Key Points
HPA-1a immunization is unlikely to occur in HPA-1a− pregnant women who do not express HLA-DRB3*01:01.
Even in HPA-1a-immunized pregnant women, the risk for severe fetal/neonatal outcomes is low for mothers who are HLA-DRB3*01:01−.
Abstract
The most common, severe cases of fetal and neonatal alloimmune thrombocytopenia among whites are caused by antibodies against human platelet antigen 1a (HPA-1a). The aims of this systematic review and meta-analysis are to determine the association between maternal HLA-DRB3*01:01 and: (1) HPA-1a-alloimmunization and (2) neonatal outcome in children born of HPA-1a-immunized women. A systematic literature search identified 4 prospective and 8 retrospective studies. Data were combined across studies to estimate pooled odds ratios (ORs) and the associated 95% confidence intervals (CIs). The population represented by the prospective studies was more than 150 000. In the prospective studies, there were 64 severely thrombocytopenic newborns (platelet count <50 × 109/L) of whom 3 had intracranial hemorrhage. The mothers of all 64 children were HLA-DRB3*01:01+. The number of severely thrombocytopenic children born of HPA-1a-alloimmunized women in the retrospective studies was 214; 205 of whom were born of HLA-DRB3*01:01+ women. For HLA-DRB3*01:01− women, the OR (95% CI) for alloimmunization was 0.05 (0.00-0.60), and for severe neonatal thrombocytopenia 0.08 (0.02-0.37). This meta-analysis demonstrates that the risk of alloimmunization and of having a child with severe thrombocytopenia are both very low for HPA-1a− women who are HLA-DRB3*01:01−.
Introduction
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is a rare but potentially serious fetal and neonatal bleeding condition. The clinical spectrum ranges from no bleeding, petechiae and ecchymoses, to gastrointestinal hemorrhage, hematuria, and intracranial hemorrhage (ICH). ICH may result in intrauterine death or lifelong disability.1 Whereas anti-HPA-1a-associated FNAIT occurs in around 1 in 1000 pregnancies,2 FNAIT-associated ICH is rarer and estimated to occur in as few as 1 in 10 000 fetuses/newborns.3 With ∼10 million annual deliveries in North America and Europe alone,4 this incidence rate translates to ∼1000 cases of ICH from HPA-1a alloimmunization every year.
FNAIT is the platelet counterpart to hemolytic disease of the fetus and newborn in which the mother becomes immunized against paternally inherited antigens on the fetus’ red blood cells. Among whites, ∼80% of FNAIT cases are caused by alloantibodies against the human platelet antigen 1a (HPA-1a). These antibodies are not only the most common but also responsible for the most severe cases of FNAIT. The HPA-1a antigen is located on the β3 integrin GPIIIa, which is present on the platelet surface as early as 16 weeks of intrauterine life.5 HPA-1a and HPA-1b are the 2 allelic variants of the β3 integrin molecule. A substitution of proline for leucine at amino acid residue 33 of the β3 integrin molecule determines the difference between HPA-1a and HPA-1b.6
For ∼3 decades, it has been known that the propensity of HPA-1a− pregnant women to develop HPA-1a antibodies is closely associated with a certain HLA type: HLA-DRB3*01:01 or HLA-DR52a, which is the serological nomenclature for the HLA molecule encoded by the HLA-DRA1 and the HLA-DRB3*01:01 chains. Elegant in vitro studies have demonstrated that peptides harboring the HPA-1a antigen fit into the cleft of the HLA molecule encoded by the HLA-DRA1 and the HLA-DRB3*01:01 chains.7,8 In addition, antigen-presenting cells expressing this HLA molecule can efficiently present peptides harboring the HPA-1a epitope for HPA-1a-specific T cells,9,10 which in turn provides help for HPA-1a-specific B cells that eventually differentiate into anti-HPA-1a-producing plasma cells.
Although the association between HPA-1a immunization and HLA-DRB3*01:01 has been recognized for many years, there is a small proportion of HPA-1a− women who become HPA-1a immunized in association with pregnancy and delivery. Whether fetal outcome of HPA-1a-immunized mothers’ pregnancies is dependent on the maternal HLA-DRB3*01:01 carrier status has not been elucidated. Therefore, the aims of this systematic review and meta-analysis were to examine the associations in HPA-1a− women between HLA-DRB3*01:01 carrier status and (1) maternal HPA-1a immunization and (2) fetal/neonatal outcome of children born of HPA-1a-immunized women. Fetal/neonatal outcome was examined both with regard to severe fetal/neonatal thrombocytopenia, as defined by platelet count <50 × 109/L, and ICH because the later outcome variable is clinically a more important outcome than platelet count.
