds that I have is the barrier that I have with taking my meds. (22-year-old female)
Intentional nonadherence.
Intentional barriers to implementation were less frequently endorsed among participants. The themes within this category included Does Not Like Taking Any Medication, Uncertainty of Hydroxyurea’s Effectiveness, and Perception Hydroxyurea Was Causing More Negative SCD-Related Outcomes. Of these themes, the most commonly cited intentional barrier to implementation of hydroxyurea was a general dislike of taking any medication. One 32-year-old male explained this aversion, “I don’t like taking pills. It was, there was no barrier [to adherence]. I could do it, it’s just that I didn’t want to.” Others pointed to subjective interpretations of whether or not hydroxyurea truly needed to be taken on a daily basis, particularly if one is not experiencing any negative SCD-related outcomes or has not experienced a crisis in a long period of time:
For a while I was taking it pretty consistently, now like I will attempt to take it when I feel like my sickle cell’s kind of rearing its head a little bit, but I mostly don’t take it, and I’m being like so real. [Laughs] (20-year-old female)
Discontinuation
Participants were considered a part of the Discontinuation phase if they ever stopped taking hydroxyurea in the past and were not taking it at the time of the interview. Twenty-nine percent (n = 26) of interview participants experienced the discontinuation phase. Participants were asked to provide their reasoning behind this decision.
Unintentional nonadherence
Similar to barriers found within the initiation phase, the majority of participants that discontinued hydroxyurea (n = 21 [81%]), endorsed factors leading to discontinuation that were categorized as unintentional. The most prominent themes within this category included Medical Team Decision (because of the lack of a clinical response), Taken off Hydroxyurea by Medical Team Due to Poor Adherence, Pregnancy, and Experience of Side Effects. The experience of adverse effects varied from leg ulcers to hair loss.
I mean, I know in other situations that some people may not experience it, but in my particular case I did have a leg ulcer as an effect of hydroxyurea, but I will say is that the hydroxyurea did help me as far as my crisis. It kept me from being in crisis a lot more than I was prior to taking it. But the side effect of that was I developed a leg ulcer, and that in itself was probably more excruciating than any crisis I’ve ever been in. (34-year-old male)
A less commonly vocalized reason for being taken off hydroxyurea was an adverse reaction identified by the medical team. As one 26-year-old female described, “I was having some issues with like my platelet count and [my doctor] wanted me to stop taking it for a while to see if it made any changes to it.”
Intentional nonadherence.
The remaining participants described intentional nonadherence that were categorized into the following themes: Changing Attitudes/Perceptions About the Safety of Hydroxyurea, Perceptions that Hydroxyurea Was Not Effective (without a medical team endorsement of this statement), and Chronic Transfusion as a Preferred Method of SCD Management. No definitive pattern of these themes was observed across demographic groups. The most common codes for this section, Changing Attitudes About Hydroxyurea, seemed to occur after the patient conducted research on his or her own or had a personal experience that changed his or her perceptions of its safety. The changing perception of hydroxyurea as a safe drug influencing the decision to discontinue is exemplified below.
Honestly, I just didn’t trust [hydroxyurea]. If I can’t touch this with my hands and I have to bypass my tongue, but it’s being ingested into my body, that’s–something about that doesn’t sound right, you know? (28-year-old female)
Medical team member’s precautions for the physical handling of the hydroxyurea pill was also viewed as concerning and caused some participants to become cautious about the drug’s overall safety and whether or not it was, in fact, harmful:
And you notice how they give [hydroxyurea] to you [as an] inpatient at the hospital, the nurses don’t even touch it; they put gloves on, they say don’t touch it. And then from two years ago to now I’ve just been full of questions like well why is it okay for me to take it they can’t even touch it? And finally, about a month ago I looked it up and I saw that it depletes bone marrow. And then that’s when I was like, ‘oh. I’m not taking it anymore’. So that, I’ve been off of it since then. (32-year-old female)
Notably, a few participants described a preference for chronic red cell transfusion therapy (chronic transfusion) over hydroxyurea for disease management. The reasoning included the perception that chronic transfusion had a better perceived effect than hydroxyurea, combined with participants’ negative attitudes about medications in general.
