Key Points
CARTs enable bioorthogonal and efficient transfection of resting primary human NK cells while preserving cell phenotype and function.
CARTs enable production of highly cytotoxic primary human CAR NK cells.
Abstract
Chimeric antigen receptor (CAR) natural killer (NK) cells are an emerging cell therapy with promising results in oncology trials. However, primary human NK cells are difficult to transfect, hampering both mechanistic studies and clinical applications of NK cells. Currently, NK cell CAR modification relies on viral vectors or cell activation. The former raises cost and tolerability issues, while the latter alters NK cell biology. Here, we report that readily synthesized and inexpensive nonviral charge-altering releasable transporters (CARTs) efficiently transfect primary human NK cells with messenger RNA without relying on NK cell activation. Compared with electroporation, CARTs transfect NK cells more efficiently, better preserve cell viability, and cause minimal reconfiguration of NK cell phenotype and function. We use CARTs to generate cytotoxic primary anti-CD19 CAR NK cells, demonstrating this technology can drive clinical applications of NK cells. To our knowledge, CARTs represent the first efficacious transfection technique for resting primary human NK cells that preserves NK cell phenotype and can enable new biological discoveries and therapeutic applications of this understudied lymphocyte subset.
Introduction
Natural killer (NK) cells are innate lymphocytes that execute the rapid elimination of neoplastic and virus-infected cells.1,2 Upon activation through a combinatorial array of germ line–encoded inhibitory and activating receptors, NK cells can directly kill their targets via targeted release of perforin- and granzyme-containing granules, as well as coordinate the downstream immune response by secreting proinflammatory cytokines like interferon γ (IFN-γ) and tumor necrosis factor α (TNFα).3,4 The rapid and robust cytotoxicity of NK cells makes them excellent assets for cancer immunotherapy, a view recently bolstered by the use of chimeric antigen receptor (CAR) NK cells in treating CD19+ lymphoid cancers with high efficacy and low toxicity.5-13
Despite the potential of NK cells in cancer immunotherapy, NK cells are notoriously difficult to manipulate, impeding both a deeper understanding of fundamental NK cell biology and clinical applications.14-18 Most clinical trials involving genetically modified NK cells use viral transduction (registered at www.clinicaltrials.gov as #NCT02944162, #NCT02892695, #NCT03056339, and #NCT03579927).5 Although viral transduction enables stable transgene expression, it is costly and laborious and induces robust NK cell activation and apoptosis by triggering innate nucleic acid sensors, limiting its practicality for mechanistic studies of NK cell biology.8,15,19-24 Furthermore, in clinical applications, viral transduction carries oncogenic potential, requiring the codelivery of caspase-based suicide switches.5,6,25 Most nonviral gene delivery methods, although easier to use for mechanistic studies, are only efficacious for immortalized NK cell lines and not primary NK cells.26-29 The most commonly used nonviral delivery method for primary NK cells is currently electroporation.8,30,31 However, electroporated NK cells generally require cytokine stimulation or expansion on genetically modified feeder cell lines for adequate transfection efficiency and viability postelectroporation,32-36 impeding the study of NK biology by altering cellular physiology and phenotype.37 We therefore sought to develop an efficacious and bioorthogonal transfection strategy for primary NK cells.
Recently, we reported the use of charge-altering releasable transporters (CARTs) for gene delivery.38,39 CARTs are multiblock oligomers consisting of ≥1 lipid block and a charge-altering block.38-45 Differing from persistently charged nonviral vectors, CARTs are initially cationic to complex polyanionic nucleic acids but biodegrade under physiological conditions (pH 7.4) to neutral products, facilitating the release of the anionic cargo.39,44 Here, we demonstrate that CARTs efficiently transfect resting primary human NK cells with messenger RNA (mRNA). We used mass cytometry (CyTOF) and multiple functional assays to show that CART-mediated transfection preserves canonical NK cell phenotype and function. We used this technique to generate robustly cytotoxic human anti-CD19 CAR NK cells, representing a proof of concept for the clinical utility of this technique.
Methods
NK cell isolation and culture
NK cells were purified from cryopreserved peripheral blood mononuclear cells by magnetic bead isolation via negative selection according to the manufacturer’s specifications (Miltenyi Biotec; catalog #130-092-657). Unless noted, NK cells were maintained in complete RPMI + 10% fetal calf serum media without additional cytokines to ensure a resting state. Cell culture was performed at 37°C/5% CO2 in a humidified environment.
CART/mRNA polyplexes
CART O5:N6:A9 (referred to as O5-b-N6:A9 in a previous report38 ), BDK-O7:N7:A13, and D13:A11 were synthesized as previously reported and stored as 2-mM stock solutions in dimethyl sulfoxide at −20°C.38,39 Briefly, the CARTs were synthesized by organic ring-opening polymerization of the respective carbonate and morpholinone monomers using a 1-(3,5-Bis-trifluoromethyl-phenyl)-3-cyclohexyl-thiourea and diazabicycloundecene catalyst system. The polymerizations were performed at room temperature in toluene in a glove-box environment.38 As a sample procedure to prepare the CART/mRNA polyplexes, 0.42 μL of enhanced green fluorescent protein (GFP) mRNA (1 μg/μL) was diluted into 7.26 μL of PBS (pH, 5.5). To this solution was added 0.72 μL of CART O5:N6:A9 (2 mM of dimethyl sulfoxide) to achieve a charge ratio of 10:1 (+/−, assuming all ionizable cationic groups are protonated). After mixing by finger vortex for 15 seconds, the polyplexes were added to cells and incubated for 6 hours in serum-free media.
