Key Points
FM vs FB in fludarabine-based reduced intensity transplant offers marginally superior long-term survival in AML.
Compared with FB, FM is associated with higher short-term posttransplant nonrelapse mortality, yet lower long-term leukemia relapse rate.
Abstract
There is a lack of large comparative study on the outcomes of reduced intensity conditioning (RIC) in acute myeloid leukemia (AML) transplantation using fludarabine/busulfan (FB) and fludarabine/melphalan (FM) regimens. Adult AML patients from Center for International Blood and Marrow Transplant Research who received first RIC allo-transplant between 2001 and 2015 were studied. Patients were excluded if they received cord blood or identical twin transplant, total body irradiation in conditioning, or graft-versus-host disease (GVHD) prophylaxis with in vitro T-cell depletion. Primary outcome was overall survival (OS), secondary end points were leukemia-free survival (LFS), nonrelapse mortality (NRM), relapse, and GVHD. Multivariate survival model was used with adjustment for patient, leukemia, and transplant-related factors. A total of 622 patients received FM and 791 received FB RIC. Compared with FB, the FM group had fewer transplant in complete remission (CR), fewer matched sibling donors, and less usage of anti-thymocyte globulin or alemtuzumab. More patients in the FM group received marrow grafts and had transplantation before 2005. OS was significantly lower within the first 3 months posttransplant in the FM group (hazard ratio [HR] = 1.82, P < .001), but was marginally superior beyond 3 months (HR = 0.87, P = .05). LFS was better with FM compared with FB (HR = 0.89, P = .05). NRM was significantly increased in the FM group during the first 3 months of posttransplant (HR = 3.85, P < .001). Long-term relapse was lower with FM (HR = 0.65, P < .001). Analysis restricted to patients with CR showed comparable results. In conclusion, compared with FB, the FM RIC showed a marginally superior long-term OS and LFS and a lower relapse rate. A lower OS early posttransplant within 3 months was largely the result of a higher early NRM.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment strategy for patients with acute myeloid leukemia (AML).1,2 With the conventional myeloablative conditioning (MAC) developed in the 1970 and 1980s, HCT was restricted to younger patients without comorbidities.3,4 Because the median age of AML patients is ∼68 years, most AML patients are not eligible for standard MAC. Because of both antitumor effect of preparative regimens and graft-versus-leukemia immunity in allogeneic HCT, reduced intensity conditioning (RIC) regimens were developed 2 decades ago in an effort to allow allogeneic HCT among older patients and those with comorbidities.3,5-8 The goal was to reduce nonrelapse mortality (NRM) while relying on graft-versus-leukemia activity for disease control.6,9-11 In recent years, RIC regimens are also frequently used in younger age group patients.7,12,13
Fludarabine/melphalan (FM; M dose ≤140 mg/m2) and fludarabine/busulfan (FB; B dose ≤6.4 mg/kg) are the 2 most commonly used RIC regimens in HCT.12 There have been several retrospective studies and a meta-analysis comparing the 2; however, these studies were limited by the inclusion of various age groups, disease-related factors, and graft-versus-host disease (GVHD) prophylaxis regimens.14-16 In general, the results suggested a higher NRM but better disease control with FM RIC compared with FB and a variable effect on overall survival (OS). In the current study, we analyzed individual level data from a large cohort of AML transplantation patients from Center for International Blood and Marrow Transplant Research (CIBMTR) to compare the 2 RIC regimens. We studied characteristics associated with regimen utility and comparative effectiveness. Efforts were also made to identify subgroups of patients that could derive greater benefit from either of the 2 regimens.
Patients, materials, and methods
Patient cohort
The data were from the CIBMTR transplant registry, which included AML patients aged ≥18 years that had their first allogeneic HCT using FM- or FB (IV busulfan)-based RIC regimen between 2001 and 2015 in the United States. Patients were excluded if they received cord blood, identical twin or haploidentical donors, total body irradiation as a part of conditioning, ex vivo T-cell depletion or CD34 selected grafts, or GVHD prophylaxis using posttransplant cyclophosphamide.
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry, the Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program established in 2004, which collects data from more than 450 transplantation centers worldwide. The CIBMTR transplant registry collects transplant essential data as well as comprehensive patient, disease, and other clinical information pre- and poststem transplantation per CIBMTR data collection form. Data are collected pretransplantation, 100 days posttransplantation, 6 months posttransplantation, and annually thereafter or until death.
