Key Points
Most primary EBL cases in Japan were negative for HHV8.
Primary HHV8-negative EBL affected HIV-negative elderly patients, and its prognosis was favorable after chemotherapy.
Abstract
Primary effusion-based lymphoma (EBL) presents as a malignant effusion in a body cavity. The clinicopathologic features and prognosis of primary human herpesvirus 8 (HHV8)–negative EBL remain unclear. We therefore conducted a retrospective study of 95 patients with EBL, regardless of HHV8 status, in Japan. Of 69 patients with EBL tested for HHV8, a total of 64 were negative. The median age of patients with primary HHV8-negative EBL at diagnosis was 77 years (range, 57-98 years); all 58 tested patients were negative for HIV. Primary HHV8-negative EBL was most commonly diagnosed in pleural effusion (77%). Expression of at least 1 pan B-cell antigen (CD19, CD20, or CD79a) was observed in all cases. According to the Hans algorithm, 30 of the 38 evaluated patients had nongerminal center B-cell (non-GCB) tumors. Epstein-Barr virus–encoded small RNA was positive in 6 of 45 patients. In 56 of 64 HHV8-negative patients, systemic therapy was initiated within 3 months after diagnosis. Cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or CHOP-like regimens with or without rituximab (n = 48) were the most common primary treatments. The overall response and complete response rates were 95% and 73%, respectively. Three patients did not progress without systemic treatment for a median of 24 months. With a median 25-month follow-up, the 2-year overall survival and progression-free survival rates were 84.7% and 73.8%. Sixteen patients died; 12 were lymphoma-related deaths. Thus, most EBL cases in Japan are HHV8-negative and affect elderly patients. The non-GCB subtype is predominant. Overall, primary HHV8-negative EBL exhibits a favorable prognosis after anthracycline-based chemotherapy.
Introduction
Rare large B-cell lymphomas that develop as serous effusions in the body cavity without detectable tumor masses are usually positive for human herpesvirus 8 (HHV8),1-4 and primary HHV8-positive effusion-based lymphoma (EBL) is defined as primary effusion lymphoma (PEL) according to the current World Health Organization classification. However, there have been several case reports and literature reviews on primary HHV8-negative EBL,5-53 and terms such as PEL-like lymphoma, HHV8-unrelated PEL, and primary HHV8-negative EBL have been coined to describe those cases. In addition, although cases of primary HHV8-negative EBL have been reported worldwide, more than one-half of them have been reported in Japan.5 In previous case series, primary HHV8-negative EBL usually affected HIV-negative patients, and lymphoma cells expressed pan B-cell markers; in addition, the prognosis of patients with primary HHV8-negative EBL seemed to be better than that of patients with PEL.5,6 Several reports described cases with spontaneous regression without any systemic treatment or durable regression of effusion after pleurodesis.8,11,36,38,51 These clinicopathologic features seem to be in contrast to those of PEL, in which most cases occur in HIV-infected patients, neoplastic cells usually lack pan B-cell marker expression but express plasma cell-differentiation markers,54 and prognosis is poor. However, no retrospective study with a sufficient number of cases to date has investigated the clinicopathologic features and prognosis of primary HHV8-negative EBL. We therefore conducted this retrospective study to determine the clinicopathologic features and prognosis of cases of primary HHV8-negative EBL in Japan.
Patients and methods
This study was approved by the relevant institutional review board or ethics committee of each institution. Informed consent was waived because of the retrospective nature of the study.
Patient selection
In this study, 95 patients with EBL diagnosed between 1990 and 2014 were registered from 52 institutions in Japan. EBL was defined as large-cell lymphoma that presented with effusion in the body cavity (pleural effusion, pericardial effusion, and ascites) without the formation of a mass in adjacent structures, including the serosa or any extracavitary lesions at the time of diagnosis. The diagnosis of EBL was made on pathologic examination of cell block specimens obtained from the body cavity effusion and/or cytology combined with flow cytometric immunophenotyping. Clinical, pathologic, and immunophenotypic data were obtained from the case report form, and clinicopathologic features were retrospectively analyzed. We excluded 5 patients from the study for the following reasons: mass formation in the adjacent structure in 2 patients, bone marrow involvement in 1 patient, and no objective evidence of lymphoma on morphology or immunophenotyping in 2 patients (Figure 1). The data of 13 patients have previously been reported in the English literature.9-11,26,29,46,55,56
Histologic and immunohistochemical staining
Of 95 cases of EBL, immunohistochemical analysis of cell block specimens and flow cytometric immunophenotyping were performed at participating centers for 69 patients and 83 patients, respectively. Fifty-nine cases were assessed by using both methods. In addition to the diagnosis made by the pathologists at the participating centers, central pathology review was performed on 55 of 95 cases of EBL, including 45 of 64 cases of primary HHV8-negative EBL. Unstained slides were available for 35 patients, stained slides were available for 23, and pathologic images were available for 13. For 20 patients, we could not obtain unstained slides but we did obtain stained slides or pathologic images. Of these 20 patients, only stained slides were available for 9, only pathologic images were available for 9, and both pathologic images and stained slides were available for 2. The HHV8 status was assessed via immunohistochemical staining with the anti–LANA-1 antibody (n = 50) and/or polymerase chain reaction for HHV8 (n = 45) by using the body cavity effusion sample. Of the 23 patients who were assessed for HHV8 status by using both methods, 22 patients had concordant results. One patient had discordant results, as shown by the positive result obtained by immunohistochemistry and the negative result obtained by polymerase chain reaction. This patient was classified into the HHV8-positive group.