Materials and methods
This review was conducted according to the PRISMA Statement for systematic reviews and meta-analyses.11
Information sources and search
MEDLINE, EMBASE, and Cochrane databases were searched from 1946 until March 2019. The literature search was conducted with the support of an information specialist (see supplemental Information about search strategy). All citations were reviewed in duplicate (J.K.-K. and N.S.) to determine eligibility. Studies fulfilling all of the following criteria were included: (1) original research; (2) 5 or more newborns with FNAIT; (3) description of maternal HLA-DRB3 type (carrier status of the HLA-DRB3*01:01 allele or expression of HLA-DR52a), maternal HPA-1a-immunization status, and fetal/neonatal outcome (platelet count and ICH/intrauterine death); and (4) published in English.
The extracted data from eligible studies were reviewed by 2 of the authors (J.K.-K. and N.S.). Where disagreements occurred, 4 reviewers (J.K.-K., D.F., M.K., and N.S.) performed a full manuscript review, and eligibility was independently reassessed. Remaining disagreements were resolved by consensus.
Assessment of risk of bias
Synthesis of results
In the presence of methodological and clinical homogeneity, data were combined across studies to estimate the pooled odds ratios (ORs) and the associated 95% confidence intervals (CIs) for the outcomes of HPA-1a immunization and severe thrombocytopenia. ORs > 1 indicated an increased risk of maternal alloimmunization or severe neonatal outcome and ORs < 1 the opposite. We used the Mantel-Haenszel method for random effects model in anticipation of potential heterogeneity.
Subgroup analysis was conducted to determine whether the odds of severe neonatal thrombocytopenia in HPA-1a-immmunized women differed according to whether the pregnancy was prospectively screened, or identified as at risk for FNAIT retrospectively.14 Statistical heterogeneity was assessed using χ2 test and quantified using the I2 statistic.15 An I2 > 50% and 75% was considered a priori to reflect moderate and high statistical heterogeneity, respectively. Data analyses were performed using Review Manager (version 5.3; the Cochrane Collaboration, Oxford, United Kingdom).
Proportions of alloimmunized women giving birth to severely thrombocytopenic children were expressed with corresponding 95% CIs, calculated according to the “score method.”16
Results
Study selection and characteristics
Figure 1 summarizes the study selection, and the search terms are detailed in supplemental Information about search strategy. Of 6226 full-text articles, 19 were identified as potentially eligible. Of these 19 articles,17-35 7 were excluded for the following reasons: in 1 prospective study, only 2 cases of FNAIT were reported22 ; 1 paper included data of neonates with FNAIT and severe thrombocytopenia but did not present specific data on HLA-DRB3*01:0117 ; and 5 papers contained data that had been published in other papers.18,20,23-25 Characteristics of studies that were not included in the review are presented in supplemental Table 1.
A total of 12 studies met our full eligibility criteria. Four studies were prospective screening studies,26,29,32,35 in which all pregnant women within a certain geographical area and time period were HPA-1a typed to identify women at risk of having their pregnancy complicated by FNAIT. Seven19,21,27,28,31,33,34 studies were retrospective studies from platelet laboratories that had received mother/child samples for FNAIT investigation. One study reported results from 13 mother/child samples: 8 pairs of samples sent for FNAIT investigation and 5 pairs referred because a sister of the mother had had a child with FNAIT.30
The characteristics of studies reporting maternal HLA-DRB3*01:01 status in HPA-1a-immunized women and controls are presented in Table 1. The association between HLA-DRB3*01:01 carrier status and alloimmunization was recently discussed in the clinical guidelines for FNAIT management developed by Lieberman et al36 ; however, the current study represents an update and extension of our previous review. One prospective study29 did not report the number of HLA-DRB3*01:01+ and HLA-DRB3*01:01− women, but estimates were calculated from the reported positive and negative predictive values (supplemental Information regarding study29 ). The nonimmunized controls in the 3 prospective studies29,32,35 and in the study by L'Abbé et al27 consisted of nonimmunized HPA-1a− women who had given birth to an HPA-1a+ child. For the remaining retrospective studies, 2 used healthy hematopoietic stem cell donors as controls,21,34 1 used individuals included in the 11th International Histocompatibility Workshop,19 1 used a control group of 24 women who delivered term newborns with thrombocytopenia and/or suspicion of FNAIT, but for whom the diagnosis of FNAIT was excluded,28 and the last study used a control group consisting of 5 HPA-1a+ and 5 HPA-1a− individuals.33 Of the 5 HPA-1a− individuals, there were 4 females, 3 of whom had previously given birth to a nonthrombocytopenic child and 1 had never been pregnant.33
Characteristics of studies reporting maternal HLA-DRB3*01:01 carrier status in HPA-1a-immunized women and controls
| First author, yReference . | Country . | No. of HPA-1a-immunized women . | No. of nonimmunized controls* . | ||
|---|---|---|---|---|---|
| No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | ||
| Prospective studies | |||||
| Williamson, 199835 | United Kingdom | 43 | 1 | 80 | 261 |
| Maslanka, 200329 | Poland | 12 | 0 | 29† | 71† |
| Turner, 200532 | United Kingdom | 18 | 7 | 89 | 189 |
| Retrospective studies | |||||
| Valentin, 199033 | France | 18 | 0 | 6 | 4 |
| L'Abbé, 199227 | Canada | 32 | 3 | 3 | 7 |
| Braud, 199419 | France | 51 | 1 | 32 | 147 |
| Loewenthal, 201328 | Israel | 20 | 3 | 2 | 22 |
| Delbos, 201621 | France | 38 | 7 | 33 | 67 |
| Wienzek-Lischka, 201734 | Germany | 99 | 2 | 23 | 77 |
| First author, yReference . | Country . | No. of HPA-1a-immunized women . | No. of nonimmunized controls* . | ||
|---|---|---|---|---|---|
| No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | ||
| Prospective studies | |||||
| Williamson, 199835 | United Kingdom | 43 | 1 | 80 | 261 |
| Maslanka, 200329 | Poland | 12 | 0 | 29† | 71† |
| Turner, 200532 | United Kingdom | 18 | 7 | 89 | 189 |
| Retrospective studies | |||||
| Valentin, 199033 | France | 18 | 0 | 6 | 4 |
| L'Abbé, 199227 | Canada | 32 | 3 | 3 | 7 |
| Braud, 199419 | France | 51 | 1 | 32 | 147 |
| Loewenthal, 201328 | Israel | 20 | 3 | 2 | 22 |
| Delbos, 201621 | France | 38 | 7 | 33 | 67 |
| Wienzek-Lischka, 201734 | Germany | 99 | 2 | 23 | 77 |
Control groups were different for the prospective studies and retrospective studies; see text.