Study end points
The primary study end point was OS defined as time from transplant to death from any cause. Patients were censored at time of last follow-up. The secondary end points included leukemia-free survival (LFS) defined as time to leukemia relapse or death from any cause. Patients were censored at last follow-up. NRM was defined as time to death without evidence of persistent disease or leukemia relapse, with leukemia relapse as competing risk event. Patients were censored at last follow-up. Relapse was defined as recurrence of leukemia after transplant, meeting 1 or more of the following criteria per CIBMTR: ≥5% blasts in the marrow or peripheral blood, extramedullary disease, or disease presence determined by a physician upon clinical assessment. NRM is considered as a competing risk event and patients were censored at last follow-up. Hematological complete remission (CR) was defined by CIBMTR as meeting all of the following response criteria for at least 4 weeks: <5% blasts in the bone marrow; no blast in blood; normal maturation of all cellular components in the bone marrow; no extramedullary disease (eg, central nervous system, soft-tissue disease); absolute neutrophil count (ANC) ≥1000/µL, platelets ≥100 000/µL; and transfusion independent. Acute GVHD (aGVHD) included occurrence of grade 2, 3, and/or 4 skin, gastrointestinal, or liver abnormalities fulfilling the consensus criteria of aGVHD.17 Chronic GVHD (cGVHD) was defined as occurrence of symptoms in any organ system fulfilling the diagnostic criteria of cGVHD.18 For both acute and chronic GVHD, patients were censored at time of subsequent transplant or last follow-up. GVHD-free and relapse-free survival (GRFS) is a composite end point in which events include grade 3-4 aGVHD, cGVHD, relapse or death, whichever occurs first. Patients were censored at time of subsequent HCT or last follow-up.
Statistical methods
Busulfan and melphalan dosing.
In the FB group, because of having too few patients (n = 50) receiving oral busulfan who were all transplanted in early years, only patients received IV busulfan were included in the analysis. The 2 most commonly used dosing regimens, 3.2 (n = 122) and 6.4 mg/kg (n = 402), were identified. We compared demographics characteristics between the 2 groups and respective survival at 5 years and did not detect any statistically significant difference. Therefore, the 2 dose groups were combined in the analysis.
Similarly, the 2 most common dosing groups of melphalan, 100 (n = 69) and 140 mg/m2 (n = 493) in the FM group were compared with respect to demographics and survival outcomes. Similarly, in univariate analysis, no statistically significant differences were detected and therefore the 2 dose groups were combined.
Baseline characteristics.
Patient, disease, and transplant-related factors were compared between the FM and FB using the χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. The probabilities of disease-free and OS were calculated using the Kaplan-Meier estimator and were plotted using adjusted Kaplan-Meier curves. The probabilities of the secondary end points were generated using cumulative incidence estimates that were accounted for competing. Cox proportional hazards regression were used to estimate hazard ratio (HR) for outcomes between the 2 treatment groups. The variables to be considered in the multivariate models included age, sex, Karnofsky Performance Scale at time of HCT, clinical onset of AML, cytogenetics, disease status before HCT, time to achieve CR1, year of HCT, donor type, graft type, donor/recipient sex match and cytomegalovirus serostatus, GVHD prophylaxis, and in vivo T-cell depletion. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. When the test indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplant time course into 2 periods, using the maximized partial likelihood method to find the most appropriate breakpoint. Backward stepwise model selection was used to identify all significant risk factors. Each step of model building contained the main effect of conditioning regimen. Factors of significance level <5% were kept in the final model. Potential interactions between main effect and significant risk factors were also tested. Direct adjusted probabilities of OS and LFS, and direct adjusted cumulative incidence probability function (CIF) of relapse and NRM were generated from the final regression models stratified by conditioning regimen and weighted averages of covariate values using the pooled sample proportion as the weight function. These adjusted probabilities estimated likelihood of outcomes in populations with similar prognostic factors. Among the secondary outcomes, GRFS and GVHD comparing FM vs FB were also reported.
Outcome reporting.