Definition of clinical characteristics
In this study, all patients with EBL were defined as having stage IV disease according to the Ann Arbor staging system because they had disseminated extralymphatic involvement.57 Moreover, while determining the International Prognostic Index,58 body cavity effusion sites were counted as one extranodal site regardless of the number of involved body cavities.
Treatment response
In this retrospective study, the response to initial treatment was assessed. A complete response (CR) was defined as the disappearance of body cavity effusion on imaging, and a partial response (PR) was defined as the reduction in body cavity effusion. Progressive disease was defined as an increase in body cavity effusion. All other situations were considered stable disease.
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis to the date of death due to any cause. Progression-free survival (PFS) was measured from the date of diagnosis until the date of progression, relapse, or death due to any cause. In patients who did not receive any systemic treatment within 3 months from diagnosis, failure of the first systemic treatment was considered to be an event for PFS. The OS and PFS were estimated by using the Kaplan-Meier method. The Cox proportional hazards model was used to determine the significance of multiple variables. Factors were analyzed in a univariate analysis, and all factors with P < .1 were retained in the multivariate model. All P values were 2-sided, and P < .05 was considered to indicate statistical significance. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).59
Results
Patient characteristics
Ninety patients with EBL were identified. Five patients had primary HHV8-positive EBL (ie, PEL), and 64 patients had primary HHV8-negative EBL. The HHV8 status in body cavity effusions could not be evaluated in the remaining 21 patients (Figure 1).
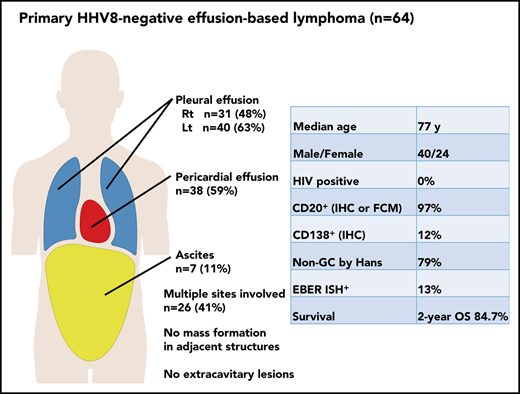
The characteristics of the patients with PEL and primary HHV8-negative EBL in our series of EBL are listed in Table 1. Because most patients with EBL were negative for HHV8, we focused on primary HHV8-negative EBL in the subsequent analysis. Among patients with primary HHV8-negative EBL, there was a predominance of male patients, with a median age of 77 years (range, 57-98 years) at presentation. Sixty-one patients (95%) were aged ≥60 years. None of the patients was HIV positive. Of 61 patients tested for hepatitis C virus (HCV), only 1 patient (2%) was positive for HCV antibodies. Body cavity effusions with lymphoma cells most commonly arose within the pleural cavity (observed in 49 of the 64 patients with primary HHV8-negative EBL [77%]), followed by the pericardial cavity (38 patients [59%]) and peritoneal cavity (7 patients [11%]) (Table 2). Among the 49 patients with pleural effusion, 22 (34%) had bilateral pleural effusion, 9 (14%) had right-sided pleural effusion only, and 18 (28%) had left-sided pleural effusion only. Multiple body cavities were involved in 26 patients at diagnosis, and 4 patients had pleural, pericardial, and peritoneal effusions.
