Key Points
Largest cohort of childhood mature B-NHL/AL with CNS disease reporting prevalence, clinical pattern, and outcome.
In patients with CNS disease, those with blasts in the CSF or LDH value above twice the upper normal value are at higher risk of failure.
Abstract
To identify the factors influencing outcome in childhood mature B-cell non-Hodgkin lymphoma and acute leukemia (B-NHL/AL) with central nervous system (CNS) disease (CNS+), we analyzed patients <18 years with newly diagnosed B-NHL/AL registered in 3 Lymphomes Malins B studies in France between 1989 to 2011. CNS+ was diagnosed on fulfillment of ≥1 of the following criteria: any L3 cerebrospinal fluid (CSF) blasts (CSF+), cranial nerve palsy, isolated intracerebral mass but also clinical spinal cord compression, and cranial or spinal parameningeal extension. Two hundred seventeen out of 1690 patients (12.8%) were CNS+. CNS+ was significantly associated with male gender, head/neck locations, Burkitt histology, high initial lactate dehydrogenase (LDH) level, and bone marrow involvement. CSF+ was the most frequent pattern of CNS+ (45%). For the 217 CNS+ patients, the 5-year event-free survival (EFS) and overall survival rates (95% confidence interval) were 81.5% (75.8% to 86.1%) and 83.9% (78.4% to 88.2%), respectively. In multivariate analysis, among CNS+ patients, low EFS was associated with CSF+, high initial LDH level, and poor response to cyclophosphamide, oncovin (vincristine), prednisone prephase. These findings have been considered for patient’s stratification in the international randomized phase 3 trial Inter-B-NHL-ritux 2010 for children and adolescents with high-risk B-NHL/AL with CNS+ CSF+ patients only receiving intensified chemotherapy.
Introduction
The involvement of central nervous system (CNS) affects at diagnosis ∼10% of children and adolescents with mature B-cell non-Hodgkin lymphoma and acute leukemia (B-NHL/AL).1-4 It is associated with adverse outcome in several multicenter trials, with event-free-survival (EFS) ranging from 45% to 75%.1,2,4,5 Moreover, although CNS disease is defined by common international criteria,6 the strict definition differs depending on the different cooperative groups.1,2,4,5 The aims of this study were to determine the prevalence, clinical patterns, and factors influencing outcome in the largest cohort of CNS+ B-NHL/AL patients. Therefore, we analyzed 1690 patients with newly diagnosed B-NHL/AL registered in 3 successive Lymphomes Malins B (LMB) trials between 1989 and 2009. Among them, CNS+ disease was diagnosed in 217 (12.8%).
Methods
Diagnosis, classification, and staging
The LMB89, FAB (French-American-British)/LMB96 and LMB2001 prospective studies included patients <18 years, with mature B-NHL, as Burkitt/Burkitt-like lymphoma, diffuse large B-cell lymphoma, other high-grade B lymphoma, and L3 acute leukemia (B-AL). Pathology was reviewed by national/international experts in LMB89 and LMB96. From the FAB/LMB96 study, only French patients were included in the current analysis.
Minimal workup included thoracoabdominal computed tomography scan, bone marrow (BM) aspirates and biopsies (for large B-cell lymphoma), cerebrospinal fluid (CSF) cytology, and lactate dehydrogenase (LDH) level (≤ or > 2N the upper limit of the institution’s normal range). Other imaging, such as cerebral computed tomography or magnetic resonance imaging were performed as clinically indicated. Staging was based on St Jude’s classification.7 In the 3 protocols, CNS+ disease was diagnosed based on fulfillment of ≥1 of the following criteria: any L3 CSF blasts (CSF+), cranial nerve palsy (CNP) that cannot be explained by extradural lesion, isolated intracerebral mass (ICM), or clinical spinal cord compression. In LMB96 and LMB2001 studies, patients with cranial or spinal tumor and parameningeal extension beyond a meninx were also considered as CNS+ irrespective of clinical symptoms.
Patients with CNS+ were assigned to high-risk group C in the 3 LMB protocols. LMB89 and LMB96 have been previously reported.3,7 Treatment of CNS+ patients did not vary significantly among the 3 protocols, except in (1) LMB89, cranial irradiation (24 Gy) was delivered to patients with CSF+ or CNP; (2) in FAB/LMB96, half of the patients were randomized to receive the reduced-intensity chemotherapy arm (cytarabine 2 g vs 3 g/m2 dose × 4 days and etoposide 100 mg vs 200 mg/m2 dose × 4 days during the consolidation phase, and shortened maintenance); and (3) in LMB2001, treatment of CNS+ patients was similar to standard FAB/LMB96 group C with high-dose methotrexate administrated over 24 hours instead of 4 hours.