These figures were not reported, but were calculated from the positive and negative predictive values that were provided in the paper. For details, see supplemental Information regarding study.29
The characteristics of the 9 studies21,26,28-32,34,35 reporting maternal HLA-DRB3*01:01 carrier status and fetal/neonatal outcome of children born of HPA-1a-immunized women are presented in Table 2. There were 4 prospective26,29,32,35 and 5 retrospective studies.21,28,30,31,34 One of the retrospective studies30 contained 2 populations with different risk profiles: 8 mother/child samples from cases where FNAIT was suspected (high risk of FNAIT) and 5 pairs of samples from referred cases in which a sister of the mother had had a child with FNAIT (low risk of FNAIT). For simplicity, this study was listed together with the retrospective studies in Table 2.
Characteristics of studies reporting maternal HLA-DRB3*01:01 carrier status and fetal/neonatal outcome in HPA-1a-immunized women
| First author, yReference . | Country . | Years of data collection . | No. with platelet counts ≥50 × 109/L . | No. with platelet counts <50 × 109/L (no. with ICH/death) . | Comments . | ||
|---|---|---|---|---|---|---|---|
| No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | ||||
| Prospective studies | |||||||
| Williamson, 199835 | United Kingdom | September 1993-September 1994 | 23 | 0 | 10 (1) | 0 | |
| Maslanka, 200329 | Poland | — | 9 | 0 | 3 (1) | 0 | |
| Turner, 200532 | United Kingdom | August 1999-March 2001 | 11 | 7 | 5 (0) | 0 | Only fetal platelet counts from 23 of 25 cases. |
| Kjeldsen-Kragh, 201926 | Norway | December 1995-March 2004 | 73 | 11 | 46 (1) | 0 | DRB3*01:01 status was unknown for 1 woman who gave birth to a child with ICH, platelet count of 26 × 109/L. HPA-1a-immunized women were offered cesarean section 2-4 wk before term. |
| Retrospective studies | |||||||
| Loewenthal, 201328 | Israel | May 2001-January 2011 | 0 | 1 | 18 (0*) | 1 (0*) | None of the women were treated with IVIG. |
| Peterson, 201330 † | United States | — | 2 | 4‡ | 0 | 4 (0)§ | A selection of women with low-avidity HPA-1a antibodies. |
| Delbos, 201621 | France | 1987-2012 | — | — | 38 (14) | 7 (2) | All FNAIT cases had platelet counts <50 × 109/L. No information about other obstetric or neonatal conditions that could cause ICH. |
| Sainio, 201731 | Finland | 1986-2010 | 11 | 0 | 60* (8*) | 0 | ICH in 2 infants born to DRB3*01:01− mothers without HPA-1a antibodies. Both cases had associated obstetric problems (severe asphyxia and subependymal hemorrhage associated with vacuum extraction). |
| Wienzek-Lischka, 201734 | Germany | — | 10 | 1* | 89 (21) | 1* (0) | According to the authors, none of the women were treated with IVIG. |
| First author, yReference . | Country . | Years of data collection . | No. with platelet counts ≥50 × 109/L . | No. with platelet counts <50 × 109/L (no. with ICH/death) . | Comments . | ||
|---|---|---|---|---|---|---|---|
| No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | No. of HLA-DRB3*01:01+ . | No. of HLA-DRB3*01:01− . | ||||
| Prospective studies | |||||||
| Williamson, 199835 | United Kingdom | September 1993-September 1994 | 23 | 0 | 10 (1) | 0 | |
| Maslanka, 200329 | Poland | — | 9 | 0 | 3 (1) | 0 | |
| Turner, 200532 | United Kingdom | August 1999-March 2001 | 11 | 7 | 5 (0) | 0 | Only fetal platelet counts from 23 of 25 cases. |
| Kjeldsen-Kragh, 201926 | Norway | December 1995-March 2004 | 73 | 11 | 46 (1) | 0 | DRB3*01:01 status was unknown for 1 woman who gave birth to a child with ICH, platelet count of 26 × 109/L. HPA-1a-immunized women were offered cesarean section 2-4 wk before term. |
| Retrospective studies | |||||||
| Loewenthal, 201328 | Israel | May 2001-January 2011 | 0 | 1 | 18 (0*) | 1 (0*) | None of the women were treated with IVIG. |
| Peterson, 201330 † | United States | — | 2 | 4‡ | 0 | 4 (0)§ | A selection of women with low-avidity HPA-1a antibodies. |
| Delbos, 201621 | France | 1987-2012 | — | — | 38 (14) | 7 (2) | All FNAIT cases had platelet counts <50 × 109/L. No information about other obstetric or neonatal conditions that could cause ICH. |