Clinical features of primary HHV8-negative EBL and PEL
| Clinical characteristics . | Primary HHV8-negative EBL . | PEL . |
|---|---|---|
| Total, n | 64 | 5 |
| Age, y | ||
| Median | 77 | 77 |
| Range | 57-98 | 38-99 |
| Sex, male/female, n | 40/24 | 4/1 |
| ECOG performance status score 0-1 | 33/62 (53) | 4/5 (80) |
| B symptoms | 8/61 (13) | 2/4 (50) |
| Fever | 4/61 (7) | 1/4 (25) |
| Night sweats | 2/61 (3) | 0/4 (0) |
| Weight loss | 2/61 (3) | 1/4 (25) |
| Dyspnea | 46/64 (72) | 4/5 (80) |
| Cough | 11/64 (17) | 0/5 (0) |
| Abdominal distention | 3/64 (5) | 1/5 (20) |
| Pedal edema | 17/64 (27) | 1/5 (20) |
| IPI score 3-5 | 48/61 (79) | 3/5 (60) |
| Hemoglobin level ≤10 g/dL | 11/64 (17) | 2/5 (40) |
| Elevated serum lactate dehydrogenase level | 42/63 (67) | 3/5 (60) |
| Serum creatinine level ≥1.5 mg/dL | 11/62 (18) | 1/5 (20) |
| Serum C-reactive protein level >5.0 mg/dL | 11/61 (18) | 1/5 (20) |
| Serum sIL2-R level >5000 U/L | 4/56 (7) | 0/5 (0) |
| HIV antibody positive | 0/58 (0) | 2/4 (50) |
| HBs antigen positive | 0/62 (0) | 0/5 (0) |
| HCV antibody positive | 1/61 (2) | 1/5 (17) |
| History of body cavity fluids | 12/60 (20) | 1/5 (17) |
| Underlying medical condition leading to fluid retention | 30/64 (47) | 2/5 (40) |
| Cirrhosis | 2/64 (3) | 0/5 (0) |
| Myocardial infarction/angina pectoris | 7/64 (10) | 1/5 (20) |
| Arrhythmia | 17/64 (27) | 1/5 (20) |
| Renal dysfunction | 7/64 (11) | 1/5 (20) |
| Hypothyroidism | 5/64 (8) | 0/5 (0) |
| Clinical characteristics . | Primary HHV8-negative EBL . | PEL . |
|---|---|---|
| Total, n | 64 | 5 |
| Age, y | ||
| Median | 77 | 77 |
| Range | 57-98 | 38-99 |
| Sex, male/female, n | 40/24 | 4/1 |
| ECOG performance status score 0-1 | 33/62 (53) | 4/5 (80) |
| B symptoms | 8/61 (13) | 2/4 (50) |
| Fever | 4/61 (7) | 1/4 (25) |
| Night sweats | 2/61 (3) | 0/4 (0) |
| Weight loss | 2/61 (3) | 1/4 (25) |
| Dyspnea | 46/64 (72) | 4/5 (80) |
| Cough | 11/64 (17) | 0/5 (0) |
| Abdominal distention | 3/64 (5) | 1/5 (20) |
| Pedal edema | 17/64 (27) | 1/5 (20) |
| IPI score 3-5 | 48/61 (79) | 3/5 (60) |
| Hemoglobin level ≤10 g/dL | 11/64 (17) | 2/5 (40) |
| Elevated serum lactate dehydrogenase level | 42/63 (67) | 3/5 (60) |
| Serum creatinine level ≥1.5 mg/dL | 11/62 (18) | 1/5 (20) |
| Serum C-reactive protein level >5.0 mg/dL | 11/61 (18) | 1/5 (20) |
| Serum sIL2-R level >5000 U/L | 4/56 (7) | 0/5 (0) |
| HIV antibody positive | 0/58 (0) | 2/4 (50) |
| HBs antigen positive | 0/62 (0) | 0/5 (0) |
| HCV antibody positive | 1/61 (2) | 1/5 (17) |
| History of body cavity fluids | 12/60 (20) | 1/5 (17) |
| Underlying medical condition leading to fluid retention | 30/64 (47) | 2/5 (40) |
| Cirrhosis | 2/64 (3) | 0/5 (0) |
| Myocardial infarction/angina pectoris | 7/64 (10) | 1/5 (20) |
| Arrhythmia | 17/64 (27) | 1/5 (20) |
| Renal dysfunction | 7/64 (11) | 1/5 (20) |
| Hypothyroidism | 5/64 (8) | 0/5 (0) |
Unless otherwise indicated, data are n/N (%).
ECOG, Eastern Cooperative Oncology Group; HBs, hepatitis B virus surface; IPI, International Prognostic Index; sIL2-R, soluble interleukin 2 receptor.
