Key Points
Age-adapted high-dose chemotherapy and autologous stem cell transplantation is safe and highly effective in elderly patients with PCNSL.
Introduction
The median age of immunocompetent patients with primary diffuse large B-cell lymphoma of the central nervous system (PCNSL) is 67 years,1 and higher age is associated with a substantially worse prognosis.2 Induction chemo(immuno)therapy followed by high-dose chemotherapy and autologous stem cell transplantation (HCT-ASCT) is considered a standard treatment for younger patients with PCNSL and results in high rates of long-term remission or cure.3-8 Nevertheless, such approaches are usually only offered to patients aged <65 years.9 Retrospective data show promising results of HCT-ASCT in elderly PCNSL patients,10 but this approach has not been investigated in prospective clinical studies. To address this gap, we designed a pilot trial for an age-adapted approach to investigate the feasibility of a short induction treatment followed by consolidating HCT-ASCT.
Methods
This open-label, single-arm pilot trial was conducted at 2 centers in Germany. The study protocol was approved by both the leading and local ethics committees. All participants provided written informed consent. The trial was registered at www.drks.de (DRKS00008900). Eligibility criteria were immunocompetence, histologically proven PCNSL of B-cell immunophenotype excluding isolated primary vitreoretinal lymphoma, age >65 years, Eastern Cooperative Group Performance Status (ECOG-PS) ≤2, Cumulative Illness Rating Scale–Geriatric score <6 (without consideration of symptoms directly caused by PCNSL), no active hepatitis B or C disease, adequate bone marrow and hepatic function (maximum National Cancer Institute Common Terminology Criteria version 4.0 grade 1 alterations), creatinine clearance ≥60 mL/min, and eligibility for HCT-ASCT according to the treating physician.
The main objective was evaluation of the feasibility of the study procedures. To determine feasibility, we evaluated (serious) adverse events, the rate of toxicity (according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0), recruitment rate, and the percentage of patients completing the protocol. Further outcomes included: rate of complete remission (CR) 30 days after HCT-ASCT, progression-free survival (PFS), with PFS being defined as time from start of treatment until progression, relapse, or death from any cause, whichever occurs first; overall survival (OS) as time from start of treatment until death from any cause; and rate of neurotoxicity determined by using the Mini–Mental State Examination and the panel of neuropsychological tests of the International PCNSL Collaborative Group.11 Treatment consisted of 2 21-day cycles of induction chemotherapy (supplemental Figure 1). During induction treatment, patients received rituximab IV at 375 mg/m2 on days 0 and 4; high-dose methotrexate IV at 3.5 g/m2 on day 1; and cytarabine IV at 2 g/m2, twice a day on days 2 and 3. Peripheral blood stem cells stimulated with granulocyte-colony stimulating factor were collected after the first induction cycle, processed, and stored according to local guidelines. Addressing the advanced age of the study population, patients achieving at least stable disease after 2 cycles of induction therapy proceeded directly to HCT-ASCT. Based on retrospective data showing the feasibility of busulfan-based HCT-ASCT in elderly patients with systemic lymphoma, we chose busulfan in combination with thiotepa as the conditioning regimen.12 Consolidation treatment consisted of busulfan IV at 3.2 mg/kg on days −7 and −6 and thiotepa IV at 5 mg/kg on days −5 and −4. Stem cell reinfusion was performed according to standard procedures on day 0. Response assessment by brain magnetic resonance imaging according to the response criteria of the International PCNSL Collaborative Group13 was performed after the second cycle and on day 30 after HCT-ASCT. Disease status was then assessed every 3 months during the first year and every 6 months in years 2 to 5. Minimum follow-up after treatment completion was 12 months. We chose 14 patients as a convenient sample size without a formal sample size calculation.