Statistical analysis
Characteristics of patients with or without CNS disease were compared using χ2 test. The primary efficacy end point was EFS, defined as duration from start of chemotherapy to the first of the following events: death of any cause, relapse, progressive disease, second malignant neoplasm or biopsy-positive residual disease following the second consolidation course. Patients without any of these events were censored at the date of last follow-up. The secondary efficacy end point was overall survival (OS), defined as time from the start of chemotherapy to death due to any cause or to the date of the last follow-up. EFS and OS were estimated with the Kaplan-Meier method.8 The 95% confidence intervals (CIs) of the survival rates were calculated with the Rothman method.9 The prognostic analysis of EFS was done using a log-rank test for univariate analyses and Cox model for multivariate analysis.10-12 The Cox model was stratified for the 3 studies. Hazard ratios from the multivariate Cox model were calculated.
The 3 protocols (LMB89, LMB96, and LMB2001) were approved by all of the local institutional review boards, and written informed consent was obtained from all patients or their parent or legal guardian, in accordance with the Declaration of Helsinki.
Results and discussion
Patient characteristics are summarized in Tables 1 and 2. A total of 217 out of the 1690 patients (12.8%) with newly diagnosed B-NHL/AL were CNS+, including 67 out of 561 (12%) in LMB89, 52 out of 384 (14%) in FAB/LMB96, and 98 out of 745 (13%) in LMB2001. CNS disease was significantly associated with male sex (P = .007), head and neck locations (P < .0001), Burkitt histology (P = .0003), high initial LDH >2N (P < .0001), and BM involvement (P < .0001) (Table 1). The rate of CNS+ in the current series is similar to a cohort of 201 B-NHL/AL patients treated at St. Jude Children’s Hospital, including 27 CNS+ patients (13.4%),2 and a cohort of 462 patients with disseminated small non–cleaved-cell lymphoma and B-cell leukemia, including 49 CNS+ patients (10.6%) treated on Children’s Cancer Group protocols,4 but higher than in the Berlin-Frankfurt-Münster (BFM) experience (7.5% in 1092 BL/B-AL).1 Of note, the rate of CNS+ was 10.5% in the current LMB series using BFM CNS diagnosis criteria (any CSF+, CNP or ICM).
CNS disease according to patient characteristics and B-NHL subtype
| . | Total no. of patients . | CNS+, n (%) of patients . | CNS−, n (%) of patients . | CNS− vs CNS+, P . |
|---|---|---|---|---|
| Study | 1690 | 217 (13) | 1473 (87) | .73 |
| LMB89 | 561 | 67 (12) | 494 (88) | |
| LMB96 (SFOP only) | 384 | 52 (14) | 332 (86) | |
| LMB2001 | 745 | 98 (13) | 647 (87) | |
| Sex | .007 | |||
| Male | 1303 | 183 (84) | 1120 (76) | |
| Age, y | .67 | |||
| >15 | 158 | 22 (10) | 136 (9) | |
| Histology* | .0003 | |||
| BL/B-LL | 1327 | 191 (88) | 1136 (77) | |
| DLBCL | 262 | 14 (6) | 248 (17) | |
| B high-grade NOS | 99 | 12 (6) | 87 (6) | |
| Primary site | <.0001 | |||
| Head and neck | 311 | 65 (30) | 246 (17) | |
| Abdomen | 916 | 50 (23) | 866 (59) | |
| Other | 463 | 102 (47) | 361 (25) | |
| BM involvement, % † | <.0001 | |||
| No | 1281 | 84 (39) | 1197 (81) | |
| 1-25 | 111 | 21 (10) | 90 (6) | |
| >25 | 296 | 112 (52) | 184 (13) | |
| LDH‡ | <.0001 | |||
| LDH >2N | 787 | 151 (73) | 636 (45) |
| . | Total no. of patients . | CNS+, n (%) of patients . | CNS−, n (%) of patients . | CNS− vs CNS+, P . |
|---|---|---|---|---|
| Study | 1690 | 217 (13) | 1473 (87) | .73 |
| LMB89 | 561 | 67 (12) | 494 (88) | |
| LMB96 (SFOP only) | 384 | 52 (14) | 332 (86) | |
| LMB2001 | 745 | 98 (13) | 647 (87) | |
| Sex | .007 | |||
| Male | 1303 | 183 (84) | 1120 (76) | |
| Age, y | .67 | |||
| >15 | 158 | 22 (10) | 136 (9) | |
| Histology* | .0003 | |||
| BL/B-LL | 1327 | 191 (88) | 1136 (77) | |
| DLBCL | 262 | 14 (6) | 248 (17) | |
| B high-grade NOS | 99 | 12 (6) | 87 (6) | |
| Primary site | <.0001 | |||
| Head and neck | 311 | 65 (30) | 246 (17) | |
| Abdomen | 916 | 50 (23) | 866 (59) | |
| Other | 463 | 102 (47) | 361 (25) | |
| BM involvement, % † | <.0001 | |||
| No | 1281 | 84 (39) | 1197 (81) | |
| 1-25 | 111 | 21 (10) | 90 (6) | |
| >25 | 296 | 112 (52) | 184 (13) | |
| LDH‡ | <.0001 | |||
| LDH >2N | 787 | 151 (73) | 636 (45) |
2N, twice the normal value; NOS, not otherwise specified; SFOP, Société Française d’Oncologie Pédiatrique.