| Sainio, 201731 | Finland | 1986-2010 | 11 | 0 | 60* (8*) | 0 | ICH in 2 infants born to DRB3*01:01− mothers without HPA-1a antibodies. Both cases had associated obstetric problems (severe asphyxia and subependymal hemorrhage associated with vacuum extraction). |
| Wienzek-Lischka, 201734 | Germany | — | 10 | 1* | 89 (21) | 1* (0) | According to the authors, none of the women were treated with IVIG. |
Numbers obtained from the authors.
This study contained 2 populations with different risk profiles; see text.
Three of these were infants born of women whose sister previously had had a child with FNAIT. Two women received IVIG treatment during pregnancy.
Data from 2 severely thrombocytopenic children were also included in the analysis, despite HPA-1a typing lacking for these 2 children. According to the authors, the child’s platelet type in case 5 was HPA-1a/b and not HPA-1a/a as indicated in the paper.30
HLA-DRB3*01:01 carrier status as a risk factor for HPA-1a immunization
Three prospective and 6 retrospective studies examined the HLA-DRB3*01:01 type in HPA-1a-immunized women and controls (Table 1).
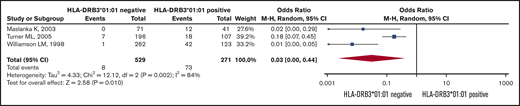
Six retrospective studies19,21,27,28,33,34 used different control groups, and for this reason we did not pool the results from these studies. Figure 2 shows that the pooled ORs (95% CI) for becoming HPA-1a immunized is 0.03 (95% CI, 0.0-0.44; I2 = 84%) for HPA-1a− and HLA-DRB3*01:01− women, as opposed to women expressing this HLA allele (Figure 2).
Risk of HPA alloimmunization in HPA-1a− mothers. Forest plot of HLA-DRB3*01:01 status and risk of HPA alloimmunization in HPA-1a− mothers.
Risk of HPA alloimmunization in HPA-1a− mothers. Forest plot of HLA-DRB3*01:01 status and risk of HPA alloimmunization in HPA-1a− mothers.
HLA-DRB3*01:01 carrier status and fetal/neonatal outcome in HPA-1a-immunized women
Table 2 summarizes the results from 9 studies of HPA-1a-immunized women, in which fetal/neonatal outcome had been reported in relation to maternal HLA-DRB3*01:01 status.
In the 4 prospective studies, there were 64 newborns with severe thrombocytopenia (platelet count <50 × 109/L) born of HPA-1a-immunized, HLA-DRB3*01:01+ mothers, and of these 64 cases, 3 suffered ICH (4.7%; 95% CI, 1.6-12.9). None of the 18 children born of HPA-1a-immunized, HLA-DRB3*01:01− women in the prospective studies were severely thrombocytopenic (0.0%; 95% CI, 0.0-17.6).
In the 5 retrospective studies,21,28,30,31,34 there were 205 severely thrombocytopenic children born of HLA-DRB3*01:01+ women, and 43 of these children suffered ICH, equivalent to 21.0% (95% CI, 16.0-27.1). Of the children born of HPA-1a-immunized, HLA-DRB*01:01− women, there were 13 neonates with platelet counts <50 × 109/L of whom 2 suffered ICH (15.4%; 95% CI, 4.3-42.2). Figure 3 shows the result of the meta-analysis of 2 prospective studies26,32 and 2 retrospective studies that reported fetal/neonatal thrombocytopenia.28,34 The results from the other 2 prospective studies,29,35 and the study by Sainio et al31 could not be included in the meta-analysis because there were no severely thrombocytopenic children born of HLA-DRB3*01:01− women in these studies. In 1 of the other retrospective studies,21 the authors only included mother/child samples from cases where the child had suffered severe thrombocytopenia (ie, newborns with platelet counts <50 × 109/L). Thus, for the meta-analysis, it was not possible to determine which proportion of the newborns with FNAIT were severely thrombocytopenic. Furthermore, the study by Peterson et al30 contained 2 populations with different risk profiles: mother/child samples from cases where FNAIT was suspected (high risk of FNAIT) and pairs of samples from referred cases where a sister had had a child with FNAIT (low risk of FNAIT). In addition, all women in this study30 were anti-HPA-1a− by standard methods for analyzing platelet antibodies, but they were positive when subjected to surface plasmon resonance (SPR) analysis. This type of analysis is extremely sensitive, but laborious and requires special equipment that limits its use to special research laboratories. For these 2 reasons, this study30 was considered inappropriate to include in the meta-analysis.
