Key Points
HLA-specific alloantibodies can be maintained despite profound CD19+ cell aplasia, likely due to production by CD19− plasma cells.
Introduction
Antibodies against foreign HLA can be pathogenic in several clinical contexts, most notably in transplantation.1 In organ and hematopoietic stem cell transplant, HLA antibodies can cause graft rejection.1,2 Preexisting HLA antibodies limit the availability of compatible donors and, following transplantation, de novo, donor-specific HLA antibodies result in acute and chronic allograft rejection.1,3 HLA antibodies can also cause refractoriness to platelet transfusion and are implicated in transfusion-related acute lung injury.4 Thus, much attention has been devoted to developing effective therapeutics to prevent and eradicate HLA antibodies.
Although there have been significant advances in the field of immunosuppression, we remain unable to reliably eliminate HLA antibodies.5,6 Corticosteroid, plasmapheresis, IV immunoglobulin, and rituximab have been insufficient to eradicate these antibodies, prompting the development of several new agents.7 Greater insight into the biology underlying formation and maintenance of HLA antibodies is critical to therapy development and understanding their mechanisms of action and failure.
HLA alloantibodies may form following blood transfusion, organ/tissue transplantation, or pregnancy.1 B cells that recognize foreign HLA activate and differentiate into memory B cells (MBCs) and, ultimately, antibody-secreting plasma cells (PCs). MBCs are long lived and can regenerate or differentiate into PCs.7 Most early PCs die within weeks of an initial immune response. However, some PCs may be spared, becoming long-lived PCs (LLPCs) that survive for years to decades.8,9 The factors that determine whether LLPCs are formed are incompletely understood, particularly in humans.7 MBCs alone may be sufficient to sustain long-lived responses.10 Alternatively, antibody responses may be maintained by the combination of MBCs and LLPCs.11
An incomplete understanding of the alloantibody response has hampered the development of therapies that effectively eradicate HLA antibodies. Although B-cell depletion using rituximab can reduce alloantibody titers, it fails to fully eradicate these antibodies because it does not completely eliminate B cells, especially MBCs, or because it does not directly target PCs.1,5,6,12-18 Lack of PC targeting may not be a problem if the PCs are short lived. If LLPCs are formed, however, these could maintain antibodies for years and would need to be directly eliminated in addition to elimination of MBCs.
It has not yet been formally established that the immune response to allo-HLA generates LLPCs. To address this, we present a case of iatrogenic immunosuppression that severs the link between B cells and PCs by virtue of targeting CD19 via chimeric antigen receptor (CAR) T-cell therapy.19,20 CD19 is a pan–B-cell antigen, but PCs can be divided into CD19+ and CD19− populations, the latter thought to contain LLPCs.21,22 Successful response to CART-19 therapy is often accompanied by sustained CD19+ cell aplasia as an expected side effect that can last for years.19,23 We have shown complete CD19+ cell depletion from lymphoid compartments, including bone marrow, spleen, lymph nodes, and organ-specific lymphoid aggregates.24 B cells as well as CD19+ PCs are lost, leaving only CD19− PCs. This presents an opportunity to evaluate the role of B cells vs LLPCs in the maintenance of antibody responses. Indeed, protective vaccine-induced antibodies were found to be maintained during CD19+ cell aplasia, indicating CD19− PCs are sufficient for maintaining these antibodies.23,24 To determine if HLA antibodies can also be maintained by LLPCs independent of the B-cell compartment, we screened ∼30 CART19-treated patients who experienced sustained CD19+ cell aplasia for preexisting HLA antibodies and identified only 1 subject with anti-HLA antibodies for investigation.
Methods
B-cell measurement
B cells were measured by flow cytometry, and B-cell aplasia was defined as <1% CD19+CD20+ B cells of peripheral blood mononuclear cells. However, the frequency of circulating B cells in this patient was 0% at all timepoints except for month 22 (0.1%).