Site of involvement in primary HHV8-negative EBL
| Variable . | No. of patients (%) . |
|---|---|
| Site of involvement | |
| Right pleural effusion | 31 (48) |
| Left pleural effusion | 40 (63) |
| Pericardial effusion | 38 (59) |
| Ascites | 7 (11) |
| Single body cavity lesion | 38 (59) |
| Bilateral pleural effusion | 5 (8) |
| Right pleural effusion | 5 (8) |
| Left pleural effusion | 13 (20) |
| Pericardial effusion | 12 (19) |
| Ascites | 3 (5) |
| Multiple body cavity lesion | 26 (41) |
| Bilateral pleural effusion + pericardial effusion | 13 (20) |
| Left pleural effusion + pericardial effusion | 5 (8) |
| Right pleural effusion + pericardial effusion | 4 (6) |
| Bilateral pleural effusion + pericardial effusion + ascites | 4 (6) |
| Variable . | No. of patients (%) . |
|---|---|
| Site of involvement | |
| Right pleural effusion | 31 (48) |
| Left pleural effusion | 40 (63) |
| Pericardial effusion | 38 (59) |
| Ascites | 7 (11) |
| Single body cavity lesion | 38 (59) |
| Bilateral pleural effusion | 5 (8) |
| Right pleural effusion | 5 (8) |
| Left pleural effusion | 13 (20) |
| Pericardial effusion | 12 (19) |
| Ascites | 3 (5) |
| Multiple body cavity lesion | 26 (41) |
| Bilateral pleural effusion + pericardial effusion | 13 (20) |
| Left pleural effusion + pericardial effusion | 5 (8) |
| Right pleural effusion + pericardial effusion | 4 (6) |
| Bilateral pleural effusion + pericardial effusion + ascites | 4 (6) |
Pathologic findings
Pathologic findings of patients with primary HHV8-negative EBL were evaluated. The detailed characteristics of lymphoma cells are described in Table 3. For central pathology review, stained and/or unstained slides or pathologic images were available for 45 patients with primary HHV8-negative EBL. All the patients had a large cell centroblastic morphology. All the patients expressed pan B-cell antigens (CD19, CD20, or CD79a), and 97% (62 of 64) expressed CD20 on flow cytometric analysis or immunohistochemical studies. In addition, flow cytometric analysis revealed immunoglobulin light-chain restriction in 70% of the patients (ie, 35 of 50 tested patients). Epstein-Barr virus–encoded small RNA in situ hybridization revealed positive results in 13% of patients. The Ki-67 proliferation index was assessed in 35 patients, and the median Ki-67 proliferation index was 73.5% (range, 3%-93%). The cell-of-origin classification based on the Hans algorithm was evaluated in 38 patients whose immunophenotype in cell block specimens was available; 30 patients (79%) had a non–germinal center B-cell phenotype, and 8 patients (21%) had a germinal center B-cell phenotype. On conventional karyotyping, 35 (71%) of 49 patients carried a complex karyotype. Moreover, on fluorescent in situ hybridization, translocations involving MYC, BCL2, and BCL6 were detected in 7 (19%) of 36, 1 (17%) of 6, and 2 (22%) of 9 patients, respectively. Only 1 patient had concurrent translocations involving MYC, BCL2, and BCL6, and none of the patients had double translocation.
Pathologic features of 64 patients with primary HHV8-negative EBL
| Variable . | Immunohistochemistry . | Flow cytometry . |
|---|---|---|
| CD3 | 1/35 (3) | 2/48 (4) |
| CD5 | 2/20 (10) | 14/51 (27) |
| CD10 | 5/41 (12) | 11/51 (22) |
| CD19 | — | 46/52 (88) |
| CD20 | 49/53 (92) | 50/53 (94) |
| CD30 | 1/32 (3) | 4/35 (11) |
| CD38 | 8/32 (25) | 6/11 (55) |
| CD79a | 26/28 (93) | — |
| CD138 | 4/34 (12) | 0/6 (0) |
| BCL2 | 8/9 (89) | — |
| BCL6 | 14/36 (39) | — |
| MUM1 | 27/38 (71) | — |
| κ+/λ- | — | 23/50 (46) |
| κ-/λ+ | — | 12/50 (24) |
| κ-/λ- | — | 12/50 (24) |
| EBER ISH | 6/45 (13) | — |
| Ki-67 proliferation index (N = 35), % | ||
| Median | 73.5 | — |
| Range | 3-93 | — |
| Cell-of-origin classification according to the Hans algorithm | ||
| Germinal center phenotype | 8/38 (21) | — |
| Non–germinal center phenotype | 30/38 (79) | — |
| BCL2/IGH (FISH) | 1/6 (17) | — |
| BCL6/IGH (FISH) | 2/9 (22) | — |
| MYC rearrangement (FISH) | 7/36 (19) | — |
| Variable . | Immunohistochemistry . | Flow cytometry . |
|---|---|---|
| CD3 | 1/35 (3) | 2/48 (4) |
| CD5 | 2/20 (10) | 14/51 (27) |
| CD10 | 5/41 (12) | 11/51 (22) |
| CD19 | — | 46/52 (88) |
| CD20 | 49/53 (92) | 50/53 (94) |
| CD30 | 1/32 (3) | 4/35 (11) |
| CD38 | 8/32 (25) | 6/11 (55) |
| CD79a | 26/28 (93) | — |
| CD138 | 4/34 (12) | 0/6 (0) |
