This article has a companion Point by Scott.
Introduction
Nearly 40 years ago when allogeneic hematopoietic cell transplantation (allo-HCT) starting gaining traction as a viable clinical modality, it was the court of last resort. By the time patients got to transplant, they had exhausted most available therapeutic options. Transplantation was the last line of defense, combining very high doses of chemo-radiotherapy with the often-toxic effects of alloimmunity. After allo-HCT in those days, patients were usually in no shape to tolerate additional antitumor treatment, and there were few, if any, novel strategies to treat or prevent relapse.
Over the past 20 years, the landscape has changed considerably. Transplantation is typically performed earlier in the disease course, less toxic conditioning regimens have been adopted, and supportive care in terms of antimicrobial treatment/prophylaxis has improved considerably so that patients today are better candidates to receive tumor-directed treatment posttransplant. More important, a whole slew of new antineoplastic agents are now available for study and/or use. These include tyrosine kinases targeting pathogenic mutations, epigenetic modifiers, checkpoint inhibitors, selective bcl-2 inhibitors, antibody-drug conjugates, and bispecific antibodies, not to mention unmodified and engineered cellular products.
Given the poor outcome of patients who relapse after allo-HCT, it is tempting to take advantage of the availability of these agents and apply them earlier to try to prevent relapse rather than react to it. I was charged with the task of arguing against maintenance therapy, which to some may sound like arguing against world peace, but the issue is far more nuanced, particularly because we infrequently have the benefit of prospective randomized trials on which to base our decisions. Also, the value of maintenance strategies has not been convincingly demonstrated in the transplant-naive setting so it is hard to argue they should be used indiscriminately posttransplant.
Maintenance after autologous hematopoietic transplantation
Maintenance therapy after transplantation has been most comprehensively studied in the autologous setting. Several prospective randomized trials have established that lenalidomide decreases rates of disease recurrence and improves both progression-free survival (PFS) in patients undergoing auto-HCT for multiple myeloma despite an increased risk of secondary malignancies.1 In lymphoma, the argument for maintenance is murkier. Brentuximab vedotin (BV) has been demonstrated to improve PFS but not overall survival (OS) after auto-HCT for Hodgkin lymphoma, so its ultimate value has been a subject of debate.2 Rituximab (R) has been evaluated as maintenance after auto-HCT for both diffuse large B-cell lymphoma and follicular lymphoma with no impact on PFS or OS for those having received prior rituximab.3,4 In contrast, there appears to be benefit in patients with mantle cell lymphoma.5 A joint consensus project sponsored by American Society of Blood and Marrow Transplantation (American Society for Transplantation and Cellular Therapy), Center for International Blood and Marrow Transplant Research, and European Society for Blood and Marrow Transplantation examining maintenance endorsed BV maintenance for BV-naive high-risk Hodgkin lymphoma patients, R maintenance for mantle cell lymphoma patients transplanted in first remission, R maintenance for follicular lymphoma patients who are R naive (a vanishingly small population), and no maintenance for diffuse large B-cell lymphoma.6 Further refinement of these recommendations will emanate from analyses identifying high-risk populations, such as those with minimal residual disease (MRD) after auto-HCT, to determine who truly benefits from maintenance.
Risks of maintenance therapy after allo-HCT
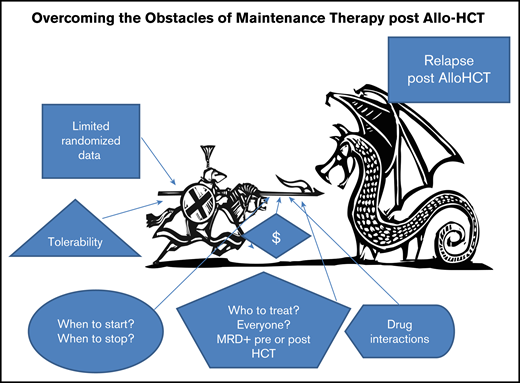
The nature of allo-HCT raises the ante in terms of potential adverse consequences of maintenance strategies. Early after allo-HCT, patients are typically prescribed medications that are immune suppressive, can affect hematopoietic recovery, and can compromise renal and hepatic function. Many proposed maintenance agents will elicit additive toxicities and could be associated with serious drug interactions, particularly with calcineurin inhibitors, mTor inhibitors, and azoles. Patients after allo-HCT are more prone to opportunistic infections and of course graft-versus-host disease (GVHD), complicating both the initiation and completion of maintenance therapy. In addition to clinical risk, there is the issue of financial toxicity. Many maintenance strategies incorporate expensive drugs and duration of treatment has not been determined, adding to the already substantial costs of allo-HCT. As well, patients who have undergone the ordeal of a transplant might find the burden of long-term administration of additional medications onerous, affecting their quality of life (Figure 1).
Who should receive maintenance therapy?
Should it be based on biologic/genomic characteristics at diagnosis, on MRD pretransplant, or rather on MRD detection early posttransplant (which should truly be considered preemptive therapy)? The answer to that question could depend on the gene(s) mutated, as well as on treatment variables such as conditioning intensity (reduced-intensity vs myoablative) and GVHD prophylaxis strategies (ie, in vivo or ex vivo T-cell depletion of use of posttransplant cyclophosphamide), which might itself affect the risk of posttransplant disease relapse. Trial design to determine study size to adequately address these questions will depend on the risk of relapse in each circumstance.
What strategies should we deploy?
If a targetable or “actionable” mutation has been identified, we might be tempted to use agents approved in the active disease setting, such as inhibitors of BCR-ABL, FLT-3, IDH1, or IDH2.