I don’t like doing blood transfusions either, but I’ve decided not to take the pills because I’ve tried taking or doing the pills first, and the pills do not keep me feeling as good as transfusions do. (45-year-old male)
Discussion
Whether in the initiation, implementation, or discontinuation phase of hydroxyurea therapy, more individuals with SCD who participated in this study described barriers leading to unintentional nonadherence (70%) than intentional nonadherence (30%), suggesting the majority of barriers to adherence are not the result of conscious decision-making on the part of the patient. Participants in the initiation phase most commonly stated that they never started taking hydroxyurea because it was not introduced by their provider; similarly, in previous studies, individuals with SCD reported never beginning hydroxyurea therapy because it was not recommended by their provider.31,41 Reasons that hydroxyurea may have not been introduced include provider barriers such as a lack of awareness leading to underprescribing,42 providers’ beliefs about a patient’s likelihood to be adherent,9 or providers with experience and knowledge making decisions that included consideration of a patient’s clinical characteristics.43 Among participants in the implementation phase (those currently receiving hydroxyurea therapy), the most common barrier leading to unintentional nonadherence was forgetting to take hydroxyurea, a key barrier that has been well recognized and described in the literature.44-48 Participants reported facing competing life demands, also described by Thornburg and colleagues,49 which contributed to forgetting to take hydroxyurea. More than 80% of participants in the discontinuation phase mentioned stopping hydroxyurea because their health care team recommended it. This finding is similar to a report by Haywood et al41 in adults with SCD who were formerly taking hydroxyurea and stopped taking it. Their primary reason was a doctor’s recommendation.
Among participants who reported barriers leading to intentional nonadherence, a general dislike of taking medications was described by participants in all 3 phases. In addition to a general dislike of taking medications, some participants in the initiation phase described never starting hydroxyurea because of a fear of adverse effects. A fear that hydroxyurea is not safe and that it has adverse effects are well-known barriers to hydroxyurea adherence,31,44 along with negative beliefs about the medication (eg, that hydroxyurea is not effective).22,44,46
Participants in this study who were in the implementation phase described negative beliefs as a barrier that contributed to intentional nonadherence. Few participants in the discontinuation phase described barriers leading to intentional nonadherence; however, the majority described their changing attitudes and beliefs about hydroxyurea as the primary reason for discontinuation. This finding is similar to findings reported by Badawy et al22 that among adolescents and young adults with SCD, increased concerns about hydroxyurea were associated with decreased adherence. A small percentage of individuals with intentional nonadherence in the discontinuation phase described preferring chronic red cell transfusion to hydroxyurea as a disease-modifying therapy, a finding not described previously in the literature on barriers to hydroxyurea adherence. Given the time and resource commitment required by patients for chronic red cell transfusion therapy, the preference for chronic red cell transfusion over hydroxyurea suggests either a high level of negative perceptions or experiences with hydroxyurea or a higher perceived benefit of chronic red cell transfusion therapy by patients electing to discontinue hydroxyurea in favor of chronic red cell transfusion therapy.
In this study, half the participants in the implementation phase reported no barriers to taking hydroxyurea. Previous reports indicated that ∼90% of parents or caregivers of children with SCD endorsed barriers to hydroxyurea therapy for their children45 ; a substantially lower number of participants in this study identified barriers to hydroxyurea therapy. This could be attributed to sample differences in this study. For example, the population in this study may be more adherent, as determined by more objective measures. Because the purpose of this study was to explore perceived barriers that contributed to intentional and unintentional nonadherence, data were not collected on participants’ actual or self-reported adherence rates.