Results and discussion
Overall, 37 patients with initially suspected newly diagnosed PCNSL aged >65 years presented at the 2 participating centers between December 2015 and September 2017. Eighteen-patients (48.6%) did not fulfill trial inclusion criteria due to renal insufficiency (n = 5), Cumulative Illness Rating Scale–Geriatric score >6 (n = 4), ECOG PS >2 (n = 3), ineligibility for HCT-ASCT due to advanced age (>80 years; n = 2), active hepatitis B disease (n = 1), concomitant cancer (n = 1), inadequate bone marrow function (n = 1), and concomitant monoclonal B-cell lymphomatosis (n = 1). In addition, 2 patients (5%) would have fulfilled inclusion criteria of the trial but were treated within the MATRix/International Extranodal Lymphoma Study Group-43 trial.14 Initially, 17 consecutive patients were registered in the trial; 3 of them were excluded afterward from the full analysis set due to detection of systemic lymphoma manifestations (n = 2) and active hepatitis B disease (n = 1) during staging evaluation. Our screening process resulted in inclusion of 14 patients. Median age and ECOG-PS were 74 years (range, 69-79 years) and 1 (range, 0-2), respectively. A summary of patient characteristics and treatment outcome is shown in Table 1.
Patient characteristics and treatment outcome
| Patient . | Age, y/sex . | Line of therapy . | Initial ECOG-PS . | CIRS score* . | IELSG PS . | Ocular involvement . | Induction cycle . | Response to induction . | Response to HCT-ASCT . | Relapse . | Survival, mo . | MMST before therapy . | Last MMST . | Last ECOG-PS . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69/M | First-line | 1 | 10 | 3 | No | 1 | CR | NA | No | 46 | 24 | ND | 1 | NA |
| 2 | 72/M | First-line | 1 | 5 | 1 | No | 2 | CR | CR | No | 45 | 27 | 29 | 0 | NA |
| 3 | 79/F | First-line | 1 | 4 | 2 | No | 2 | CR | CR | No | 40 | 28 | 30 | 1 | NA |
| 4 | 70/M | First-line | 1 | 5 | 1 | No | 2 | PR | CR | No | 38 | 18 | 27 | 1 | NA |
| 5 | 74/F | First-line | 1 | 6 | 2/4† | No | 2 | PR | CR | No | 32 | 28 | 30 | 0 | NA |
| 6 | 78/F | First-line | 1 | 4 | 2 | ND | 2 | PR | CR | No | 47 | 30 | 30 | 1 | NA |
| 7 | 70/F | First-line | 1 | 6 | 3 | No | 2 | PR | uCR | No | 47 | 29 | 30 | 2 | NA |
| 8 | 76/M | First-line | 2 | 4 | 2/4† | ND | 2 | uCR | CR | No | 46 | ND | 30 | 0 | NA |
| 9 | 79/F | First-line | 0 | 4 | 3 | ND | 2 | PR | uCR | No | 36 | 28 | 30 | 0 | NA |
| 10 | 73/M | First-line | 0 | 4 | 3 | No | 2 | PR | uCR | Yes | 19 | 30 | NA | NA | PCNSL |
| 11 | 73/F | First-line | 0 | 5 | 1/4 | No | 2 | PR | PR | No | 31 | ND | 18 | 1 | NA |
| 12 | 71/F | First-line | 2 | 6 | 3 | No | 2 | PR | CR | No | 29 | 23 | 30 | 2 | NA |
| 13 | 73/M | First-line | 2 | 4 | 3/4‡ | No | 2 | PR | PR | No | 26 | 16 | 19 | 2 | NA |
| 14 | 74/F | First-line | 1 | 4 | 3 | No | 2 | PR | uCR | No | 26 | 28 | 28 | 0 | NA |