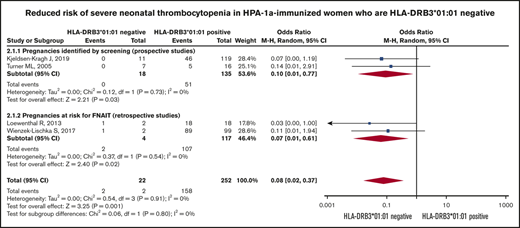
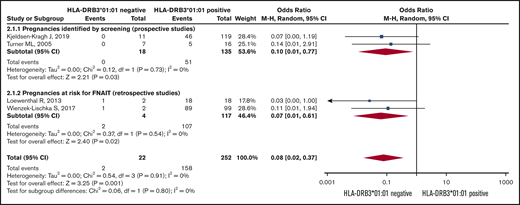
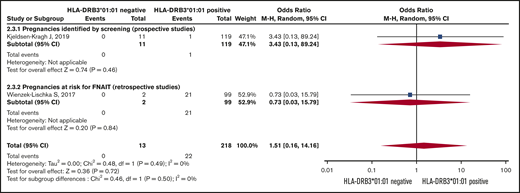
Risk of severe neonatal thrombocytopenia. Forest plot of maternal HLA-DRB3*01:01 status and risk of severe neonatal thrombocytopenia in HPA-1a-immunized women subgrouped according to whether the pregnancy was prospectively screened or retrospectively identified as at risk for FNAIT.
Risk of severe neonatal thrombocytopenia. Forest plot of maternal HLA-DRB3*01:01 status and risk of severe neonatal thrombocytopenia in HPA-1a-immunized women subgrouped according to whether the pregnancy was prospectively screened or retrospectively identified as at risk for FNAIT.
The Forest plot (Figure 3) shows that the pooled OR (95% CI) for having a severely thrombocytopenic child is 0.08 (0.02-0.37; I2 = 0) for women who are HPA-1a− and HLA-DRB3*01:01−, as opposed to women expressing this HLA allele.
The meta-analysis for ICH was based on 2 studies (Figure 4).26,34 The women’s HLA-DRB3*01:01 carrier status did not significantly influence the risk of having a child with ICH.
Risk of intracranial hemorrhage. Forest plot of maternal HLA-DRB3*01:01 status and risk of intracranial hemorrhage in children born of HPA-1a− mothers.
Risk of intracranial hemorrhage. Forest plot of maternal HLA-DRB3*01:01 status and risk of intracranial hemorrhage in children born of HPA-1a− mothers.
Quality of studies (risk of bias)
The quality assessment of studies is shown in Table 3. Sample size was not predetermined in any study, neither were blinded outcome assessments performed, nor confounding factors analyzed. For the 8 retrospective studies,19,21,27,28,30,31,33,34 where platelet laboratories had received mother/child samples for FNAIT investigation, there is a potential risk of a selection bias toward the more severe FNAIT cases because HLA typing would have been conducted after the risk of FNAIT had been identified.