Histology was available for 1688 out of 1690 patients.
BM involvement was available for 1688 out of 1690 patients.
LDH level was available for 1618 out of 1690 patients.
Prognostic factors for outcome in 217 patients with CNS disease
| . | CNS+, n (%) of patients . | 5-y EFS, % . | Univariate analyses, P . |
|---|---|---|---|
| Sex | |||
| Female | 34 (16) | 91 | .10 |
| Male | 183 (84) | 80 | |
| Age (median age: 8.4 y), y | |||
| <9 | 120 (55) | 82 | .55 |
| ≥9 | 97 (45) | 80 | |
| Histology | |||
| BL/B-LL | 191 (88) | 80 | .19 |
| DLBCL | 14 (6) | 100 | |
| B high-grade NOS | 12 (6) | 92 | |
| LDH levels* | |||
| LDH <2N | 57 (27) | 95 | .002 |
| LDH ≥2N | 151 (73) | 76 | |
| CSF status (including isolated CSF; n = 54) | |||
| Negative | 120 (55) | 90 | .0007 |
| Positive | 97 (45) | 71 | |
| CNP (including isolated CNP; n = 34) | |||
| No | 125 (58) | 83 | .40 |
| Yes | 92 (42) | 79 | |
| Spinal cord compression (including isolated spinal cord compression; n = 4) | |||
| No | 187 (86) | 82 | .56 |
| Yes | 30 (14) | 77 | |
| ICM†(including isolated ICM; n = 7) | |||
| No | 190 (88) | 80 | .13 |
| Yes | 25 (12) | 92 | |
| PME (including isolated PME; n = 28) | |||
| No | 149 (69) | 82 | .97 |
| Yes | 68 (31) | 81 | |
| BM involvement | |||
| No | 84 (39) | 81 | .77 |
| Yes | 133 (61) | 78 | |
| COP response | |||
| Poor response (<20%) | 11 (5) | 45 | .0008 |
| Good response (>20%) | 206 (95) | 83 |
| . | CNS+, n (%) of patients . | 5-y EFS, % . | Univariate analyses, P . |
|---|---|---|---|
| Sex | |||
| Female | 34 (16) | 91 | .10 |
| Male | 183 (84) | 80 | |
| Age (median age: 8.4 y), y | |||
| <9 | 120 (55) | 82 | .55 |
| ≥9 | 97 (45) | 80 | |
| Histology | |||
| BL/B-LL | 191 (88) | 80 | .19 |
| DLBCL | 14 (6) | 100 | |
| B high-grade NOS | 12 (6) | 92 | |
| LDH levels* | |||
| LDH <2N | 57 (27) | 95 | .002 |
| LDH ≥2N | 151 (73) | 76 | |
| CSF status (including isolated CSF; n = 54) | |||
| Negative | 120 (55) | 90 | .0007 |
| Positive | 97 (45) | 71 | |
| CNP (including isolated CNP; n = 34) | |||
| No | 125 (58) | 83 | .40 |
| Yes | 92 (42) | 79 | |
| Spinal cord compression (including isolated spinal cord compression; n = 4) | |||
| No | 187 (86) | 82 | .56 |
| Yes | 30 (14) | 77 | |
| ICM†(including isolated ICM; n = 7) | |||
| No | 190 (88) | 80 | .13 |
| Yes | 25 (12) | 92 | |
| PME (including isolated PME; n = 28) | |||
| No | 149 (69) | 82 | .97 |
| Yes | 68 (31) | 81 | |
| BM involvement | |||
| No | 84 (39) | 81 | .77 |
| Yes | 133 (61) | 78 | |
| COP response | |||
| Poor response (<20%) | 11 (5) | 45 | .0008 |
| Good response (>20%) | 206 (95) | 83 |
Bold P values are statistically significant (P < .05).