Thus, the relationship between number of (or lack of) perceived barriers and adherence rates was not explored. However, previous studies have reported that greater numbers of barriers are associated with lower adherence rates.21,42,44,46 Future studies may be beneficial in better understanding whether individuals with SCD or caregivers who perceive no barriers to hydroxyurea adherence do, in fact, have higher adherence rates.
Barriers to hydroxyurea adherence from the perspectives of children with SCD and their parents and caregivers or adolescents and young adults with SCD have been more commonly reported than barriers from the perspective of adults with SCD. Although many of the barriers reported by participants in this study have been described previously in the literature, this study introduces the role of patient agency or power in nonadherence by examining barriers leading to intentional and unintentional nonadherence among those who have never taken hydroxyurea, those currently receiving hydroxyurea therapy, and those who have discontinued hydroxyurea therapy. This deeper understanding of barriers to hydroxyurea adherence can be applied in the clinical setting to inform treatment strategies specific to a patient’s phase (initiation, implementation, or discontinuation) and intentional vs unintentional nonadherence barriers. Table 3 outlines examples of strategies that may be applied in each phase to address intentional or unintentional nonadherence within the phases of adherence. As seen in Table 4, several strategies to address adherence barriers may have an impact on various types of adherence. These cross-influencing strategies might be prime candidates for testing in more rigorous interventional studies.
Distribution of codes by participants’ history of hydroxyurea use
| . | Any hydroxyurea history* . | No hydroxyurea history† . | Total coded by nonadherence type . | |||||
|---|---|---|---|---|---|---|---|---|
| No. of patients . | No. of times coded . | % . | No. of patients . | No. of times coded . | % . | No. of times coded . | % . | |
| Total | 78 | 12 | ||||||
| Intentional nonadherence | 25 | 30.1 | 7 | 38.8 | 32 | 31.1 | ||
| Unintentional nonadherence | 58 | 69.9 | 11 | 31.1 | 69 | 67.9 | ||
| Total codes | 83 | 82.2 | 18 | 17.8 | 101 | 100 | ||
| . | Any hydroxyurea history* . | No hydroxyurea history† . | Total coded by nonadherence type . | |||||
|---|---|---|---|---|---|---|---|---|
| No. of patients . | No. of times coded . | % . | No. of patients . | No. of times coded . | % . | No. of times coded . | % . | |
| Total | 78 | 12 | ||||||
| Intentional nonadherence | 25 | 30.1 | 7 | 38.8 | 32 | 31.1 | ||
| Unintentional nonadherence | 58 | 69.9 | 11 | 31.1 | 69 | 67.9 | ||
| Total codes | 83 | 82.2 | 18 | 17.8 | 101 | 100 | ||
Five individuals endorsed both intentional and unintentional nonadherence barriers.
One participant endorsed intentional and unintentional nonadherence barriers.
