This article has a companion Counterpoint by Soiffer.
Introduction
Relapse remains the most common cause of failure following allogeneic hematopoietic cell transplantation (HCT) regardless of indication, particularly for high-risk acute leukemia (AL).1 Increasing conditioning intensity has largely failed due to increased treatment-related mortality.2 Limited options were available for maintenance therapy because of excessive toxicity, particularly following myeloablative conditioning. The development of less toxic therapy for ALs led to the emergence of several agents potentially useful for maintenance post-HCT. This issue is particularly relevant in patients receiving reduced-intensity conditioning (RIC) given the early and higher relapse rates observed in these patients.3 The emergence of options for maintenance therapy has raised several key questions beyond effectiveness. These include issues regarding duration, start time, interactions with clinical sequela of graft-versus-host disease, engraftment, hematopoietic toxicity, and potential impacts upon donor allograft effect. Despite these concerns, relapse remains the major cause of death, and there are immense costs to the patients from both a monetary and psychological perspective.
What is maintenance therapy?
While this seems self-explanatory, once we delve deeper, there are likely disagreements in how we define this term. Under what circumstances is treatment maintenance vs active, and how would one apply that using RIC? With RIC, persistent disease at day 28 may be expected and not indicative of disease progression. Additional issues regarding measurements of minimal residual disease (MRD) and minimal identifiable disease (MID)4,5 raise concerns about whether treatment is given for maintenance or relapse. Most post-HCT trials have considered maintenance to be treatment given to prevent rather than treat relapse. Relapse was defined based upon morphologic findings rather than presence of MRD or MID using flow cytometry, fluorescence in situ hybridization, karyotype, molecular analysis, or mutational studies. Given the disparate approaches used at academic centers to measure MRD/MID, it is difficult to design multicenter trials examining maintenance therapy that exclude patients with MRD/MID. For these reasons, maintenance therapy remains treatment given for patients without morphologic evidence of malignancy. As our ability to measure MRD/MID improves, there should be more universal adoption of these approaches, thus increasing the appropriate assignment of patients into clinical trials.
Who should receive maintenance therapy?
How are patients at high risk of post-HCT relapse defined? Disease-related factors such as cytogenetics,6 remission status,7 and mutations8 are known to be associated with higher rates of post-HCT relapse. More recent analyses have indicated that any measurable disease pre-HCT4,5 is associated with an increased risk of relapse. Additionally, HCT-related factors such as donor source9 and conditioning intensity3 have been associated with risk of relapse. In choosing who should receive maintenance therapy, it makes sense to focus on patients who are at higher risk while acknowledging that it may be less effective in this population; this would include patients in whom the expected 1-year risk of relapse would be >30%.
TKIs in patients with BCR/ABL+ ALL
A single-center retrospective analysis assessing maintenance tyrosine kinase inhibitors (TKIs) in 32 patients with various stages of BCR/ABL+ acute lymphoblastic leukemia (ALL), including active disease, using a variety of conditioning regimens and donor sources did not show a benefit in relapse-free survival (RFS).10 The European Society for Blood and Marrow Transplantation (EBMT) performed a retrospective analysis evaluating post-HCT TKI maintenance in 60 patients with BCR/ABL+ ALL in first complete remission (CR).11 The use of TKIs as prophylaxis was associated with a lower risk of relapse (hazard ratio [HR], 0.40; 95% confidence interval [CI], 0.21-0.76; P = .01) and improved overall survival (OS) (HR, 0.42; 95% CI, 0.23-0.76; P = .004). A retrospective analysis from the Center for International Blood and Marrow Transplant Research evaluating conditioning intensity included 43 patients who received maintenance TKIs (RIC, n = 21; myeloablative conditioning, n = 22).12 There was no impact on the 3-year relapse rate, but the data were analyzed separately based upon conditioning intensity. The major limitations include potential selection bias, lack of data regarding MRD-directed therapy, and the small number of patients who received maintenance. The German multicenter ALL group performed a randomized phase 2 trial comparing maintenance imatinib (n = 26) vs MRD-directed imatinib (n = 29) in patients with BCR/ABL+ ALL in CR following HCT.13 Maintenance imatinib significantly reduced the incidence of molecular recurrence (40% vs 69%, P = .046). There were no significant differences OS or RFS between the study arms.