BL/BLL, Burkitt lymphoma/Burkitt-like lymphoma; COP, cyclophosphamide, oncovin (vincristine), prednisone; DLBCL, diffuse large B-cell lymphoma; PME, parameningeal extension.
LDH level was available for 208 out of 217 patients.
ICM status was available for 215 out of 217 patients.
Median age at diagnosis was 8.4 years (range, 4 months to 18 years). Fifty-four (25%) had CSF+ only, 34 (16%) had isolated (ie, the only feature of CNS disease) CNP, 7 (3%) had isolated ICM, 28 (13%) had isolated cranial or spinal parameningeal extension (including 4 patients with clinical symptoms of spinal cord compression), 5 (2%) had isolated chin palsy, and 6 had other isolated lesions. Eighty-three patients (38%) had multiple criteria of CNS disease. Only 5 patients were considered with primary CNS lymphoma. Blasts in the CSF was the prevailing pattern of CNS involvement in 97 CSF+ patients (45%). Although the rate of CNS+ was stable during the 3 periods, the rate of CSF+ significantly decreased in the last LMB study; 37 (55%), 26 (50%), and 34 (35%) patients had CSF+ in the LMB89, LMB96, and LMB2001 study, respectively (P = .02). While there is no obvious explanation, the use of modern imaging such as magnetic resonance imaging may explain that in the more recent LMB study, more patients with less advanced CNS+ CSF− disease may be diagnosed.
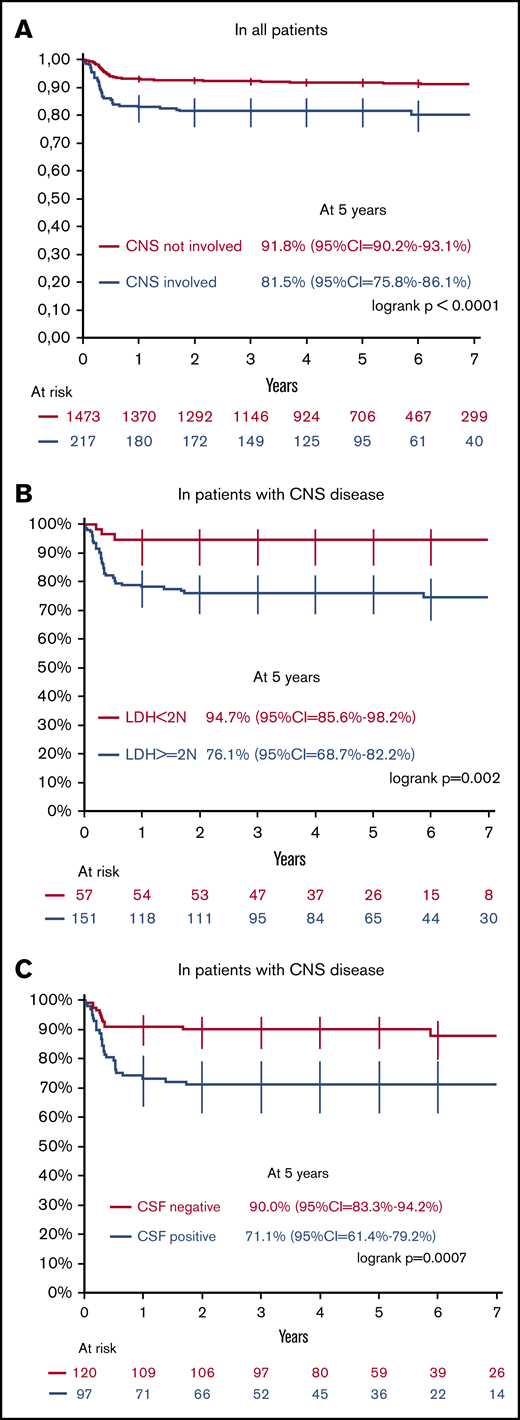
The median follow-up was 5.1 years (interquartile range, 3.6 to 6.8 years) for all 1690 patients registered in the 3 LMB studies. The 5-year OS rate was 94.5% (95% CI, 93.2% to 95.6%) for CNS− patients vs 83.9% (95% CI, 78.4% to 88.2%) for CNS+ patients (P < .0001) and the 5-year EFS rates were respectively 91.8% (95% CI, 90.2% to 93.1%), and 81.5% (95% CI, 75.8% to 86.1%) (P < .0001) (Figure 1A). Among the 217 CNS+ patients, 41 had an event. Ten patients died of treatment-induced toxicity (6%, 5.8%, and 3.1%, in LMB89, LMB96, and LMB2001, respectively). Twenty-eight children had suffered from relapse or progression, and CNS was the most frequent site of failure (61%). Notably, none of the 28 patients with isolated cranial or spinal parameningeal extension had CNS relapse. Three remaining patients had a second malignancy (MLL-AF4 acute lymphoblastic leukemia, myeloproliferative disorder, and CNS primitive neuroectodermal tumor after previous cranial irradiation).