BCR-ABL
A CIBMTR registry analysis found no benefit in terms of relapse rates, locoregional-free survival, and OS for maintenance therapy posttransplant in chronic-phase chronic myeloid leukemia patients who received tyrosine kinase inhibitor (TKI) therapy before allo-HCT.7 In Ph+ acute lymphoblastic leukemia (ALL), a single-institution retrospective study failed to show benefit for posttransplant TKI.8 The CIBMTR found TKI use did not reduce 3-year cumulative incidence of relapse among 197 patients with Ph+ ALL undergoing allo-HCT in CR1.9 In contrast, an EBMT study of 473 patients with Ph+ ALL in CR1, of which 60 patients received maintenance therapy with TKIs, imatinib administration was dently associated with a lower relapse rate and improvements in locoregional-free survival and OS.10 Based on this, and scattered reports of tolerability and efficacy of TKI maintenance after allo-HCT for Ph+ ALL, the Acute Leukemia Working Party of EBMT issued a position statement supporting maintenance.11 However, the authors do state it is reasonable to delay initiation until detection of bcr-abl transcript posttransplant, effectively endorsing a preemptive strategy that is markedly different than maintenance. A prospective phase 2 study of 55 patients comparing maintenance and preemptive, MRD-triggered imatinib in Ph+ ALL demonstrated equivalent rates of hematological relapse and survival in both arms, although the maintenance strategy reduced molecular recurrence compared with preemptive therapy.12 Feasibility is also a consideration. One study examining maintenance nilotinib was terminated after only accruing 57 patients, with only 13 able to complete a full year of treatment.13
FLT3-ITD
Large registry analyses have demonstrated that FLT3-ITD mutations found in AML patients at diagnosis confer a higher risk of relapse after allo-HCT than in wild-type AML patients.14 Several TKIs that target FLT3-ITD have been studied as maintenance after transplant with potentially superior outcomes reported with sorafenib when compared with historical controls.15-18 A relatively small (83 total patients) prospective controlled randomized trial reported superior outcomes for patients in complete hematologic remission receiving sorafenib compared with placebo.19 However, little information has been provided as yet about FLT3 allelic frequencies at diagnosis, MRD pretransplant, or persistent/recurrent MRD after allo-HCT in this study, making it unclear what subset of patients benefit. Nonetheless, these and other single-arm data have led some to advocate blanket TKI use, even substituting other FLT3 inhibitors only partially tested after allo-HCT. This had in part affected participation in the large phase 3 prospective randomized trial of gilteritinib maintenance (NCT0299720.2) though it appears accrual is complete. This study includes a PCR-based MRD assay for FLT3-ITD and should provide critical insights to identify what patients if any should receive maintenance.20
IDH1/IDH2
Ivosidenib and enasidenib are recently approved agents targeting IDH1 and IDH2, respectively.21,22 They are reasonably well tolerated and could theoretically be used as maintenance agents after allo-HCT for patients whose leukemia carries those mutations. However, before jumping on that bandwagon, we must first understand the implication of these mutations of the dynamics of relapse after allo-HCT and systematically test tolerability of these agents in this setting. Trials doing just that are under way (NCT03515512, NCT03564821) but there is no evidence to support use of these agents at this point.
How about using nontargeted approaches with potential immune modulatory activity?
Hypomethylating agents
Azacitidine can induce remissions in patients who relapse after transplant.23 Activity of azacitidine for posttransplant relapse led to a series of small posttransplant prophylactic or preemptive trials. In patients with falling CD34 chimerism, azacitidine administration has been suggested to delay relapse. Induction of a CD8+ T-cell response after azacitidine correlated with protection from relapse.24,25 Yet another study combined azacitidine with sequential donor lymphocyte infusion.26 Although acute and chronic GVHD were noted, relapse rates were low and OS promising. The problem with these uncontrolled studies is that, of course, they are uncontrolled, and trying to perform a case control analysis is fraught with difficulties. Unfortunately, in a recently reported prospective randomized study of azacitidine (n = 93) vs observation (n = 94) after transplantation, there was no difference in disease recurrence. Relapse-free survival curves were virtually superimposable.27 On the other hand, an oral azacytidine formulation has been reported to be beneficial in a randomized trial as maintenance after induction chemotherapy for AML so hypomethylating agents likely need to be revisited after allo-HCT.28,29 Currently there is interest in testing hypomethylating agents with a variety of partners including agents like venetoclax, checkpoint inhibitors, and monoclonal antibodies, although evidence of benefit has yet to be demonstrated.
Conclusion
We perform transplants to cure patients of their malignancies. It makes complete sense that we do everything in our power to prevent relapse, but we must approach the problem methodically. We must resist widespread adoption of maintenance therapy after allo-HCT in the absence of understanding who will benefit as that will impede progress toward improving future outcomes for our patients.
Send data sharing requests via e-mail to the corresponding author, Robert J. Soiffer (robert_soiffer@dfci.harvard.edu).
Authorship
Contribution: R.J.S. wrote and edited the manuscript.
Conflict-of-interest disclosure: R.J.S. serves on the Board of Directors for Kiadis and Be The Match/National Marrow Donor Program; provided consulting for Gilead, Rheos Therapeutics, Cugene, Jazz, Mana Therapeutics, VOR Biopharma, and Novartis; and Data Safety Monitoring Board for Juno/Celgene.
Correspondence: Robert J. Soiffer, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: robert_soiffer@dfci.harvard.edu.