Lipofection and electroporation
Lipofectamine 2000 (L2000) and L3000 (ThermoFisher) were used according to the manufacturer’s instructions. NK cells were electroporated as previously described.32 The full procedure is described in supplemental Methods. Unless noted, the same final dose of mRNA per well was used for all conditions in all comparative assays.
Flow cytometry
Antibodies used for flow cytometric analyses are listed in supplemental Table 1. The LIVE/DEAD Fixable Yellow Staining Kit (ThermoFisher) was used as a viability stain. Target cells were stained with CellTrace Violet (ThermoFisher) before coculture with NK cells. Two hours after addition of cancer cell lines or cytokine stimulation cocktail, brefeldin, monensin (eBioscience), and anti-CD107a antibody were added, and the cells were incubated for an additional 4 hours. Cells were surface stained for 20 minutes at room temperature, fixed with 2% paraformaldehyde, permeabilized with eBioscience Permeabilization Buffer, and stained intracellularly for 30 minutes at room temperature. Data were acquired on an Aurora flow cytometer (Cytek Biosciences) and analyzed by FlowJo software (version 10.6.1).
CyTOF
NK cells were stained for CyTOF analysis as previously described,37,46 using the panel shown in supplemental Table 2. Bead normalization, bead removal, and debarcoding were performed using the premessa R package. NK cells were gated from debarcoded files according to supplemental Figure 3. Hyperbolic sine-transformed data were used to perform uniform manifold approximation and projection (UMAP) dimensionality reduction. The first 6 principal components were used to calculate the k-nearest neighbors of each cell, from which a shared nearest-neighbors graph was constructed and clustered using the Louvain method for community detection.47 The Simpson Diversity Index was calculated as previously described.48
Results
CARTs transfect resting NK cells more efficiently than commercial reagents
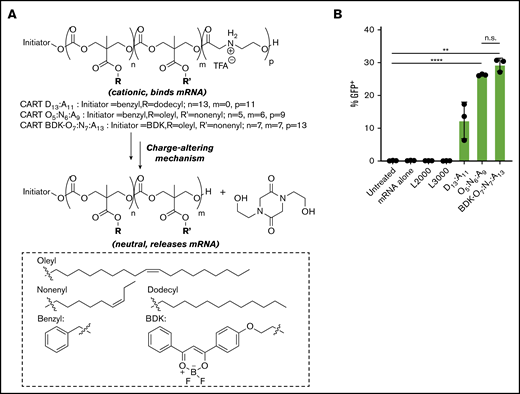
CARTs are synthetic, biodegradable, and dynamic delivery vectors, based on di- or triblock oligomers, consisting of ≥1 lipid block followed by a charge-altering block (Figure 1A).38-41 The charge-altering block is initially polycationic for complexation to polyanionic mRNA but rearranges to neutral diketopiperazine small molecules at a pH of 7.4, facilitating the release of the anionic mRNA while avoiding toxicities associated with persistent cations.38,49,50 These delivery vectors can be readily synthesized in 2 steps from monomers and their chemical composition tuned as dictated by research and clinical needs. The first-generation single-lipid CART D13:A11 has been used for efficient mRNA delivery in vitro (>95% transfection in many cultured cells) and in vivo (Figure 1A).39 However, decreased transfection efficiencies were observed in lymphocytes compared with other cell types, a common trend among transfection reagents.38,51 Through high-throughput screening, we previously discovered that CARTs containing both unsaturated C18 oleyl and C9 nonenyl lipids (CART O5:N6:A9 and CART BDK-O7:N7:A13) resulted in enhanced transfection of T and B lymphocytes, significantly outperforming single-lipid CARTs and commercial reagents.38 We therefore hypothesized that CARTs could serve as a novel delivery system for highly transfection-recalcitrant resting NK cells and facilitate as well as enhance the use of genetically modified NK cells for clinical applications.
CARTs robustly transfect resting NK cells with mRNA. (A) Charge-altering rearrangement and structures of single-lipid and mixed-lipid CARTs. (B) Percentage of transfection of primary resting human NK cells from 3 donors (500 000 cells per well in 100 μL of serum-free RPMI) using L2000, L3000, CART D13:A11, CART O5:N6:A9, or CART BDK-O7:N7:A13. CART/mRNA polyplexes were formulated at a 10:1 charge ratio (+/−, assuming all ionizable cationic groups are protonated). NK cells were incubated with the complexes for 6 hours before analysis by flow cytometry. Error bars represent ±standard deviation. **P < .01, ****P < .0001. n.s., not significant via paired Student t test at the 2-sided P = .05 level.
CARTs robustly transfect resting NK cells with mRNA. (A) Charge-altering rearrangement and structures of single-lipid and mixed-lipid CARTs. (B) Percentage of transfection of primary resting human NK cells from 3 donors (500 000 cells per well in 100 μL of serum-free RPMI) using L2000, L3000, CART D13:A11, CART O5:N6:A9, or CART BDK-O7:N7:A13. CART/mRNA polyplexes were formulated at a 10:1 charge ratio (+/−, assuming all ionizable cationic groups are protonated). NK cells were incubated with the complexes for 6 hours before analysis by flow cytometry. Error bars represent ±standard deviation. **P < .01, ****P < .0001. n.s., not significant via paired Student t test at the 2-sided P = .05 level.