EFS by CNS status, and in CNS involved, by LDH value and blast in CSF. EFS according to CNS disease in 1690 B-NHL/AL patients and 217 CNS+ B-NHL/AL patients (A) according to LDH value at diagnosis (B) and blasts in the CSF (C) among CNS+ patients only.
EFS by CNS status, and in CNS involved, by LDH value and blast in CSF. EFS according to CNS disease in 1690 B-NHL/AL patients and 217 CNS+ B-NHL/AL patients (A) according to LDH value at diagnosis (B) and blasts in the CSF (C) among CNS+ patients only.
Age, sex, histology, clinical patterns of CNS involvement (ICM, CNP, or parameningeal extension) and BM involvement were not prognostic. By contrast, CSF involvement, initial LDH level, and response to COP prephase were significantly associated with EFS in univariate analysis (Table 2). In the multivariate analysis, all remained significantly associated with EFS, with hazard ratios (95% CIs) of 2.03 (1.03; 3.99; P = .041) for CSF+, 4.58 (1.39; 15.12; P = .013) for initial LDH >2N (Figure 1B-C), and 0.31 (0.13; 0.76; P = .011 for COP prephase response >20%). By contrast, in the BFM experience from 1986 to 2002, no risk factors for treatment failure were identified.1 Indeed, neither the initial pattern of CNS disease (CSF+ vs ICM without blast vs CNP only) nor the LDH level (>1000 U/L or <1000 U/L) had an impact on relapse risk in a smaller series of 81 CNS+ patients.
The current series represents the largest series of uniformly diagnosed and treated pediatric B-NHL/AL CNS+ patients. CSF blasts and initial LDH >2N were strong predictors of treatment failure. These findings have been considered for patient stratification in the international randomized phase 3 trial Inter-B-NHL-ritux 2010 for children and adolescents with high-risk B-NHL/AL with CNS+ CSF+ patients receiving high-dose methotrexate administrated over 24 hours.
For data sharing requests, e-mail the corresponding author (veronique.minard@gustaveroussy.fr).
Acknowledgments
The authors thank the members of the French review panel (C. Bayle, J. Bosq, M. Raphael, and M. J. Terrier-Lacombe), the French data managers of the LMB89 and FAB/LMB96 (N. Dupouy) and LMB2001 (Y. Oubouzar and I. Villa) studies, and all the investigators who treated patients and participated in the studies.
This study was supported by grants from Gustave Roussy, Association pour la Recherche Contre le Cancer, La Ligue Nationale Contre le Cancer (FAB/LMB96), and Société Française des Cancers de l’Enfant et de l’Adolescent & Enfants et Santé (LMB2001).
Authorship
Contribution: C.P., A.B., G.L., C.C., and J.M. conceived the study; M.S. and V.M.-C. wrote the manuscript; A.A. performed analyses; and Y.B., N.A., A.B., A.C., C.C., V.G., S.H., G.L., T.L., J.M., C.P., and V.M.-C. provided study materials or patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Veronique Minard-Colin, Department of Pediatric and Adolescent Oncology and U1015, Gustave Roussy, 114 rue Edouard Vaillant, 94800 Villejuif, France; e-mail: veronique.minard@gustaveroussy.fr.