Antibody measurements
Total immunoglobulins were measured by immunoassays in the clinical laboratory at the Hospital of the University of Pennsylvania. Vaccine/pathogen-associated IgG levels were measured using enzyme-linked immunosorbent assays according to manufacturers’ instructions: anti-measles IgG (Serion Immunodiagnostica), anti-mumps IgG (Calbiotech), anti-rubella IgG (Phoenix Pharmaceuticals), anti-Streptococcus pneumoniae capsular polysaccharide IgG (The Binding Site), anti-Haemophilus influenzae type B capsular polysaccharide IgG (The Binding Site), anti-tetanus toxoid IgG (The Binding Site). HLA-specific antibodies were measured using the HLA class II single-antigen bead assay (One λ).
Results and discussion
The patient is a 57-year-old woman with a 7-year history of stage IV follicular lymphoma. Her course included 2 remissions with rituximab-based regimens. Upon relapse, she was treated with ibrutinib, a brief trial of an anti-CD22 immunoconjugate, which was discontinued due to neutropenia, the phosphoinositide-3-kinase inhibitor, idelalisib, and radiation therapy with partial response. She was enrolled in a phase 2A trial of CART-19 at the University of Pennsylvania (Penn; ClinicalTrials.gov, #NCT0203083420 ). Following lymphodepletion with mediastinal radiation therapy (400 cGy mantle field) and cyclophosphamide (1 g/m2), she received an infusion of 1.79 × 108 autologous CAR T cells. She is currently 5 years post-CAR T-cell infusion and remains in remission and clinically well. Prior to receiving CAR T cells, she had a history of red blood cell and platelet transfusions, no history of transplant, and no reported history of pregnancy.
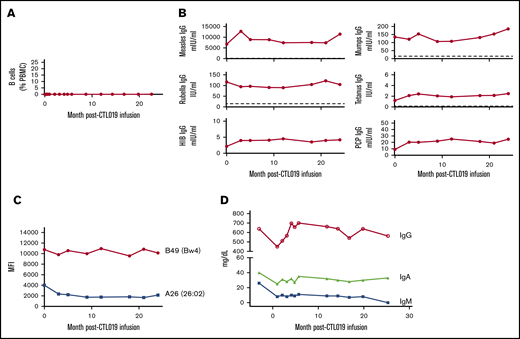
In addition to tumor remission, the patient experienced sustained B-cell aplasia following CART infusion. CD19+ cells remained undetectable for at least 2 years in the blood (Figure 1A) and in a 3-month bone marrow sample (not shown). Preexisting immunoglobulin G (IgG) against Tetanus toxoid, Measles, Mumps, Rubella, Hinfluenzae type B, and Spneumoniae was stable through the 2-year testing period (Figure 1B). Diphtheria IgG was undetectable pretreatment and remained undetectable (not shown). A screen for HLA-directed antibodies in the pretreatment serum demonstrated HLA class I reactivity only. Evaluation using single-antigen beads showed antibodies to HLA-A26 (26:02) and to the Bw4 epitope, with the highest mean fluorescence intensity of 10 761 against HLA-B49. These HLA-specific antibodies remained stable for 2 years despite the absence of B cells (Figure 1C). Total serum IgG and IgA were also stable. However, her serum IgM, which was 26 mg/dL 3 months prior to CART infusion, declined further, becoming undetectable at month 25 (Figure 1D), and remains undetectable to date. The lack of adequate bone marrow specimens in the patient limited direct evaluation of LLPCs; however, our prior studies in CART-19–treated subjects support the persistence of CD19− PCs despite deep and durable B-cell aplasia.
B-cell and antibody levels following CD19-directed CAR T-cell treatment. (A) Immediately prior to and following CAR T-cell infusion, B cells were measured in the blood by flow cytometry. Levels of protective IgG (B), HLA IgG (C), and total immunoglobulins (D) were measured prior to and at indicated times following CAR T-cell infusion. Dotted lines represent protective antibody levels. HIB, Hinfluenzae type B; MFI, mean fluorescence intensity; PBMC, peripheral blood mononuclear cells; PCP, pneumococcal capsular polysaccharide.