For both primary and secondary outcomes, OS, LFS, relapse, NRM, and GVHD (acute and chronic) were reported for the entire cohort and patients who achieved CR pretransplant (CR cohort). For reporting OS and NRM, outcomes were divided into short- (≤3 months) and long-term (>3 months) periods. The 2 survival curves crossed at 3 months, suggesting model-defined time dependence resulting from violation of the proportionality assumption of the Cox model. We therefore reported early vs late period divided at 3-month posttransplant.
Sensitivity analyses.
The analyses included comparison limited to patients with reported comorbidity index (HCT-CI) available who were transplanted in 2007 and beyond, and among patients who received matched sibling or well-matched unrelated donors. Center effect in the comparison was studied in the comparison using random-effects model in the framework of extended Cox multivariate regression analysis and for OS and LFS; center effect was not a statistically significant factor (P > .05).
Results
Baseline characteristics
The cohort consisted of 622 AML patients who received transplant with the FM RIC and 791 with the FB RIC regimen. Baseline characteristics of the 2 treatment groups in the entire cohort and CR cohort are summarized in Table 1 and supplemental Table 1, respectively. In the entire cohort, the median age of the FM group was 59 years (range, 18-76); and the median age of FB group was 61 years (range, 18-77). Notably, compared with the FB group, the FM group had more patients with active disease (36% vs 18%), with mismatched donors (24% vs 15%), with bone marrow grafts (19% vs 9%), received transplant before 2005 (29% vs 12%), and without anti-thymocyte globulin (ATG)/alemtuzumab (58% vs 48%). However, there were fewer patients in CR1 (46% vs 62%) and matched sibling donor grafts (21% vs 30%) in the FM group compared with that of FB group. The remaining characteristics were comparable between the 2 groups for both entire cohort and CR cohort.
Baseline characteristics in AML patients receiving FM, FB RIC regimen for allogeneic HCT (full cohort)
| . | FM . | FB . | P . |
|---|---|---|---|
| Patient-related | |||
| No. of patients | 622 | 791 | |
| No. of centers | 73 | 80 | |
| Age at transplant, y | |||
| Median (range) | 59 (18-76) | 61 (18-77) | <.001 |
| Male sex, n (%) | 359 (58) | 445 (56) | .58 |
| Karnofsky score, n (%) | .19 | ||
| <90 | 290 (47) | 336 (43) | |
| ≥90 | 311 (50) | 416 (52) | |
| Disease status before HCT, n (%) | |||
| Primary induction failure | 120 (19) | 86 (11) | <.001 |
| CR1 | 285 (46) | 484 (62) | |
| ≥CR2+ | 110 (18) | 163 (20) | |
| Relapse | 106 (17) | 57 (7) | |
| MRD status before transplant | <.001 | ||
| Negative | 127 (20) | 257 (33) | |
| Positive | 40 (6) | 63 (8) | |
| Not in CR1 | 138 (22) | 190 (24) | |
| NA before 2007 | 310 (50) | 244 (31) | |
| Cytogenetic score | .96 | ||
| Favorable | 36 (6) | 50 (6) | |
| Intermediate | 323 (52) | 425 (54) | |
| Poor | 200 (32) | 266 (34) | |