Suggestions for interventions by adherence category
| Components of adherence . | Strategies for addressing nonadherence . | ||
|---|---|---|---|
| Intentional . | Unintentional . | ||
| Initiation | Decides not to start | • Open provider-patient discussions about potential adverse effects of hydroxyurea, history, efficacy, and pros and cons compared with other treatments, including discussions about the potential effects of having untreated SCD. | • Educate non-hematologists about hydroxyurea indications for SCD. |
| • Have informal Q & A sessions with peers who are currently taking hydroxyurea. | • Open provider-caregiver discussions about hydroxyurea’s benefits, risks, and limitations and how these will be monitored while receiving treatment. | ||
| • Explore personal concerns and beliefs to help alleviate general aversion or fear of medication. | • For pediatric patients whose caregivers are considering hydroxyurea, hold informal Q & A sessions with other caregivers who opted to have their child take hydroxyurea. | ||
| • Respect the final decision of the individual and be open to future discussions. | |||
| Implementation | Adherence while taking | • Involve patient in reviewing laboratory results and changes to results as they start taking hydroxyurea. | • Provide tools to help prompt individuals when to take their medication (eg, phone app, incorporation into daily routine). |
| • Continue open and ongoing provider-patient conversations about concerns, worries, or perceived effects resulting from taking hydroxyurea. | • Open provider-patient/caregiver discussions about adherence challenges and potential strategies to addressing barriers. Use strength- based approaches to build on patient’s strengths to overcome adherence challenges. | ||
| • Collaborate with patients to identify ways to address barriers as they arise; use strength-based approaches to build on patient’s strengths to overcome adherence challenges. | • Continue routine discussions about any experienced adverse effects or concerns about the impact of taking hydroxyurea on the patient. | ||
| • Acknowledge and respect the challenges in maintaining a daily medication regimen. | |||
| Discontinuation | Decision to discontinue taking | • Have open discussion with patient/caregiver on factors leading to this decision. | • Have open discussion with patients and caregivers on factors leading to this decision and what it means for future opportunities and treatment modalities. |
| • Provide options for patients to continue hydroxyurea in the future. | |||
| • Respect the final decision of the individual and be open to future discussions. | |||
| Components of adherence . | Strategies for addressing nonadherence . | ||
|---|---|---|---|
| Intentional . | Unintentional . | ||
| Initiation | Decides not to start | • Open provider-patient discussions about potential adverse effects of hydroxyurea, history, efficacy, and pros and cons compared with other treatments, including discussions about the potential effects of having untreated SCD. | • Educate non-hematologists about hydroxyurea indications for SCD. |
| • Have informal Q & A sessions with peers who are currently taking hydroxyurea. | • Open provider-caregiver discussions about hydroxyurea’s benefits, risks, and limitations and how these will be monitored while receiving treatment. | ||
| • Explore personal concerns and beliefs to help alleviate general aversion or fear of medication. | • For pediatric patients whose caregivers are considering hydroxyurea, hold informal Q & A sessions with other caregivers who opted to have their child take hydroxyurea. | ||
| • Respect the final decision of the individual and be open to future discussions. | |||
| Implementation | Adherence while taking | • Involve patient in reviewing laboratory results and changes to results as they start taking hydroxyurea. | • Provide tools to help prompt individuals when to take their medication (eg, phone app, incorporation into daily routine). |
| • Continue open and ongoing provider-patient conversations about concerns, worries, or perceived effects resulting from taking hydroxyurea. | • Open provider-patient/caregiver discussions about adherence challenges and potential strategies to addressing barriers. Use strength- based approaches to build on patient’s strengths to overcome adherence challenges. | ||
| • Collaborate with patients to identify ways to address barriers as they arise; use strength-based approaches to build on patient’s strengths to overcome adherence challenges. | • Continue routine discussions about any experienced adverse effects or concerns about the impact of taking hydroxyurea on the patient. | ||
| • Acknowledge and respect the challenges in maintaining a daily medication regimen. | |||
| Discontinuation | Decision to discontinue taking | • Have open discussion with patient/caregiver on factors leading to this decision. | • Have open discussion with patients and caregivers on factors leading to this decision and what it means for future opportunities and treatment modalities. |
| • Provide options for patients to continue hydroxyurea in the future. | |||
| • Respect the final decision of the individual and be open to future discussions. | |||
In 2013, a National Institutes of Health Consensus Statement on hydroxyurea treatment of SCD identified only 3 studies that addressed barriers to hydroxyurea, none of which tested interventions.50 Currently, the majority of interventions that address barriers to hydroxyurea adherence are designed for children or adolescents and young adults and have undergone pilot testing but have not reported testing via adequately powered randomized control trials.44,51-54 Importantly, the SCDIC is currently testing a mobile health (mHealth) intervention designed to address the major unintentional barrier: forgetting to take the drug.55,56 Findings from our qualitative study may be used in future research through development and testing of multimodal interventions for adults with SCD to improve initiation of hydroxyurea or adherence to hydroxyurea by including the role of patient agency and specifically addressing barriers leading to intentional or unintentional nonadherence.