FLT3 inhibitors as maintenance therapy in patients with AML
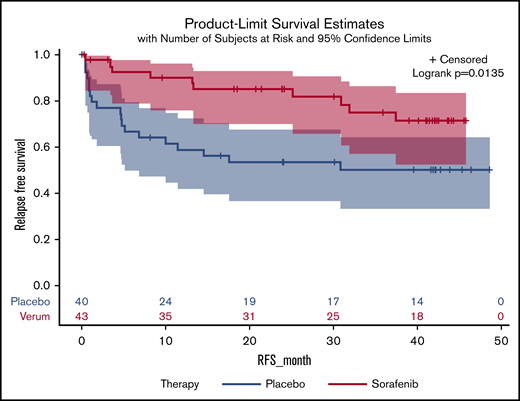
Midostaurin is the first FLT3 inhibitor approved for treatment in patients with acute myeloid leukemia (AML). The approval was based on a randomized trial showing improved OS in patients who received midostaurin in combination with induction and consolidation chemotherapy.14 Patients enrolled into this trial had the midostaurin stopped prior to HCT, and this study was not designed to address the role of post-HCT maintenance. Interestingly, there was a continued OS benefit in those patients who underwent HCT and received pre-HCT therapy with midostaurin. Several preliminary studies evaluated the use of FLT3 inhibitors for prevention of relapse post-HCT,15,16 Recently, 2 randomized phase 2 studies have been completed in patients with FLT3-ITD–mutated AML. The SORMAIN trial randomized 83 patients to either sorafenib (n = 43) or placebo (n = 40) and was stopped early due to slow accrual.17 There was a significantly improved RFS (HR, 0.39; 95% CI 0.18-0.85; P = .0135) (Figure 1) but no significant improvement in OS (HR, 0.52; 95% CI 0.24-1.1; P = .09). The RADIUS trial randomized 60 patients to receive either post-HCT midostaurin or standard of care (SOC).18 There were no significant differences in RFS (HR, 0.46; 95% CI, 0.12-1.86; P = .27) between the 2 arms of the trial. The estimated 2-year RFS was 85% for midostaurin and 76% for SOC (HR, 0.60; 95% CI 0.17-2.14; P = .4297; Figure 2). The estimated 2-year OS was 85% with midostaurin and 76% with SOC (HR, 0.58; 95% CI 0.19-1.79; P = .34). There was a 40% reduction in relapse risk and 42% reduction in death with midostaurin. Both studies excluded patients who received prior treatment with FLT-3 inhibitors and excluded patients who had morphologic evidence of post-HCT relapse. The BMT-CTN 1506 trial randomizes patients with FLT3-ITD–mutated AML who were previously treated with a FLT3 inhibitor to either gilteritinib or placebo(NCT02997202). While the results are currently unknown it was recently announced this pivotal study has completed accrual.19
RFS following HCT in patients with FLT3+AML randomized to receive sorafenib or placebo.
RFS following HCT in patients with FLT3+AML randomized to receive sorafenib or placebo.
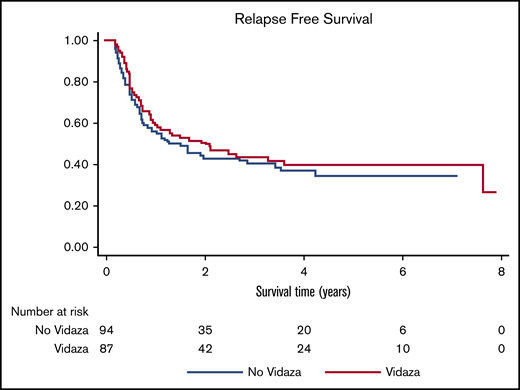
RFS following HCT in patients with MDS or AML randomized to receive maintenance azacitidine vs supportive care.