Quality assessment of all 12 included studies
| First author, yReference . | Adequate description of study population . | Tests conducted before FNAIT diagnosed . | Sample size predetermined . | All eligible patients screened . | Were normal samples included? . | Clear definitions of associations . | Blind outcome assessment . | Reproducibility of testing assessed . | Missing data reported . | Confounding factors analyzed . |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||||
| Williamson, 199835 | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Maslanka, 200329 | No | Yes | No | Yes | No | Yes | No | No | No | No |
| Turner, 200532 | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Kjeldsen-Kragh, 201926 | Yes | No | No | No | No | Yes | No | No | Yes | No |
| Retrospective studies | ||||||||||
| Valentin, 199033 | No | No | No | No | Yes | Yes | No | No | No | No |
| L'Abbé, 199227 | Yes | Yes | No | No | Yes | Yes | No | No | No | No |
| Braud, 199419 | No | No | No | Yes | Yes | Yes | No | No | No | No |
| Loewenthal, 201328 | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes |
| Peterson, 201330 | Yes | No | No | Yes | No | Yes | No | No | Yes | No |
| Delbos, 201621 | Yes | No | No | Yes | Yes | Yes | No | No | No | Yes |
| Sainio, 201631 | Yes | No | No | No | Yes | Yes | No | No | No | Yes |
| Wienzek-Lischka, 201734 | Yes | No | No | Yes | No | Yes | No | No | No | No |
| First author, yReference . | Adequate description of study population . | Tests conducted before FNAIT diagnosed . | Sample size predetermined . | All eligible patients screened . | Were normal samples included? . | Clear definitions of associations . | Blind outcome assessment . | Reproducibility of testing assessed . | Missing data reported . | Confounding factors analyzed . |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||||
| Williamson, 199835 | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Maslanka, 200329 | No | Yes | No | Yes | No | Yes | No | No | No | No |
| Turner, 200532 | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Kjeldsen-Kragh, 201926 | Yes | No | No | No | No | Yes | No | No | Yes | No |
| Retrospective studies | ||||||||||
| Valentin, 199033 | No | No | No | No | Yes | Yes | No | No | No | No |
| L'Abbé, 199227 | Yes | Yes | No | No | Yes | Yes | No | No | No | No |
| Braud, 199419 | No | No | No | Yes | Yes | Yes | No | No | No | No |
| Loewenthal, 201328 | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes |
| Peterson, 201330 | Yes | No | No | Yes | No | Yes | No | No | Yes | No |
| Delbos, 201621 | Yes | No | No | Yes | Yes | Yes | No | No | No | Yes |
| Sainio, 201631 | Yes | No | No | No | Yes | Yes | No | No | No | Yes |
| Wienzek-Lischka, 201734 | Yes | No | No | Yes | No | Yes | No | No | No | No |
Table 4 describes the GRADE assessment. The quality of evidence for the outcome of thrombocytopenia was moderate, low for HPA alloimmunization, and very low for ICH.
Overall quality of the included studies assessed by using the GRADE approach
| Quality assessment . | No. of patients, n/N (%) . | Effect . | Quality . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies . | Design . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Other considerations . | HLA-DRB3*0101− . | Control . | Relative OR (95% CI) . | Absolute . | |
| HLA-DRB3*0101−to predict risk of HPA alloimmunization in HPA 1a−women | |||||||||||
| 3 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 8/529 (1.5) | 73/271 (26.9) | 0.03 (0.0-0.44) | 258 fewer per 1000 (from 130 fewer to 269 fewer) | ⊕⊕○○ LOW |
| HLA-DRB3*0101−to predict risk of severe neonatal thrombocytopenia in HPA alloimmunized women | |||||||||||
| 4 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | Strong association* | 2/22 (9.1) | 158/252 (62.7) | 0.08 (0.02-0.37) | 508 fewer per 1000 (from 244 fewer to 594 fewer) | ⊕⊕⊕○ MODERATE |
| HLA-DRB3*01:01−to predict risk of intracranial hemorrhage | |||||||||||
| 2 | Observational studies | Serious† | Serious†,‡ | No serious indirectness | Serious† | None | 0/13 (0) | 22/218 (10.1) | 1.51 (0.16- 14.16) | 44 more per 1000 (from 83 fewer to 513 more) | ⊕○○○ VERY LOW |
| Quality assessment . | No. of patients, n/N (%) . | Effect . | Quality . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies . | Design . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Other considerations . | HLA-DRB3*0101− . | Control . | Relative OR (95% CI) . | Absolute . | |
| HLA-DRB3*0101−to predict risk of HPA alloimmunization in HPA 1a−women | |||||||||||
| 3 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 8/529 (1.5) | 73/271 (26.9) | 0.03 (0.0-0.44) | 258 fewer per 1000 (from 130 fewer to 269 fewer) | ⊕⊕○○ LOW |
| HLA-DRB3*0101−to predict risk of severe neonatal thrombocytopenia in HPA alloimmunized women | |||||||||||
| 4 | Observational studies | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | Strong association* | 2/22 (9.1) | 158/252 (62.7) | 0.08 (0.02-0.37) | 508 fewer per 1000 (from 244 fewer to 594 fewer) | ⊕⊕⊕○ MODERATE |
| HLA-DRB3*01:01−to predict risk of intracranial hemorrhage | |||||||||||
| 2 | Observational studies | Serious† | Serious†,‡ | No serious indirectness | Serious† | None | 0/13 (0) | 22/218 (10.1) | 1.51 (0.16- 14.16) | 44 more per 1000 (from 83 fewer to 513 more) | ⊕○○○ VERY LOW |
Relative risk < 0.5
One study was a prospective screening study and the other was a retrospective study of neonates suspected of having FNAIT.
The ORs of the individual studies are not in the same direction.
Discussion
Main findings
The current systematic review and meta-analysis has confirmed that the risk for becoming HPA-1a immunized is much lower for women who are HPA-1a− and HLA-DRB3*01:01−, as opposed to HPA-1a− women expressing this HLA allele.