To examine whether CARTs can transfect primary NK cells without activation, we compared the efficacy of single-lipid CART D13:A11 and mixed-lipid CART O5:N6:A9 to deliver mRNA encoding a reporter gene. We first selected firefly luciferase–encoding mRNA, because the firefly luciferase protein can be quantified by bioluminescence imaging. CART/mRNA polyplexes were readily prepared by simply mixing the CARTs and mRNA. The polyplexes were then applied by addition to NK cells in serum-free media. Six hours posttransfection, CART D13:A11– and CART O5:N6:A9–transfected NK cells had ∼20- and ∼50-fold increases in luciferase expression over untreated NK cells, respectively, suggesting efficient cytosolic delivery of the mRNA followed by its translation into functional luciferase (supplemental Figure 1).
To further compare the ability of CARTs and commercial reagents to deliver reporter mRNA into NK cells, we used GFP mRNA to quantify protein expression and the percentage of transfected cells. We again tested single-lipid CART D13:A11 as well as mixed-lipid CARTs O5:N6:A9 or BDK-O7:N7:A13 and compared the delivery efficiency of GFP mRNA with that of commercial transfection reagents L2000 and L3000. Although minimal GFP was detectable in lipofectamine-transfected cells (<1%), ∼10% of NK cells exhibited GFP expression (GFP+) when transfected with CART D13:A11, and 26% and 29% GFP+ NK cells were observed with O5:N6:A9 and BDK-O7:N7:A13 mixed-lipid CARTs, respectively (Figure 1B). This trend is consistent with our previous finding that mixed-lipid CARTs enhance gene delivery into lymphocytes38 and indicated that the mixed-lipid CARTs were the most robust of the transfection systems tested. Therefore, we used mixed-lipid CART O5:N6:A9 or BDK-O7:N7:A13 for all future experiments.
CART-mediated transfection of resting NK cells is efficient and scalable
Next, we optimized and evaluated the scalability of this transfection technique in primary human NK cells using mixed-lipid CART O5:N6:A9. We observed that increasing cell density improved the viability of CART O5:N6:A9–complexed GFP mRNA-transfected cells without significantly affecting transfection efficacy (Figure 2A-B). We used a density of 500 000 NK cells per well for all following experiments, because this density enabled a balance between viability, transfection efficacy, and conservation of reagents. Additionally, we found that replacing serum-free media with serum-containing media after only 2 or 4 hours did not substantially decrease transfection efficacy while improving cell viability (supplemental Figure 2A-B), indicating that shortening the serum-free incubation can preserve viability without sacrificing substantial efficacy.
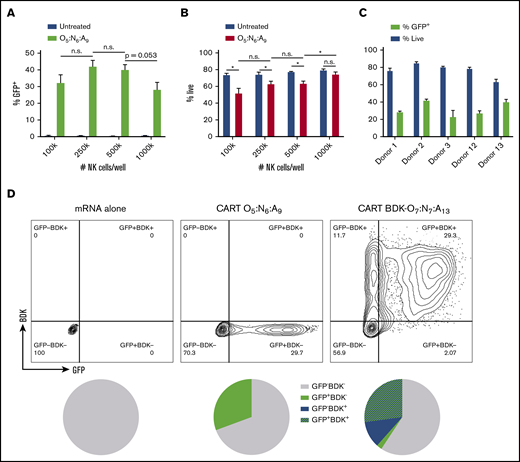
CART transfection of resting NK cells is scalable and selectable. Unless noted, CART/mRNA polyplexes were prepared at a 10:1 (+/−) charge ratio and added to primary resting human NK cells (500 000 cells per well in 100 μL of serum-free RPMI in 96-well plates) and analyzed by flow cytometry after 6 hours. Effect of NK cell density on percentage of GFP+ cells (A) and viability (B). (C) Effect of NK cell donor on CART O5:N6:A9–mediated GFP mRNA transfection. (D) Dot plot of GFP expression vs BDK fluorescence when delivering CART BDK-O7:N7:A13/GFP mRNA polyplexes to primary resting NK cells. Percentage of NK cells (gated for live cells) that were BDK−GFP−, BDK−GFP+, BDK+GFP−, or BDK+GFP+. All measurements were run at least in triplicate. Error bars represent ±standard deviation. *P < .05 by unpaired Student t test with Bonferroni’s correction for multiple testing. n.s., not significant at P = .05.
CART transfection of resting NK cells is scalable and selectable. Unless noted, CART/mRNA polyplexes were prepared at a 10:1 (+/−) charge ratio and added to primary resting human NK cells (500 000 cells per well in 100 μL of serum-free RPMI in 96-well plates) and analyzed by flow cytometry after 6 hours. Effect of NK cell density on percentage of GFP+ cells (A) and viability (B). (C) Effect of NK cell donor on CART O5:N6:A9–mediated GFP mRNA transfection. (D) Dot plot of GFP expression vs BDK fluorescence when delivering CART BDK-O7:N7:A13/GFP mRNA polyplexes to primary resting NK cells. Percentage of NK cells (gated for live cells) that were BDK−GFP−, BDK−GFP+, BDK+GFP−, or BDK+GFP+. All measurements were run at least in triplicate. Error bars represent ±standard deviation. *P < .05 by unpaired Student t test with Bonferroni’s correction for multiple testing. n.s., not significant at P = .05.
We evaluated the extent of interdonor heterogeneity in cell viability and transfection efficacy using the optimized transfection conditions. We treated primary resting NK cells from 5 different human donors with CART O5:N6:A9/GFP mRNA polyplexes. Across the donors, viability ranged between 60% and 85% and transfection efficacy between 20% and 40% (Figure 2C), indicating that CART-mediated mRNA transfection is broadly applicable to human NK cells regardless of donor.