RFS following HCT in patients with MDS or AML randomized to receive maintenance azacitidine vs supportive care.
APR-246 in patients with AML or MDS with mutated p53
Perhaps the biggest risk factor for post-HCT relapse in patients with AML is the presence of mutated p53.20 What if there were a way to restore normal p53 function? Fortunately, APR-246 is in development specifically for patients with myeloid malignancies and mutated p53. The early trial results look promising, with a CR rate as high as 80% in patients with AML and myelodysplastic syndrome (MDS).21 There is currently a phase 2 trial evaluating combination therapy with azacitidine and APR-246 given post-HCT for patients with MDS and AML and mutated p53 (NCT03931291). The primary end point of the study is 1-year RFS.
HMAs in patients with AML or MDS
There have been several early-phase trials suggesting that hypomethylating agent (HMA) therapy can prevent imminent relapse22,23 and treat early relapse post-HCT.24 This combined with the low toxicity of HMAs and the potential improvement in graft-versus-leukemia post-HCT25 led to development of HMAs for post-HCT maintenance. Investigators from MD Anderson Cancer Center recently completed a randomized trial comparing azacitidine (n = 93) to SOC (n = 94) in patients with MDS or AML.26 There was no significant difference in RFS at 1 year, with 2.07 years (azacitidine) vs 1.28 years (SOC) (P = .43) (Figure 2). Azacitidine was dosed at 32 mg/m2 daily for 5 consecutive days. Azacitidine maintenance was planned for 1 year; however, only 29% completed 1 year of therapy. Among the patients who stopped azacitidine early, the reasons were relapse (47%), toxicity (18%), patient preference (15%), and infection (11%). There was a trend toward improved RFS in patients who received at least 9 cycles of post-HCT maintenance. Oral delivery may improve efficacy, compliance, and tolerability, resulting in improved outcomes.27,28 Combination therapy with more targeted agents such as specific FLT3 inhibitors, isocitrate dehydrogenase inhibitors, and APR-246 may be warranted.
ProDLI
Initial trials with prophylactic donor lymphocyte infusions (proDLI) had promising results, particularly when combined with RIC.29 A retrospective matched-pair analysis from the EBMT compared 89 patients who received proDLI to 89 control patients with AL.30 There was no advantage in giving proDLI in standard-risk AL. However, patients with high-risk AML who received proDLI had significantly improved 5-year RFS (HR, 0.387; 95% CI, 0.116-0.898; P = .027) Interestingly, both relapse incidence and treatment-related mortality were reduced in patients who received proDLI, but there was a nonsignificant trend toward increased chronic graft-versus-host disease.
Conclusion
To date, studies showing significantly improved outcomes with maintenance therapy include the EBMT assessment using TKIs showing lower relapse rates, the German multicenter ALL group study showing lower rates of molecular recurrence, the SORMAIN trial showing improved RFS, and the EBMT matched-pair analysis of proDLI showing improved RFS. So why would one argue for maintenance therapy in patients with high-risk AL? Relapse is an important end point to both patients and physicians. Furthermore, medical decisions should not be solely based upon survival end points from small single-center randomized trials without appropriate consideration of the context such as compliance with scheduled interventions. This reminds me of a review of randomized trials evaluating parachutes to prevent gravitational challenge.31 No one was willing to be randomized to the placebo arm. In a discussion with a well-respected senior colleague, it was stated that no one needs 2 parachutes. The irony is that indeed any person who engages in skydiving does wear 2 parachutes, because skydiving is perilous, much like high-risk AL. A renewed focus on novel conditioning regimens and the incorporation of novel agents into post-HCT maintenance strategies is needed.
Authorship
Contribution: B.L.S. wrote and reviewed the article as sole author.
Conflict-of-interest disclosure: B.L.S. reports income for advisory roles from Celgene, Alexion, Agios, Jazz, and Novartis.
Correspondence: Bart L. Scott, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: bscott@fhcrc.org.