| BCL2 | 8/9 (89) | — |
| BCL6 | 14/36 (39) | — |
| MUM1 | 27/38 (71) | — |
| κ+/λ- | — | 23/50 (46) |
| κ-/λ+ | — | 12/50 (24) |
| κ-/λ- | — | 12/50 (24) |
| EBER ISH | 6/45 (13) | — |
| Ki-67 proliferation index (N = 35), % | ||
| Median | 73.5 | — |
| Range | 3-93 | — |
| Cell-of-origin classification according to the Hans algorithm | ||
| Germinal center phenotype | 8/38 (21) | — |
| Non–germinal center phenotype | 30/38 (79) | — |
| BCL2/IGH (FISH) | 1/6 (17) | — |
| BCL6/IGH (FISH) | 2/9 (22) | — |
| MYC rearrangement (FISH) | 7/36 (19) | — |
Unless otherwise indicated, data are n/N (%).
EBER ISH, Epstein-Barr virus–encoded small RNA in situ hybridization; FISH, fluorescent in situ hybridization.
Initial treatment and response
Systemic therapy was initiated within 3 months after the diagnosis in 56 patients. Of these 56 patients, 48 (86%) received first-line systemic therapy with combination chemotherapy of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or a CHOP-like regimen. Among the remaining 8 patients, rituximab alone, rituximab with etoposide, prednisolone alone, and hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) were administered to 4, 2, 1, and 1 patient, respectively. In the 56 patients treated with systemic therapy, the overall response rate was 95%, with 73% of the patients achieving CR. Twelve patients achieved PR. One patient who achieved PR was examined for cytology of residual effusion, and it was positive for residual lymphoma. There was no significant difference in CR rates between patients treated with anthracycline-containing regimens and those receiving nonanthracycline-containing systemic treatments. Of the remaining 8 patients, 2 were transferred to another hospital within 1 week after diagnosis; 1 failed to achieve a response after drainage alone and died within 1 month following diagnosis. The clinical courses of the remaining 5 patients are described in supplemental Table 1. Five patients (4 patients treated with effusion drainage alone and 1 patient who had no treatment, including drainage, because of spontaneous regression) did not receive systemic treatment within 3 months after diagnosis. All of the 5 patients achieved a response, including 3 who achieved CRs; however, 2 of them eventually relapsed at 4 and 29 months after diagnosis. One who relapsed achieved a CR by the second drainage alone and died at 22 months without evidence of lymphoma relapse. Three patients did not experience disease progression after successful drainage therapy or spontaneous regression for a median of 24 months (range, 5-147 months).
Survival and prognostic factors
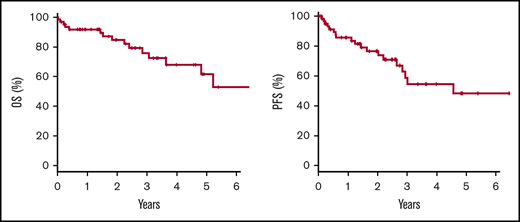
Over a median follow-up of 25 months (range, 0-147 months), the 2-year OS and PFS rates were 84.7% (95% confidence interval, 71.3-92.1) and 73.8% (95% confidence interval, 58.7-84.1), respectively (Figure 2). Eighteen patients showed disease progression after achieving a response at a median of 9.7 months (range, 1.0-48.2 months) (Table 4). In 12 patients, body cavity effusion was observed at relapse with or without mass formation. The same body cavity effusion was the only site of relapse in 8 patients. Mass formation was observed in 8 patients at the time of relapse: in the central nervous system (CNS) in 3 patients, and in the adrenal glands, liver, kidneys, muscle, and adjacent pleura in 1 patient each. None of the patients who experienced CNS relapse received CNS prophylaxis. In terms of treatment at relapse, 2 patients were managed with drainage alone, and 1 of them who had been treated with drainage alone as an initial therapy achieved CR (patient number 2, supplemental Table 1). Another patient who had been treated with CHOP with rituximab as initial treatment did not respond to drainage alone and died of lymphoma. Of the remaining 15 patients whose treatment at relapse was reported, cytotoxic chemotherapy, rituximab alone, radiotherapy, and prednisolone alone were used for relapsed disease in 10, 1, 3, and 1 patient. Median survival time after relapse was 13 months (range, 2-73 months). Sixteen patients died, with the causes of death being lymphoma in 12 patients, chronic renal failure in 1 patient, and unknown in 3 patients. These 3 patients had no evidence of primary HHV8-negative EBL relapse at the time of death.