Significantly, NK cells activated and expanded for 7 days on the modified K562 feeder line K562-mbIL15-41BBL52 had nearly double the transfection efficacy of resting NK cells when transfected with CART O5:N6:A9–complexed GFP mRNA (supplemental Figure 2C). However, CART-mediated delivery is the only method for efficient delivery of mRNA to NK cells in the absence of activating cytokines; therefore, we focused our efforts on delivery to resting NK cells, because this is a unique capability of this approach.
Coselection of CART-transfected NK cells
Enrichment for transfected cells often requires cotransfection of a selectable marker that decreases the transfection efficacy of the marker of biological interest.53 We hypothesized that CARTs may represent a versatile method for coselection without the delivery of additional cargo because of their chemical tunability. We therefore transfected NK cells with GFP mRNA using a mixed-lipid CART (BDK-O7:N7:A13) functionalized with a difluoroboron diketonate (BDK) fluorophore.38,40,45 BDK is covalently incorporated into every CART oligomer by using it as the alcohol initiator for the organocatalytic ring-opening polymerization that provides the initiator-lipid-cationic CART vectors.38 We found that CART BDK-O7:N7:A13 had similar transfection efficacy to CART O5:N6:A9 in resting NK cells (Figure 1B) and that a vast majority of GFP+ NK cells were also BDK+ (Figure 2D). These data indicate that CART BDK-O7:N7:A13 represents a robust coselection strategy for mRNA transfection.
CART-mediated mRNA transfection causes minimal changes to NK cell proteomic phenotype
NK cells are a remarkably diverse population of innate lymphocytes, with potentially between 6000 and 30 000 NK cell subsets in an individual.54 Because this diverse repertoire can be rapidly and dramatically modulated by common in vitro interventions, including low-dose (2 ng/mL) interleukin-15 (IL-15),37 we hypothesized that established transfection techniques may alter NK cell proteomic phenotype.55
To define how different transfection techniques affect the NK cell repertoire, we transfected unactivated NK cells with GFP-encoding mRNA using commercial reagent L2000 or CART O5:N6:A9. We also electroporated primary NK cells according to a recently published protocol that demonstrated efficient transfection using a widely available electroporation device (Lonza 4D Nucleofector).32 To match this method and other protocols for electroporating NK cells, we stimulated NK cells with IL-2 for 24 hours before electroporation.32 We found a large discrepancy between the dose of mRNA we used for CART-mediated transfection (31 ng per well) and the dose published for electroporation (10 000 ng per well),30 and therefore, IL-2–stimulated NK cells were electroporated with both doses of mRNA. Additionally, we included NK cells maintained in RPMI with 10% fetal calf serum as a negative control to represent the baseline NK cell repertoire. Specifically, to evaluate surface phenotype, we transfected NK cells with GFP-encoding mRNA via L2000, electroporation, or CART O5:N6:A9 in serum-free media, changed the media in all conditions 6 hours posttransfection, let the cells rest for 12 hours, and then performed flow cytometric analysis for viability and transfection efficacy and CyTOF for NK cell repertoire analysis.
Significantly, between the tested transfection techniques, GFP expression was detected only in the CART-transfected NK cells and the NK cells electroporated with the 322-fold higher dose of mRNA, indicating CART-mediated transfection is several orders of magnitude more efficient in terms of reagent use (Figure 3A). Furthermore, we observed that CART-transfected cells maintained substantially higher viability than electroporated cells (Figure 3A). Nonlinear dimensionality reduction indicated that CART-transfected NK cells were phenotypically similar to untreated NK cells, suggesting minimal off-target effects (Figure 3B). However, electroporated cells were dramatically phenotypically distinct from cells in the remaining conditions (Figure 3B). Hierarchical clustering by mean marker expression did not separate CART-transfected NK cells from untreated cells, whereas the first branch of this clustering separated all electroporated samples (Figure 3C). Electroporated cells across all donors displayed reduced expression of activating NK cell receptors 2B4, NKp46, and NTB-A and increased expression of activation markers CD38, CD69, and HLA-DR, as well as target cell apoptosis–inducing Fas-L (Figure 3C). Notably, there were no consistent changes in mean marker expression in CART-transfected NK cells.
CART transfection outperforms electroporation and causes minimal reconfiguration of NK cell phenotype. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) Flow cytometric analysis of NK cell viability (top) and transfection efficacy (bottom) 18 hours posttransfection with GFP-encoding mRNA. (B) Uniform manifold approximation and projection (UMAP) dimensionality reduction of CyTOF data faceted by treatment condition. (C) Heatmap of mean marker expression, with samples and markers hierarchically clustered. *P < .05, ***P < .001, ****P < .0001 by paired Student t test; all other comparisons were not statistically significant at P = .05 level.
CART transfection outperforms electroporation and causes minimal reconfiguration of NK cell phenotype. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) Flow cytometric analysis of NK cell viability (top) and transfection efficacy (bottom) 18 hours posttransfection with GFP-encoding mRNA. (B) Uniform manifold approximation and projection (UMAP) dimensionality reduction of CyTOF data faceted by treatment condition. (C) Heatmap of mean marker expression, with samples and markers hierarchically clustered. *P < .05, ***P < .001, ****P < .0001 by paired Student t test; all other comparisons were not statistically significant at P = .05 level.