Furthermore, the HLA-DRB3*01:01 carrier status influences fetal/neonatal outcome: a total of 237 severely thrombocytopenic children (those with platelet counts <50 × 109/L) were born of the 400 HPA-1a-immunized women who were included in 4 prospective26,29,32,35 and 4 retrospective28,30,31,34 studies (Table 2). (The study by Delbos et al21 has been excluded in this context because platelet count <50 × 109/L was used as a selection criterion.) Only 6 of these 237 children were born of HLA-DRB3*01:01− women. Accordingly, the meta-analysis of the 2 prospective32,37 and the 2 retrospective studies28,34 revealed that the pooled OR (95% CI) for having a severely thrombocytopenic child was 0.08 (0.02-0.37), for HPA-1a-immunized women who are HLA-DRB3*01:01−, as opposed to women expressing this HLA allele. The ORs determined by the subgroup analyses of the prospective and retrospective studies, respectively, were similar.
Finally, the meta-analysis did not show any association between HLA-DRB3*01:01 carrier status and risk for FNAIT-induced ICH.
The study by Delbos et al21 could not be included in the meta-analysis because this study only included cases where the newborns were severely thrombocytopenic. Yet, in this study, there were 2 HPA-1a-immunized HLA-DRB3*01:01− women who gave birth to a child with ICH. Unfortunately, clinical information was not available about these 2 children, and thus, it is not known if there were any concurrent obstetric or neonatal conditions that are known to predispose to ICH, or even if the ICHs were grade 1, which are clinically insignificant and may occur in full-term newborns as well. In addition, data for this study were collected by a large reference laboratory in France over a 25-year period.
In 1 of the included prospective studies,26 it was demonstrated, first, that the level of anti-HPA-1a was significantly lower in HLA-DRB3*01:01− women as opposed to women who have this allele, and second, that the neonatal platelet count was significantly higher in children born of HLA-DRB3*01:01− as opposed to HLA-DRB3*01:01+ women. Hence, it is possible that the effect of HLA-DRB3*01:01 carrier status on fetal/neonatal outcome is mediated via different levels in HPA-1a antibody levels. This hypothesis is in accordance with a recent systematic review that demonstrated an association between maternal anti-HPA-1a-levels and fetal/neonatal outcome.38
Strengths and limitations
The current review is the first study that systematically has examined the association between maternal HLA-DRB3*01:01 carrier status in HPA-1a− women and (1) risk of HPA-1a-alloimmunization and (2) neonatal outcome of children born of HPA-1a-immunized women. Despite FNAIT being a rare disease, the prospectively identified FNAIT cases included in the current review originated from populations comprising more than 150 000 pregnant women.26,29,32,35 However, the largest number of HPA-1a-immunized FNAIT cases were from retrospective studies where platelet immunology laboratories have received mother/child samples for FNAIT examination. Mild to moderate FNAIT cases may not be identified clinically, and platelet immunology laboratories only rarely receive samples from such cases. Thus, retrospective FNAIT studies carry an inherent risk of selection bias toward more severe neonatal cases.
In 1 of the retrospective studies,30 samples from suspected FNAIT cases where HPA-1a antibodies could not be detected by traditional serological assays were subjected to SPR analysis. In this study, the authors found that 8 of 10 HLA-DRB3*01:01− women had low-avidity HPA-1a antibodies. Hence, the proportion of HPA-1-a-immunized, HLA-DRB3*01:01− women, as well as proportion of affected newborns, was much higher in this study compared with the other retrospective studies. Thus, it is conceivable that the majority of HLA-DRB3*01:01− women, who become HPA-1a immunized, preferentially develop low-avidity HPA-1a antibodies with no or marginal clinical consequences. However, 4 of 8 HLA-DRB3*01:01− women in this study by Peterson et al30 gave birth to children with platelet counts <50 × 109/L, so there seems to be a subpopulation of HPA-1a-immunized HLA-DRB3:01:01− women who develop antibodies that can cause severe thrombocytopenia. This subpopulation seems to be very small: first, because none of the HLA-DRB3*01:01− women in the 4 prospective studies,26,29,32,35 representing more than 150 000 pregnancies, gave birth to severely thrombocytopenic newborns; and second, because only 13 of 218 severely thrombocytopenic children from the 5 retrospective studies21,28,30,31,34 were born of HLA-DRB3*01:01− women.
The results published by Kjeldsen-Kragh et al26 were based on data from the hitherto largest prospective FNAIT study.25 This was an observational study intended to reduce the morbidity and mortality associated with FNAIT. Women who were HPA-1a immunized were offered cesarean section 2 to 4 weeks before term. Hence, the neonatal platelets count in this study may not represent the natural history of FNAIT, and it is possible that platelet counts of children would have been somewhat lower if the women had given birth at term, which in principle could have influenced the number of severely thrombocytopenic children born of HLA-DRB3*01:01+ as well as HLA-DRB3*01:01− women. Also, that the number of fetal/neonatal ICH was significantly lower in this study,25 compared with previously published prospective FNAIT studies, could be due to 2- to 4-week preterm delivery, although most cases of ICH occur in utero around gestational week 28 weeks or earlier.39
Even though the meta-analysis did not reveal any significant difference in the risk of ICH for children born of women who were HLA-DRB3*01:01+ as compared with women lacking this HLA allele, this result should be interpreted cautiously because the meta-analysis was based on both a very small number of events and a small sample size, particularly with regard to the HLA-DRB3*01:01− group.