Limitations
An important limitation of this study is the lack of data captured to assess the current adherence status of participants; however, these interview participants represent a subsample of the SCDIC registry,57 wherein 42.1% of respondents (n = 462) self-reported poor adherence. Limited collection of sociodemographic characteristics was also a limitation to understanding how these variables could impact adherence decisions. Future studies should incorporate these variables into the study design to assess their impact on adherence.
In addition, the aim of this study was to explore barriers from the perspective of individuals living with SCD to provide rich descriptions of barriers and introduce new variables relevant to hydroxyurea adherence to test in future studies. Study participants in this sample were recruited via purposive sampling at SCD clinics, health fairs, and SCD advocacy groups. Although attempts were made to identify which individuals were currently engaged in care and which were not, the sampling approach led to challenges in identifying individuals not currently engaged in care, an important subgroup of patients with SCD. Given the number of sites, there was some variation in conducting the qualitative research study. For example, sites were not required to conduct all topical areas in the semi-structured interview guide; however, the hydroxyurea use and barriers section was required by all sites participating in the qualitative component of the Needs Assessment. To address these concerns, all sites were trained before collecting data to ensure fidelity in the quality of data collection, a cross-site study protocol was developed, and regular meetings were conducted to address any concerns with data collection. Using multiple coders can lead to misapplication of codes; however, duplicate coding and coding meetings twice per month were used to address this concern. The frequency of codes and themes may not necessarily represent the importance of barriers in each individual’s decision-making process. Finally, data were limited to what was provided by participants based on their personal experiences.
In conclusion, in a study of 90 individuals with SCD at multiple institutions across the United States, the role of patient agency in adherence behaviors was introduced, and barriers leading to intentional and unintentional adherence challenges for patients who are considering starting hydroxyurea therapy, those already receiving hydroxyurea therapy, and those who have discontinued hydroxyurea therapy are illustrated. Our results showed that unintentional adherence barriers were reported with greater frequency, pointing to the importance of recognizing these categories of barriers. Adherence is challenging to address, and a greater understanding of complex barriers to adherence can inform strategies in the clinical and research settings for overcoming barriers.
For data sharing requests contact Jason R. Hodges (jason.hodges@stjude.org).
Acknowledgments
The authors thank the participants for giving their time and providing their perspectives on factors involved in their adherence to hydroxyurea regimens, and they also thank the members of the SCDIC Needs Assessment Committee for their important work in developing and implementing the overall needs assessment.
This research was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants (U24 HL133948, U01 HL134042, U01 HL133994, U01 HL133964, U01 HL134007, U01 HL133997, U01 HL134004, U01 HL133990, U01 HL133996, and K01 HL125495).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.R.H., S.M.P., and S.N. developed the theoretical framework for analysis; J.R.H., S.M.P., S.N., C.N., H.K., and L.L. performed the coding and analysis of the qualitative data; S.M.P., S.N., C.N., H.K., P.T., and M.T. conducted interviews; J.S.H., P.T., M.T., A.K., L.R.S., and J.P. conceived of the research design and planning; J.R.H., S.M.P., and S.N. took the lead in writing the manuscript; S.M.B. and C.C. assisted in writing and reviewing mansucript; C.C. assisted in interviews, development of research design, analytic approach, and interview guide; and all authors provided critical feedback and helped shape the research, analysis, and manuscript.
Conflict-of-interest disclosure: J.S.H. received research support from Global Blood Therapeutics (GBT) and Novartis and consultant fees from GBT, UpToDate, and MJ Lifesciences. M.T. received consultant fees from GBT. The remaining authors declare no competing financial interests.
The current affiliation for S.N. is Walgreens Corporation, Deerfield, IL.
Correspondence: Jason R. Hodges, Department of Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS 800, Memphis, TN 38105; e-mail: jason.hodges@stjude.org.
References
Author notes
The full-text version of this article contains a data supplement.