| Patient . | Age, y/sex . | Line of therapy . | Initial ECOG-PS . | CIRS score* . | IELSG PS . | Ocular involvement . | Induction cycle . | Response to induction . | Response to HCT-ASCT . | Relapse . | Survival, mo . | MMST before therapy . | Last MMST . | Last ECOG-PS . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69/M | First-line | 1 | 10 | 3 | No | 1 | CR | NA | No | 46 | 24 | ND | 1 | NA |
| 2 | 72/M | First-line | 1 | 5 | 1 | No | 2 | CR | CR | No | 45 | 27 | 29 | 0 | NA |
| 3 | 79/F | First-line | 1 | 4 | 2 | No | 2 | CR | CR | No | 40 | 28 | 30 | 1 | NA |
| 4 | 70/M | First-line | 1 | 5 | 1 | No | 2 | PR | CR | No | 38 | 18 | 27 | 1 | NA |
| 5 | 74/F | First-line | 1 | 6 | 2/4† | No | 2 | PR | CR | No | 32 | 28 | 30 | 0 | NA |
| 6 | 78/F | First-line | 1 | 4 | 2 | ND | 2 | PR | CR | No | 47 | 30 | 30 | 1 | NA |
| 7 | 70/F | First-line | 1 | 6 | 3 | No | 2 | PR | uCR | No | 47 | 29 | 30 | 2 | NA |
| 8 | 76/M | First-line | 2 | 4 | 2/4† | ND | 2 | uCR | CR | No | 46 | ND | 30 | 0 | NA |
| 9 | 79/F | First-line | 0 | 4 | 3 | ND | 2 | PR | uCR | No | 36 | 28 | 30 | 0 | NA |
| 10 | 73/M | First-line | 0 | 4 | 3 | No | 2 | PR | uCR | Yes | 19 | 30 | NA | NA | PCNSL |
| 11 | 73/F | First-line | 0 | 5 | 1/4 | No | 2 | PR | PR | No | 31 | ND | 18 | 1 | NA |
| 12 | 71/F | First-line | 2 | 6 | 3 | No | 2 | PR | CR | No | 29 | 23 | 30 | 2 | NA |
| 13 | 73/M | First-line | 2 | 4 | 3/4‡ | No | 2 | PR | PR | No | 26 | 16 | 19 | 2 | NA |
| 14 | 74/F | First-line | 1 | 4 | 3 | No | 2 | PR | uCR | No | 26 | 28 | 28 | 0 | NA |
Overview of patients’ characteristics and treatment outcome of the 14 patients included in the trial. Remission status was assessed according to the International PCNSL Collaborative Group response criteria.
CIRS, Cumulative Illness Rating Scale–Geriatric; F, female; IELSG PS, International Extranodal Lymphoma Study Group Prognostic Score; M, male; MMST, Mini–Mental State Examination; NA, not applicable; ND, not done; PR, partial remission.
Symptoms caused by PCNSL were not considered.
Cerebrospinal fluid protein concentration unknown.
Serum lactate dehydrogenase unknown.
Overall, 13 (93%) of 14 patients completed the protocol. In 1 patient, trial treatment was stopped prematurely during the first cycle due to acute decompensated heart failure. During the first cycle, 12 (86%) of 14 patients received the full doses of the induction components; during the second cycle, 11 (85%) of 13 patients received full dose, and all 13 patients received the planned consolidation therapy without dose reductions. In cases of initial corticosteroid therapy, doses were reduced during induction treatment, with no patient remaining on corticosteroids after HCT-ASCT. Therapy was generally well tolerated, and no treatment-related deaths occurred. Grade 3 or 4 hematologic toxicities were observed in all patients. The most frequently reported adverse events (≥grade 3) were infections and gastrointestinal disorders. Nephrotoxicity was observed in 1 patient. Overall, 8 serious adverse reactions were reported, of which 4 occurred after the first cycle, 3 after the second cycle, and 1 after HCT-ASCT (supplemental Table 1). All patients recovered without sequelae.