To analyze these changes in NK cell repertoire with more granularity, we clustered the cells using the Louvain method for community detection, identifying 22 clusters in the data set (Figure 4A-B). We found that all clusters present in untreated NK cells were also represented in CART-transfected NK cells (Figure 4C) and that CART-transfected NK cells congruently had a comparable Simpson Diversity Index to untreated cells (supplemental Figure 4). However, >50% of cells in the electroporated conditions belonged to 1 of only 3 clusters (3, 21, and 22; Figure 4C), reflected in a substantially lower Simpson Diversity Index than for untreated cells (supplemental Figure 4). Collectively, these data indicate that electroporation dramatically reconfigures the NK cell repertoire. CART-mediated transfection, remarkably, is a bioorthogonal technique that does not cause off-target phenotypic reconfiguration of NK cells, while yielding superior transfection efficacy.
Louvain clustering of CyTOF data reveals that CART-transfected NK cells maintain NK cell repertoire diversity. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) UMAP dimensionality reduction plot colored by identified clusters. (B) Heatmap of mean marker expression per cluster, with rows clustered hierarchically. (C) Proportions of cells belonging to each cluster.
Louvain clustering of CyTOF data reveals that CART-transfected NK cells maintain NK cell repertoire diversity. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) UMAP dimensionality reduction plot colored by identified clusters. (B) Heatmap of mean marker expression per cluster, with rows clustered hierarchically. (C) Proportions of cells belonging to each cluster.
CART transfection preserves NK cell functional phenotype
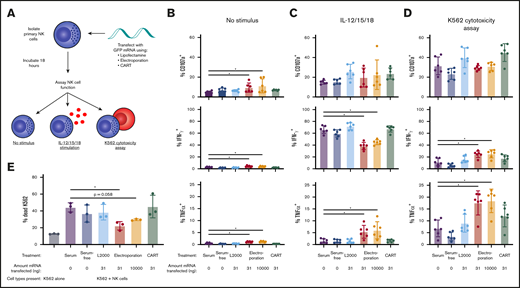
We next determined how the various transfection techniques affected the ability of NK cells to perform their canonical functions. We transfected resting or IL-2–stimulated primary NK cells with GFP-encoding mRNA using L2000, CART O5:N6:A9, or electroporation as described. We then changed the media 6 hours posttransfection and let the cells rest for another 12 hours before assaying 2 classical aspects of NK cell function: cytotoxicity in response to coculture with K562 cells and cytokine production in response to IL-12, -15, and -18 stimulation (Figure 5A).
NK cell cytotoxic potential and cytokine production profiles are preserved after CART transfection. (A) Isolated NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 or 10 000 ng of mRNA per well), or CARTs (31 ng of mRNA per well) and incubated for a total of 18 hours before assay of functional capacity by IL-12, -15, and -18 cytokine stimulation and K562 cytotoxicity assay. Percentage of NK cells with surface CD107a, intracellular IFN-γ, or intracellular TNFα without stimulus (B), after IL-12, -15, and -18 cytokine stimulation (C), or after K562 cytotoxicity assay at an effector/target (E/T) ratio of 1:3 (D). (E) Percentage of dead K562 cells after 10:1 E/T ratio coculture with transfected NK cells. *P < .05 via Wilcoxon matched-pairs signed rank test (B-D) or paired Student t test (E).
NK cell cytotoxic potential and cytokine production profiles are preserved after CART transfection. (A) Isolated NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 or 10 000 ng of mRNA per well), or CARTs (31 ng of mRNA per well) and incubated for a total of 18 hours before assay of functional capacity by IL-12, -15, and -18 cytokine stimulation and K562 cytotoxicity assay. Percentage of NK cells with surface CD107a, intracellular IFN-γ, or intracellular TNFα without stimulus (B), after IL-12, -15, and -18 cytokine stimulation (C), or after K562 cytotoxicity assay at an effector/target (E/T) ratio of 1:3 (D). (E) Percentage of dead K562 cells after 10:1 E/T ratio coculture with transfected NK cells. *P < .05 via Wilcoxon matched-pairs signed rank test (B-D) or paired Student t test (E).
Across all experimental conditions, CART-transfected resting NK cells were indistinguishable from their untransfected counterparts (Figure 5B-D). CART-transfected NK cells responded to IL-12, -15, and -18 stimulation with the same magnitude of IFN-γ production as untreated NK cells (Figure 5C). In response to coculture with K562 cells, CART-transfected NK cells degranulated, produced IFN-γ and TNFα, and killed the targets comparably to untreated NK cells (Figure 5D-E). This demonstrates that the CART transfection protocol does not alter canonical NK cell functions.
In contrast, electroporated NK cells were functionally distinct from untreated cells. In NK cells that received no further stimulus (not treated with IL-12, -15, or -18 or cocultured with K562 cells), electroporated NK cells expressed higher baseline levels of CD107a, IFN-γ, and TNFα (Figure 5B), indicating spontaneous activation. Furthermore, electroporated NK cells expressed significantly less IFN-γ and more TNFα in response to IL-12, -15, and -18 stimulation (Figure 5C), suggesting a skewing of NK cell cytokine response. Electroporated cells also expressed significantly higher levels of both IFN-γ and TNFα compared with untreated cells when cocultured with K562 cells (Figure 5D) and were slightly less able to kill their K562 targets (Figure 5E). These data collectively indicate that, unlike the reported electroporation protocol,32 CART-mediated transfection preserves canonical NK cell functions, making it a particularly suitable technique for studying NK cell biology and for therapeutic applications.
Generation of highly cytotoxic CAR NK cells using CARTs
CAR NK cells have shown promise in several clinical trials.8,9 We hypothesized that CARTs could be used to generate human CAR NK cells in vitro and designed and synthesized an mRNA encoding the anti-human CD19-41BB-CD3ζ CAR (hCAR).56 We transfected isolated resting human NK cells with this hCAR-encoding mRNA or a negative control anti-murine CD19 CAR mRNA (mCAR) using L2000 or CART BDK-O7:N7:A13 and then rested the cells for 18 hours before coculture with the target cells of interest.