High-dose IV immunoglobulin (IVIG) treatment is usually used for pregnant women known to be HPA-1a immunized to reduce the risk of severe neonatal outcome. Although this could potentially have biased the results, only 4 women received this kind of treatment; 2 in the study by Maslanka et al29 and 2 in the study by Peterson et al.30 However, both studies were not included in the meta-analysis for the reasons explained previously. Thus, administration of IVIG treatment did not bias the results.
There are many caveats considering that the studies included in this review were all performed differently and represent a time frame covering many years. If SPR analysis had been used in the prospective studies, it is possible that the number of HPA-1a-immunized women would have been higher. Moreover, the retrospective studies are subject to selection bias, and generally represent more severe clinical cases than the prospective studies.
Finally, the few prospective studies included in the meta-analyses were all from Europe. It would have been preferable to have data from prospective FNAIT studies representing different populations.
Interpretation
The overall result of the current systematic review and meta-analysis show that the odds of severe fetal/neonatal outcome are low for HPA-1a-immunized women who are HLA-DRB3*01:01−. Consequently, HLA-DRB3*01:01 typing could be used for risk stratification when counseling HPA-1a− pregnant women. This also could be relevant for a pregnant woman if she has been identified as HPA-1a− by virtue of being a blood donor, or if her sister previously has had a pregnancy complicated by FNAIT. If she is HLA-DRB3*01:01−, it is worthwhile considering if further follow-up during pregnancy is necessary. Even if she should become HPA-1a immunized during pregnancy, the risk of developing anti-HPA-1a levels in levels high enough to produce significant thrombocytopenia in the fetus/newborn is very low. However, decisions on prenatal management in this setting should of course ultimately be determined by the parents together with their physician. In case it is decided that no or minimal clinical follow-up during pregnancy should be conducted, it is essential that platelet counts are determined immediately after delivery to identify those very few newborns who may have low platelet counts.
In past years, the European Union-financed PROFNAIT Consortium worked to develop a hyperimmune anti-HPA-1a immunoglobulin G (IgG) to be used as a prophylaxis against HPA-1a immunization, analogous to anti-D, which has been used successfully during the past 5 decades for the prevention of RhD immunization and hemolytic disease of the fetus and newborn.40 The hyperimmune anti-HPA-1a IgG drug product is manufactured from plasma collected from HPA-1a-immunized women; hence, the supply of plasma for drug production is limited. It would therefore be important to reserve prophylactic treatment, when available for clinical use, for HPA-1a− women with a high risk of having a child with severe thrombocytopenia. Based on the systematic review and meta-analysis reported here, determination of the HLA-DRB3*01:01 carrier status of HPA-1a− pregnant women would appear to be an appropriate test for identification of women to be treated with hyperimmune anti-HPA-1a IgG.
In summary, this systematic review and meta-analysis has shown that HLA-DRB3*01:01 typing can serve as an important tool for risk stratification of HPA-1a− pregnant women, both for risk of immunization and of severe neonatal outcome.
Requests for data sharing should be e-mailed to the corresponding author, Jens Kjeldsen-Kragh (jkk@prophylix.com).
Acknowledgments
The authors thank Elizabeth Uleryk for the search strategy and Sylvia Torrance and Kimberly Figures for administrative assistance.
This work was supported in part by Canadian Blood Services. Canadian Blood Services did not have any role in the design, analysis, and interpretation of the data or preparation, review, and approval of the manuscript.
Authorship
Contribution: J.K.-K. drafted the initial manuscript; N.S. and D.L. performed the data search; J.K.-K. extracted and analyzed the data; N.S. performed the meta-analysis; and D.A.F., M.K., L.L., A.G., M.F.M., J.B., T.B., S.C., G.B., D.O., J.M.B., H.H., E.M., C.K., D.M.A., S.B., G.R., and H.S. participated in the interpretation and revision of critically important contents of the review and gave final approval of the version to be submitted.
Conflict-of-interest disclosure: J.K.-K. and M.K. are 2 of the founders and owners of Prophylix AS, which has been developing a hyperimmune anti-HPA-1a IgG for the prevention of fetal and neonatal alloimmune thrombocytopenia. J.K.-K. and M.K. are consultants for Rallybio IPA, LLC, which recently acquired the assets of Prophylix AS. N.S. is a consultant for Canadian Blood Services. D.O. has received research funding for the project “Towards Routine HPA-Screening in Pregnancy.” J.B. is a consultant for Superior Biologics and Rallybio IPA, LLC. The remaining authors declare no competing financial interests.
C.K. is retired from the Institut National de la Transfusion Sanguine, Paris, France.
Correspondence: Jens Kjeldsen-Kragh, Department of Clinical Immunology and Transfusion Medicine, University and Regional Laboratories, Region Skåne, Akutgatan 8, SE-22185 Lund, Sweden; e-mail: jkk@prophylix.com.
References
Author notes
The full-text version of this article contains a data supplement.