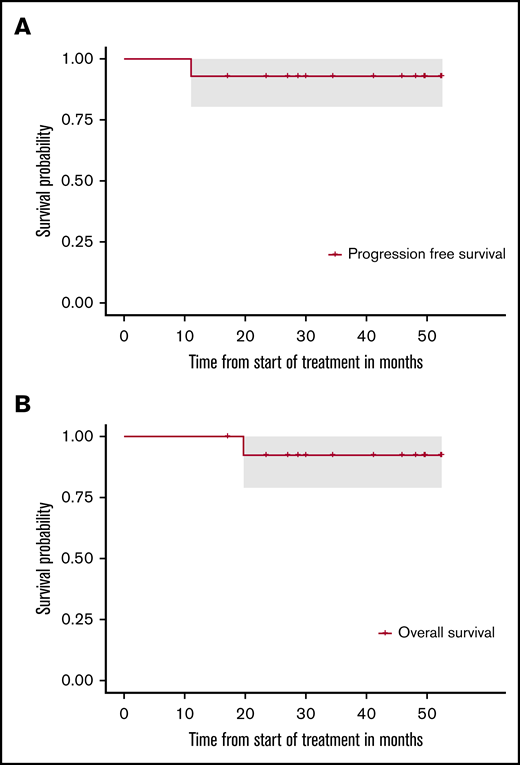
After induction, all 14 patients achieved a remission (3 with CR, 1 with unconfirmed complete remission [uCR], 10 with partial remission). Overall, 13 of 14 patients commenced HCT-ASCT; 30 days after HCT-ASCT, 11 patients achieved CR or uCR and 2 a partial remission, which converted to CR without any additional therapy after 3 months. After a median follow-up of 41 months, 1 patient who had achieved uCR after completion of therapy developed progressive disease 9 months after HCT-ASCT and died due to lymphoma progression later on. All other patients are in ongoing CR and in good mental (supplemental Figure 2) and general condition without having received additional therapy. After 24 months, respective PFS and OS rates were 92.9% (95% CI, 80.3-100) and 92.3% (95% CI, 78.9-100), respectively (Figure 1).
PFS and OS of the intention-to-treat population. (A) PFS. (B) OS. After a median follow-up of 41 months, 1 patient developed progressive disease 9 months after HCT-ASCT and died of lymphoma progression later on. All other patients are in ongoing remission.
PFS and OS of the intention-to-treat population. (A) PFS. (B) OS. After a median follow-up of 41 months, 1 patient developed progressive disease 9 months after HCT-ASCT and died of lymphoma progression later on. All other patients are in ongoing remission.
To date, a limited number of single-arm prospective studies focusing on elderly patients with PCNSL have been reported,15-23 and evidence for the best treatment approach in these elderly PCNSL patients remains sparse. Notably, a randomized trial evaluating the role of consolidation HCT-ASCT is lacking. The results of this pilot trial support feasibility and effectiveness of HCT-ASCT in selected elderly patients with newly diagnosed PCNSL. However, it should be noted that this pilot study was conducted at 2 tertiary referral centers, both very experienced in the management of PCNSL. Only 40% of all newly diagnosed PCNSL patients aged >65 years referred to the participating centers within the screening period fulfilled the trial inclusion criteria. This positive selection may explain the fact that the presented results compare favorably with previously reported HCT-ASCT trials in younger patients.3,4,24 We have initiated a phase 2 study (DRKS00011932)25 to scrutinize our results in a multicenter setting.
Data were analyzed at the Clinical Trials Unit of the University of Freiburg, and all authors had access to primary clinical trial data. Individual participant data will not be shared. All requests may be submitted to the corresponding author (Elisabeth Schorb, e-mail: elisabeth.schorb@uniklinik-freiburg.de).
Acknowledgments
The authors thank the patients and their families for kindly accepting to take part in this study.
The study was funded by a seeding grant of the Faculty of Medicine of the University of Freiburg.
Authorship
Contribution: E.S. prepared the manuscript and is the trial’s principal investigator; J.F. and G. Illerhaus are the deputy principal investigators; J.F., G. Ihorst, B.K., H.F., and G. Illerhaus participated in preparing the manuscript and study protocol; G. Ihorst and B.K. conducted the statistical analysis for the trial; E.S., F.S., J.W., and L.I. collected patient data; and all authors have read and approved the final manuscript.
Conflict-of-interest disclosure: E.S., B.K., J.F., and G. Illerhaus receive speakers honoraria from Riemser Pharma GmbH. B.K. also receives honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Elisabeth Schorb, Freiburg University Medical Center, Hugstetter Straße 55, 79106 Freiburg, Germany; e-mail: elisabeth.schorb@uniklinik-freiburg.de.
References
Author notes
The full-text version of this article contains a data supplement.