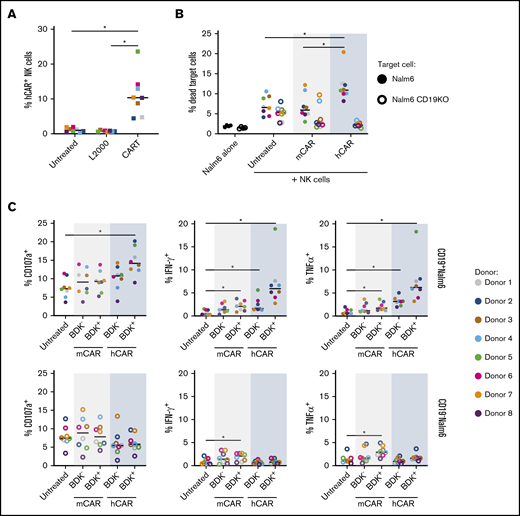
We found that CART-mediated transfection of the hCAR mRNA induced robust expression of the CAR on the NK cell surface (Figure 6A). After coculture of Nalm6 target cells (a human B lymphoblast cell line) with hCAR mRNA CART-transfected NK cells, we detected a significant increase in the percentage of dead CD19+ Nalm6 target cells (Figure 6B), demonstrating that CARTs can be used to generate cytotoxic human CAR NK cells.
CART-mediated transfection of anti-CD19 CAR generates cytotoxic human CAR NK cells. Isolated primary resting human NK cells were transfected with an mRNA encoding an hCAR or mCAR. The cells were incubated for a total of 18 hours before being cocultured with wild-type CD19+ Nalm6 (filled circles) or CD19KO Nalm6 (open circles) target cells for 6 hours followed by flow cytometric analysis. (A) hCAR expression by NK cells in the absence of target cells, as detected by fluorescein isothiocyanate–conjugated soluble human CD19. (B) Percentage of dead CD19+ wild-type Nalm6 or CD19KO Nalm6 cells after a 20:1 effector/target (E/T) ratio coculture with transfected NK cells.61 (C) CD107a, IFN-γ, and TNFα expression after 1:3 E/T ratio coculture with Nalm6 cells. For all panels, n = 8 donors. *P < .05 by Wilcoxon matched-pairs signed rank test with Bonferroni correction for multiple testing.
CART-mediated transfection of anti-CD19 CAR generates cytotoxic human CAR NK cells. Isolated primary resting human NK cells were transfected with an mRNA encoding an hCAR or mCAR. The cells were incubated for a total of 18 hours before being cocultured with wild-type CD19+ Nalm6 (filled circles) or CD19KO Nalm6 (open circles) target cells for 6 hours followed by flow cytometric analysis. (A) hCAR expression by NK cells in the absence of target cells, as detected by fluorescein isothiocyanate–conjugated soluble human CD19. (B) Percentage of dead CD19+ wild-type Nalm6 or CD19KO Nalm6 cells after a 20:1 effector/target (E/T) ratio coculture with transfected NK cells.61 (C) CD107a, IFN-γ, and TNFα expression after 1:3 E/T ratio coculture with Nalm6 cells. For all panels, n = 8 donors. *P < .05 by Wilcoxon matched-pairs signed rank test with Bonferroni correction for multiple testing.
To confirm that this enhanced cytotoxicity and cytokine production resulted from ligand-specific recognition by the transfected hCAR, we tested the ability of NK cells transfected with the mCAR to recognize human CD19+ target cells (Nalm6), and we tested the ability of CAR-transfected NK cells to respond to human CD19− target cells (CD19KO Nalm6). We found that only hCAR-transfected NK cells exhibited enhanced killing of human CD19+ Nalm6 target cells; the mCAR did not exhibit enhanced killing of human CD19+ Nalm6 cells (Figure 6B). Importantly, hCAR-transfected NK cells were significantly more lethal to human CD19+ Nalm6 cells relative to CD19− target cells (Nalm6 CD19KO; Figure 6B).
We next investigated the impact of CAR expression on NK cell degranulation and cytokine production in response to CD19+ Nalm6 target cells and CD19− Nalm6 cells by comparing CD107a, IFN-γ, and TNFα expression of CART-transfected NK cells (Figure 6C). We compared BDK− vs BDK+ cells instead of CAR− vs CAR+ cells, because coculture with the target cells induced a significant decrease in the percentage of NK cells expressing the CAR on the surface despite comparable transfection efficacy, suggesting CAR internalization or blockade of CAR staining as a result of engagement of the CD19 target ligand (supplemental Figure 5). Only CART-mediated hCAR-transfected NK cells had substantially enhanced levels of degranulation and IFN-γ and TNFα production, and this trend was observed only after coculture with CD19+ Nalm6 target cells (Figure 6C). The higher degranulation among BDK+ CART- mediated hCAR-transfected NK cells compared with untreated cells when cocultured with CD19+ Nalm6 target cells (Figure 6C) suggests a higher frequency of potentially cytolytic interactions among hCAR+ NK cells. Although BDK+ CART-mediated hCAR-transfected NK cells also expressed the highest levels of IFN-γ and TNFα when cocultured with CD19+ Nalm6 cells, the presence of hCAR NK cells seemed to induce bystander cytokine secretion, because BDK− NK cells from the CART-mediated hCAR-transfected condition expressed significantly more IFN-γ and TNFα than at baseline (Figure 6C). There was a modest but significant increase in IFN-γ and TNFα production by BDK+ mCAR-transfected NK cells, which we attribute to tonic signaling of the transfected CAR, because it is present in response to both parental and CD19KO Nalm6 target cells (Figure 6C). However, mCAR-transfected NK cells displayed no increase in degranulation or target cell killing (Figure 6B-C), implying that tonic CAR signaling does not affect cytolytic mechanisms. Collectively, these studies demonstrate that CARTs can be used to generate CAR NK cells with sharpened effector functions.