B-cell and antibody levels following CD19-directed CAR T-cell treatment. (A) Immediately prior to and following CAR T-cell infusion, B cells were measured in the blood by flow cytometry. Levels of protective IgG (B), HLA IgG (C), and total immunoglobulins (D) were measured prior to and at indicated times following CAR T-cell infusion. Dotted lines represent protective antibody levels. HIB, Hinfluenzae type B; MFI, mean fluorescence intensity; PBMC, peripheral blood mononuclear cells; PCP, pneumococcal capsular polysaccharide.
This case represents the clearest evidence in humans that anti-HLA antibodies can be maintained by LLPCs, independently of the B-cell compartment. Similar findings are reported after rituximab therapy.25 However, rituximab does not achieve complete B-cell eradication, leaving open the question of B-cell–independent antibody maintenance.12,13,15-18
Total IgG levels were also stable in this patient as previously observed. This suggests that most circulating IgG is normally derived from LLPCs, or it may imply that IgG production and/or turnover adjust to maintain a constant level even after depletion of CD19+ cells. Hill et al have shown that some patients treated with CART-19 experience modest declines in serum total IgG; despite this, many preexisting antigen-specific protective antibodies are maintained.23,24 In contrast, the decline in IgM to eventually undetectable concentrations in this patient is consistent with the idea that this isotype is primarily produced by relatively short-lived PCs and plasmablasts.
In summary, these findings clearly implicate LLPCs in the HLA-antibody response and suggest that B-cell targeted therapies (eg, CD20 or CD19-targeted therapies) are unlikely to be sufficient for eliminating alloantibodies. Effective eradication is expected to require a combination of B-cell– and PC-directed therapies. For antibody-mediated autoimmune diseases, however, CD19-targeted immunotherapy may be sufficient where autoreactive MBCs are thought to maintain a short-lived PC compartment and LLPCs are not implicated.
Data-sharing requests should be sent to the corresponding author, Vijay G. Bhoj (vbhoj@pennmedicine.upenn.edu).
Acknowledgments
The authors thank Brooke Marcellus and Caroline Kneib for assistance with measurement of HLA antibodies and members of the TCSL laboratory at the Center for Cellular Immunotherapy at Penn for measurement of B cells.
Authorship
Contribution: Z.Z. assisted with antibody measurements and editing the report; V.G.B. conceived the study and wrote the report; S.F.L. edited the report and directed the processing and biobanking of the patient sera and cells and the assessment of B cells by flow cytometry; D.M. assisted with conceptualization, data interpretation, and editing the report; S.J.S. conducted the CAR T-cell trial involving the subject, cared for the patient, and edited the report; and M.C.M. assisted with conceptualization and edited the report.
Conflict-of-interest disclosure: V.G.B. is an inventor of CAR-T–related intellectual property (IP) assigned to Tmunity, Cabaletta Bio by the University of Pennsylvania. S.F.L. is an inventor of CAR-T–related IP assigned to Novartis by the University of Pennsylvania, receives research support from Novartis and Tmunity, and receives consultancy fees from Gilead. S.J.S. receives consultancy fees, honoraria, and membership on an entity’s Board of Directors or advisory committees and research funding from Celgene; consultancy and honoraria from Dava Oncology; honoraria and research funding from Genentech; membership on an entity’s Board of Directors or advisory committees from Gilead, Pfizer; consultancy, honoraria, and research funding from Merck; honoraria, membership on an entity’s Board of Directors or advisory committees, and research funding from Novartis; consultancy, honoraria, and membership on an entity’s Board of Directors or advisory committees from Nordic Nanovector; and honoraria from OncLive, Physician’s Education Source, LLC. M.C.M. is an inventor of CAR-T–related IP assigned to Novartis by the University of Pennsylvania and receives research support from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Vijay G. Bhoj, University of Pennsylvania, South Tower Pavilion, 8-111, 3400 Civic Center Blvd, Philadelphia, PA, 19104; e-mail: vbhoj@pennmedicine.upenn.edu.