Kaplan-Meier estimates. Two-year OS was 84.7%, and PFS was 73.8% in the 64 patients with primary HHV8-negative EBL.
Kaplan-Meier estimates. Two-year OS was 84.7%, and PFS was 73.8% in the 64 patients with primary HHV8-negative EBL.
Site of progression after achievement of a response in those with primary HHV8-negative EBL
| Variable . | N = 18/64 . | No. of deaths . |
|---|---|---|
| Body cavity effusion | 12 (67%) | 8 |
| Effusion at the same site alone | 8 | 6 |
| Effusion at different sites | 2 | 0 |
| Presence of tumor mass (intracavitary or extracavitary) | 2 | 2 |
| Tumor mass without body cavity effusion | 6 (34%) | 2 |
| Variable . | N = 18/64 . | No. of deaths . |
|---|---|---|
| Body cavity effusion | 12 (67%) | 8 |
| Effusion at the same site alone | 8 | 6 |
| Effusion at different sites | 2 | 0 |
| Presence of tumor mass (intracavitary or extracavitary) | 2 | 2 |
| Tumor mass without body cavity effusion | 6 (34%) | 2 |
Results of the univariate and multivariate analyses of the risk factors for PFS are listed in supplemental Table 2. In the multivariate analysis, Eastern Cooperative Oncology Group performance status ≥2, age ≥70 years, and presence of ascites were independent risk factors for PFS.
Discussion
This analysis is the first retrospective study, to the best of our knowledge, regarding the characteristics and prognosis of primary HHV8-negative EBL, a rare large B-cell lymphoma that arises in body cavity effusion without mass formation. Although primary HHV8-negative EBL has been reported in several single case reports and small case series, it has not been defined in the current lymphoma classification. The results of the current study revealed that primary HHV8-negative EBL arose in HIV-negative elderly patients, and that lymphoma cells were positive for mature B-cell markers and negative for plasma cell markers in most cases. A majority of the patients received anthracycline-based systemic chemotherapy, and the response rate was >90%. The 2-year overall survival rate was 84.7%, higher than that observed for similarly aged patients with nodal diffuse large B-cell lymphoma (DLBCL).60
Because both PEL and primary HHV8-negative EBL are very rare diseases, their relative prevalence has not been precisely determined. In the current study in Japan, the number of patients with primary HHV8-negative EBL was ∼12 times higher than that of patients with PEL. The reason for this might be that the number of HHV8-positive individuals is much smaller in Japan than in western countries, regardless of the HIV seropositivity.61,62 The seroprevalence rate of HHV8 in the general populations in Japan is 1% to 2%, which is significantly lower than that in other countries.62-64 The HHV8 seropositivity in the general populations in sub-Saharan Africa is the highest, at ∼40%. In addition, the seroprevalence is ∼10% in Mediterranean countries; 2% to 4% in northern European, southeast Asian, and Caribbean countries; and 5% to 20% in the United States.62,64
We compared clinicopathologic features of primary HHV8-negative EBL with PEL (Table 5).65-67 Currently, the etiology of primary HHV8-negative EBL remains undetermined. The tendency of fluid retention might be associated with the development of primary HHV8-negative EBL,5 as almost one-half of the patients in the current study had an underlying medical condition that leads to fluid retention. In previous case series and literature reviews regarding primary HHV8-negative EBL, HCV infection was found in ∼30% to 40% of patients, and HCV was suggested to be a possible pathogen.7 However, in the current study, only 1 patient was positive for HCV. Therefore, on the basis of our observation, the association between primary HHV8-negative EBL and HCV infection seems unlikely.