Furthermore, we studied Raji target cells (another human B lymphoblast cell line) as an alternative to Nalm6 target cells to demonstrate that the activation and killing potential of CART-transfected CAR NK cells were consistent between different types of target cancer cells (supplemental Figure 6). With an average of 12% of CART-transfected NK cells expressing the hCAR, we detected a significant increase in the percentage of dead CD19+ Raji target cells when cocultured with hCAR mRNA CART-transfected NK cells (supplemental Figure 6A) as well as enhanced levels of NK cell degranulation and IFN-γ and TNFα production (supplemental Figure 6B-C), demonstrating the generalizability of this technique to target cancer cells of interest.
Discussion
Here, we describe a new and highly effective technique for mRNA delivery into difficult-to-transfect primary NK cells, with significant research and clinical ramifications. CART-mediated transfection is >300 times more efficient and better preserves cell viability compared with electroporation. We characterized the phenotype of CART-transfected NK cells by high-dimensional CyTOF as well as multiple assays of canonical NK cell functions. Using these methods, we show that CART-mediated transfection does not induce off-target effects and thus represents a technique for the specific phenotypic manipulation of NK cells. To our knowledge, this work represents the first demonstration of an efficacious nonviral primary NK cell transfection that does not require prior cell activation and does not change NK cell phenotype, and thus may currently be the only method for bioorthogonal manipulation of primary NK cells.
Because electroporation is the most widely used nonviral transfection method for primary lymphocytes, we benchmarked CART transfection of NK cells with electroporation. We selected the protocol described by Rautela et al,32 because it uses the 4D Nucleofector device (Lonza), which is widely available. Other groups have used electroporation with other devices to successfully generate viable and functional CAR NK cells and have used this strategy to study mechanisms of CAR NK cell cytotoxicity at the NK cell subset level.31 Our data add to this knowledge by revealing that electroporation may induce numerous off-target effects on the NK cell repertoire, skew NK cell cytokine production profiles, and deleteriously affect NK cell killing capacity. In our study, we pretreated the electroporated NK cells with IL-2, because NK cell activation seems to be required for postelectroporation viability.32-36 Interestingly, the phenotypic changes induced by electroporation were not congruent with the alterations expected to be induced by IL-2 treatment alone. For example, IL-2 treatment does not cause spontaneous secretion of IFN-γ,37 as we observed in electroporated NK cells (Figure 5B). Ultimately, these data point to electroporation-specific off-target effects on NK cell phenotype, making it unsuitable for rigorously answering some open questions of NK cell biology.
A bioorthogonal transfection strategy for resting human NK cells is necessary to address fundamental mechanistic questions in human NK cell biology. The contributions of many individual NK cell receptors in the recognition of specific target cells remain poorly understood. For example, NKp46 is reported to recognize influenza hemagglutinin,57,58 but mouse models of NKp46 deficiency have yielded differing phenotypes upon influenza infection.59,60 Because questions like this involve molecules that are rapidly modulated by common biological perturbations like cytokine treatment,37 it is important to use a transfection technique that maintains native NK cell physiology. Our high-dimensional analysis of NK cell phenotype and function after CART-mediated transfection indicates that CARTs do not induce unwanted off-target effects, making CARTs ideally suited to address basic questions in NK cell biology.
Although the ability to transfect primary resting NK cells represents a significant advance for understanding human NK cell biology, CART-mediated transfection could greatly enhance clinical efforts. We demonstrated the potential clinical utility of CARTs by generating anti-CD19 human CAR NK cells in vitro. These CAR NK cells are potently cytotoxic and more robustly activated in response to CD19+ target cells than their untransfected counterparts. Because the transfection is mRNA mediated and thus transient, this approach could mitigate the need for a suicide switch, as was used in the recent clinical trial of virally transduced CAR NK cells.5 Additional studies will need to examine whether sufficient numbers of CART-generated CAR NK cells can be readily generated and assess their lifespan in vivo to determine if this technique would be superior to lentiviral transduction for CAR NK cell preparation. As we have previously demonstrated, different CART complexes target different organs and can thus be used to selectively deliver mRNA in vivo with a high specificity for mRNA expression, for example, in the spleen.38,43,45 As a consequence, in vivo CART-mediated delivery of CAR-encoding mRNA may provide an alternative therapeutic approach for the generation of CAR NK cells as a strategy for personalized cancer immunotherapy.
In conclusion, we report that readily available CARTs enable phenotypic manipulation of primary human NK cells in vitro with a minimal effect on native NK cell biology. This method shows enhanced transfection and improved cell viability compared with other nonviral transfection methods using the same dose of mRNA. We applied the developed CART technology to generate CAR NK cells, which as required for clinical applications exhibited enhanced killing of the target cells. As described herein, readily accessible and tunable CART technology offers significant comparative advantages over other tested delivery systems and enables manipulation of NK cells without phenotypic consequences, collectively opening new opportunities for NK research and clinical applications.