Clinicopathologic characteristics of primary HHV8-negative EBL compared with clinical series of PEL
| Characteristic . | Primary HHV8-negative EBL (current study), n = 64 . | PEL65-67 . |
|---|---|---|
| HHV8 status | Negative | Positive |
| HIV infection | Negative | Positive |
| Median age, y | 77 | 4466 |
| Male | 63% | >95%66 |
| Site of involvement | Single site (59%) | Single site |
| Multiple sites (41%) | ||
| Extracavitary involvement | No | Some |
| Pan B-cell markers (CD19, CD20, CD79a) | Positive | Negative |
| Plasma cell–related markers (CD138) | Usually negative (CD138 positive; 12%) | Positive |
| Prognosis | Favorable | Poor |
| (2-y OS 84.7%) | (1-y OS 30%67 ) |
| Characteristic . | Primary HHV8-negative EBL (current study), n = 64 . | PEL65-67 . |
|---|---|---|
| HHV8 status | Negative | Positive |
| HIV infection | Negative | Positive |
| Median age, y | 77 | 4466 |
| Male | 63% | >95%66 |
| Site of involvement | Single site (59%) | Single site |
| Multiple sites (41%) | ||
| Extracavitary involvement | No | Some |
| Pan B-cell markers (CD19, CD20, CD79a) | Positive | Negative |
| Plasma cell–related markers (CD138) | Usually negative (CD138 positive; 12%) | Positive |
| Prognosis | Favorable | Poor |
| (2-y OS 84.7%) | (1-y OS 30%67 ) |
In the current study with primary HHV8-negative EBL, Epstein-Barr virus-encoded small RNA in situ hybridization was positive in 13% of cases. This number is much smaller than the number of PEL cases whose cells are mostly infected with EBV.68 EBV-positive DLBCL accounts for <5% to 15% of DLBCL cases among Asian patients, and the EBV-positive rate of DLBCL is similar to that of primary HHV8-negative EBL.69,70 EBV-positive DLBCL usually occurs in individuals aged >50 years, and the increased incidence of EBV-positive DLBCL in elderly patients is believed to be associated with immune senescence.69 Because primary HHV8-negative EBL affects elderly people, the proportion of EBV-positive patients was similar to that of DLBCL in this age group.
In the current study, MYC translocation was found in 7 (19%) of 36 patients, and the MYC translocation partner gene was not determined in most of the patients because of the lack of specimens. It was difficult to evaluate the clinical implication of MYC translocation in primary HHV8-negative EBL, because the number of patients was too small.
Almost all the patients with primary HHV8-negative EBL responded to systemic treatment, and its prognosis was clearly favorable compared with that of PEL or DLBCL in similarly aged patients.71 Among patients with primary HHV8-negative EBL, spontaneous regression or cure with drainage alone has been reported.8,11,31,35,36,38,44,51 In our series, 5 patients showed spontaneous regression or a durable response after watchful waiting or drainage alone. However, in the current study, most of the patients underwent immediate treatment with chemotherapy, including CHOP or a CHOP-like regimen with or without rituximab. Considering the results of these studies, evidence that supports the management of primary HHV8-negative EBL without chemotherapy is limited. Therefore, immunochemotherapy should be recommended for DLBCL if the patient is well enough, and effusion drainage alone should be reserved for only certain patient populations such as those who are very elderly or those with severe comorbidities. However, we believe that a certain proportion of patients with HHV8-negative EBL can be managed safely with drainage alone based on our limited observation and previous case reports.11,36,38 Further studies are needed to distinguish patients who require systemic therapy from those who can be managed with effusion drainage alone.
The sites of relapse in patients with primary HHV8-negative EBL exhibited a unique pattern, with more than one-half of the cases of relapse occurring as body cavity effusion. Eight patients developed extracavitary relapse, and notably, they were exclusively at extranodal sites, including the CNS. No patients developed relapse at nodal sites. It therefore seems that lymphoma cells of primary HHV8-negative EBL have a unique tropism to body cavity effusion and other extranodal sites.
To the best of our knowledge, this analysis is the first and largest retrospective study of primary HHV8-negative EBL. However, the study has several limitations. Only Japanese patients were included in this study; hence, the clinicopathologic features of primary HHV8-negative EBL might vary according to ethnic background. In addition, because the median follow-up was relatively short (ie, 25 months), the long-term outcomes of this clinical entity remain unknown. Moreover, genetic alterations could not be assessed in the current study because of the paucity of available samples. The genomic features of primary HHV8-negative EBL have been reported by other groups,72 and these data should be validated in different cohorts. Finally, because this was a retrospective study, possible selection bias may have affected the results, including the response to treatment and prognosis. However, because most published data are from case series with or without literature review, the results of the current retrospective study of 64 patients are the best available data on primary HHV8-negative EBL.
In summary, this is the first retrospective study to determine the characteristics and prognosis of primary HHV8-negative EBL. Primary HHV8-negative EBL is a rare B-cell lymphoma that arises in body cavity effusion without any mass formation, a clinical presentation similar to that of primary HHV8-positive EBL (ie, PEL). Primary HHV8-negative EBL exhibits contrasting clinicopathologic features from those of PEL in that EBL affects HIV-negative elderly patients, and lymphoma cells were positive for mature B-cell markers and negative for plasma cell markers in most cases. Moreover, the majority of the patients with primary HHV8-negative EBL responded to systemic treatment, and their prognosis was favorable compared with that of similarly aged patients with PEL or DLBCL.