All data are available upon request. Mass cytometry data are publicly available on ImmPort (https://www.immport.org), accession number SDY1634. Code for mass cytometry data analysis is available upon request.
Acknowledgments
The authors thank the laboratory of Crystal Mackall for providing cell lines; Colin McKinlay for materials, including assistance in the synthesis of the mixed-lipid CARTs; and Anne-Maud Ferreira and Tim Blake for materials and helpful discussion.
This work was supported by grants CA031845 (P.A.W.) and R35CA197353-04 (R.L.) from the National Cancer Institute, National Institutes of Health (NIH), and CHE848280 (P.A.W.) and CHE1607092 (R.M.W.) from the National Science Foundation (organocatalytic polymerization). C.A.B. is supported by the Chan Zuckerberg Biohub, grant 5DP1DA04608902 from the National Institute on Drug Abuse, NIH, grant 1016687 from the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Diseases, and a Bill & Melinda Gates Foundation Pilot Grant through the Stanford Human Systems Immunology Center. C.A.B. is the Tashia and John Morgridge Faculty Scholar in Pediatric Translational Medicine from the Stanford Maternal Child Health Research Institute. A.J.W is supported by grant 5T32GM007365-44 from the Stanford Medical Scientist Training Program and by the Stanford Bio-X Interdisciplinary Graduate Fellowship.
Authorship
Contribution: A.J.W., N.L.-B.W., P.A.W., and C.A.B. developed the concepts and designed the study; A.J.W. and N.L.-B.W. performed the experiments; A.J.W, N.L.-B.W, R.M.W., P.A.W., and C.A.B. analyzed data; A.J.W. and N.L.-B.W. prepared the manuscript with input from all authors; A.J.W. performed bioinformatic analyses; R.V. acquired CyTOF data; and O.A.W.H. and R.L. provided reagents.
Conflict-of-interest disclosure: R.L. is a member of the scientific advisory boards for Five Prime, Quadriga, BeiGene, GigaGen, Teneobio, Sutro, Checkmate, Nurix, Dragonfly, Abpro, Apexigen, Spotlight, 47 Inc, XCella, Immunocore, and Walking Fish. The remaining authors declare no competing financial interests.
Correspondence: Paul A. Wender, Stanford University, 333 Campus Dr, Stanford, CA 94305; e-mail: wenderp@stanford.edu; and Catherine A. Blish, Stanford University, 300 Pasteur Dr, Grant Building S131A, Stanford, CA 94305; e-mail: cblish@stanford.edu.
References
Author notes
A.J.W. and N.L.-B.W. contributed equally to this study.
The full-text version of this article contains a data supplement.

![CART transfection outperforms electroporation and causes minimal reconfiguration of NK cell phenotype. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) Flow cytometric analysis of NK cell viability (top) and transfection efficacy (bottom) 18 hours posttransfection with GFP-encoding mRNA. (B) Uniform manifold approximation and projection (UMAP) dimensionality reduction of CyTOF data faceted by treatment condition. (C) Heatmap of mean marker expression, with samples and markers hierarchically clustered. *P < .05, ***P < .001, ****P < .0001 by paired Student t test; all other comparisons were not statistically significant at P = .05 level.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/17/10.1182_bloodadvances.2020002355/1/m_advancesadv2020002355f3.png?Expires=1768354008&Signature=0SYgNzvzayOWJdiESyu8IbLsK0o4XrjLfdkhUqMHJAV~HUvDdqFpl63qr~N1PiBKPSbZlAq8EoqJeowxiqC6NrjY9VNz02SJEdi~1JngZiPLX3vby2c8j8SlwdtP7fpB-0wGBA1GzFHn1u07Z9tVV9E4WB1eJpQDNplnbzW-HiApzZVpCuOUZVOrifeabDLN8pBh5HL5ETdyZCFd-ZKl8Akzhhqxjr~o2OwbW~ls41GCz4klX0FK8YL2TkIEkRFUqLKVXha24AaVx67AdAJg4Ag22JMDy4W2TwcLL~-2fHFLgE9tlXkgdN4z9fvx573kGOmEI5Jp7GTpsc9ydQjN9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Louvain clustering of CyTOF data reveals that CART-transfected NK cells maintain NK cell repertoire diversity. NK cells (500 000 cells per well) were transfected with GFP-encoding mRNA via L2000 (31 ng of mRNA per well), electroporation (31 ng per well [Elec] or 10 000 ng of mRNA per well [Elec high]), or CART O5:N6:A9 (31 ng of mRNA per well). (A) UMAP dimensionality reduction plot colored by identified clusters. (B) Heatmap of mean marker expression per cluster, with rows clustered hierarchically. (C) Proportions of cells belonging to each cluster.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/17/10.1182_bloodadvances.2020002355/1/m_advancesadv2020002355f4.png?Expires=1768354008&Signature=qoQHhQRP0GbI85W4mmfaDowAxgVP-6y0IBa1nh1IdxS7NMzSy5BMm1gsPxFz8EfRoxLPYjKrb3riuzQq~YIxeDQb-2zv8PvmgB88n4M7aUM-rZlsreAc~BsQFa027aEYGA0DVaxamzYB123mGU50myUqwssRwCN9rjLxMs1Jf6-CfuMc1B8-9plil2bo0UtHAyx~Vt9gXhbjXbfWanyR91M4FMwLX1nUnQvlaW4GV2D0~cJzLhdbgQwxFO9SBUouBQ2N0wkQfVMLiLhpxeMNBEpKlBiEJhmaDAka58yR~IKX~bM24gH5lbn77wh~jMRab09RjdLFlvlygfZIxXew3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)