Original data may be requested by contacting the corresponding author (Koji Izutsu; e-mail: kizutsu@ncc.go.jp).
The findings of this study were presented, in part, at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 7 December 2014, as well as at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, 17 June 2015.
Acknowledgment
The authors thank the collaborators who provided the case report forms and specimens; the names of their institutions are listed in the Appendix.
Authorship
Contribution: D.K. and K.I. contributed to the study design and participated in the analysis and interpretation of the data; and all authors collected data for the study, critically reviewed the manuscript, approved the final version, and support its publication.
Conflict-of-interest disclosure: D.K. has received honoraria from Eisai, Chugai, Janssen, Sanofi, Kyowa Kirin, Celgene, Asahi KASEI Pharma, Mundipharma, and Mitsubishi Tanabe. M.O. has received scholarship donations from Chugai, Kyowa Kirin, Takeda, and Taiho. K.M. has received honoraria from Janssen, Takeda, and Celgene. G.Y. has received grants from Chugai and honoraria from Chugai, Mundipharma, Bristol Myers Squibb, Celgene, Ono, Daiichi-Sankyo, Eisai, Kyowa Kirin, and Takeda. S.T. has received grants from Chugai and Kyowa Kirin; and honoraria from Janssen, Asahi KASEI Pharma, Eisai, Otsuka Pharma, Novartis, Pfizer, MSD, Astellas Pharma, Mundipharma, Ono, Kyowa Kirin, Chugai, Celgene, Mochida Pharma, Bristol Myers Squibb, Dainihon Sumitomo, Fujimoto Pharma, and Sanofi. S.C. has received honoraria from Thyas Co, Kyowa Kirin, Astellas Pharma, Ono, Sanofi, Chugai, and Takeda. K.I. has received grants from Chugai; and honoraria from Chugai, Celgene, Eisai, Novartis, Abbvie, Janssen, Kyowa Kirin, Takeda, MSD, AstraZeneca, FUJIFILM Toyama Chemical, Ono, Nihon Mediphysics, Dainihon Sumitomo, Bayer, HUYA Japan, Bristol Myers Squibb, Mundipharma, Otsuka, Daiichi Sankyo, Astellas, and Asahi KASEI Pharma. The remaining authors declare no competing financial interests.
Correspondence: Koji Izutsu, Department of Hematology, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: kizutsu@ncc.go.jp.
Appendix: case report forms and specimens
The case report forms and specimens were received from collaborators at the following institutions: Japanese Red Cross Asahikawa Hospital, Tenshi Hospital, Hokkaido University Hospital, Niigata University Medical & Dental Hospital, Niigata Prefectural Central Hospital, Ibaraki Prefectural Central Hospital, Tsuchiura Kyodo General Hospital, Gunma Prefectural Cancer Center, Jichi Medical University Hospital, Saitama Medical Center, Jichi Medical University, International Medical Center, Saitama Medical University, Saitama Medical Center, Saitama Medical University, Saitama Red Cross Hospital, Kawaguchi Municipal Medical Center, Kameda Medical Center, NTT Medical Center Tokyo, Tokyo Metropolitan Geriatric Hospital, Showa General Hospital, Ome Municipal General Hospital, Toranomon Hospital, Yokohama Municipal Citizen’s Hospital, Kanagawa Cancer Center, St. Marianna University School of Medicine Hospital, Tokai University Hospital, Toyama University Hospital, Toyama City Hospital, University of Fukui Hospital, Fujita Health University Hospital, Nishio Municipal Hospital, Japanese Red Cross Nagoya Daini Hospital, National Hospital Organization Nagoya Medical Center, Osaka University Hospital, PL Hospital, Kitano Hospital, National Hospital Organization Osaka Minami Medical Center, Kinan Hospital, Hyogo Cancer Center, Nishiwaki City Hospital, Kaneda Hospital, Okayama Medical Center, Fukuyama City Hospital, Matsuyama Red Cross Hospital, Japanese Red Cross Kochi Hospital, Kochi Medical School Hospital, National Hospital Organization Kyushu Cancer Center, Fukuoka Higashi Medical Center, Saiseikai Fukuoka General Hospital, Iizuka Hospital, Kurume University Hospital, Saga-ken Medical Centre Koseikan, Nagasaki University Hospital, and Oita University Hospital.
References
Author notes
The full-text version of this article contains a data supplement.