| Therapy-related/secondary AML | .56 | ||
| No | 533 (86) | 689 (87) | |
| Yes | 75 (12) | 88 (11) | |
| Transplant-related, n (%) | |||
| Donor type | <.001 | ||
| HLA-identical sibling | 130 (21) | 241 (30) | |
| Well-matched unrelated (8/8) | 335 (54) | 427 (54) | |
| Partially-matched unrelated (7/8) | 115 (18) | 95 (12) | |
| Mismatched unrelated (≤6/8) | 18 (3) | 5 (< 1) | |
| Donor age at HCT (for URD only) | .006 | ||
| Median (range) | 38 (16-85) | 39 (11-82) | |
| GVHD prophylaxis | <.001 | ||
| TAC-based + MTX | 238 (38) | 493 (63) | |
| TAC-based + MMF | 132 (21) | 158 (20) | |
| CsA-based + MTX | 49 (8) | 18 (2) | |
| CsA-based + MMF | 70 (11) | 32 (4) | |
| ATG/alemtuzumab use | 259 (42) | 413 (52) | <.001 |
| Graft type | <.001 | ||
| Bone marrow | 119 (19) | 73 (9) | |
| Peripheral blood | 503 (81) | 726 (91) | |
| Year of transplant | <.001 | ||
| 2001-2005 | 180 (29) | 98 (12) | |
| 2006-2015 | 442 (71) | 693 (88) | |
| Median follow-up (range), mo | 85 (6-168) | 72 (6-169) |
| . | FM . | FB . | P . |
|---|---|---|---|
| Patient-related | |||
| No. of patients | 622 | 791 | |
| No. of centers | 73 | 80 | |
| Age at transplant, y | |||
| Median (range) | 59 (18-76) | 61 (18-77) | <.001 |
| Male sex, n (%) | 359 (58) | 445 (56) | .58 |
| Karnofsky score, n (%) | .19 | ||
| <90 | 290 (47) | 336 (43) | |
| ≥90 | 311 (50) | 416 (52) | |
| Disease status before HCT, n (%) | |||
| Primary induction failure | 120 (19) | 86 (11) | <.001 |
| CR1 | 285 (46) | 484 (62) | |
| ≥CR2+ | 110 (18) | 163 (20) | |
| Relapse | 106 (17) | 57 (7) | |
| MRD status before transplant | <.001 | ||
| Negative | 127 (20) | 257 (33) | |
| Positive | 40 (6) | 63 (8) | |
| Not in CR1 | 138 (22) | 190 (24) | |
| NA before 2007 | 310 (50) | 244 (31) | |
| Cytogenetic score | .96 | ||
| Favorable | 36 (6) | 50 (6) | |
| Intermediate | 323 (52) | 425 (54) | |
| Poor | 200 (32) | 266 (34) | |
| Therapy-related/secondary AML | .56 | ||
| No | 533 (86) | 689 (87) | |
| Yes | 75 (12) | 88 (11) | |
| Transplant-related, n (%) | |||
| Donor type | <.001 | ||
| HLA-identical sibling | 130 (21) | 241 (30) | |
| Well-matched unrelated (8/8) | 335 (54) | 427 (54) | |
| Partially-matched unrelated (7/8) | 115 (18) | 95 (12) | |
| Mismatched unrelated (≤6/8) | 18 (3) | 5 (< 1) | |
| Donor age at HCT (for URD only) | .006 | ||
| Median (range) | 38 (16-85) | 39 (11-82) | |
| GVHD prophylaxis | <.001 | ||
| TAC-based + MTX | 238 (38) | 493 (63) | |
| TAC-based + MMF | 132 (21) | 158 (20) | |
| CsA-based + MTX | 49 (8) | 18 (2) | |
| CsA-based + MMF | 70 (11) | 32 (4) | |
| ATG/alemtuzumab use | 259 (42) | 413 (52) | <.001 |
| Graft type | <.001 | ||
| Bone marrow | 119 (19) | 73 (9) | |
| Peripheral blood | 503 (81) | 726 (91) | |
| Year of transplant | <.001 | ||
| 2001-2005 | 180 (29) | 98 (12) | |
| 2006-2015 | 442 (71) | 693 (88) | |
| Median follow-up (range), mo | 85 (6-168) | 72 (6-169) |
Transplant outcomes
OS and LFS.
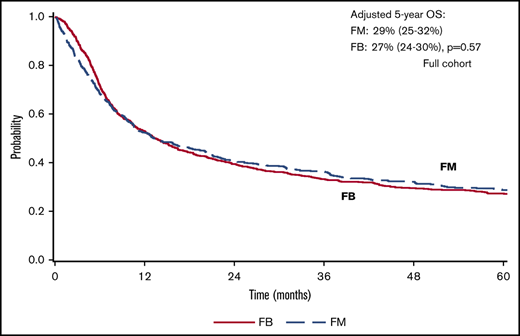
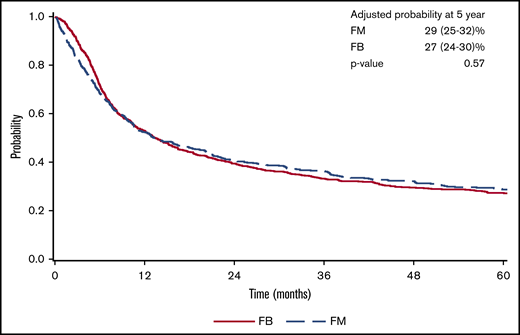
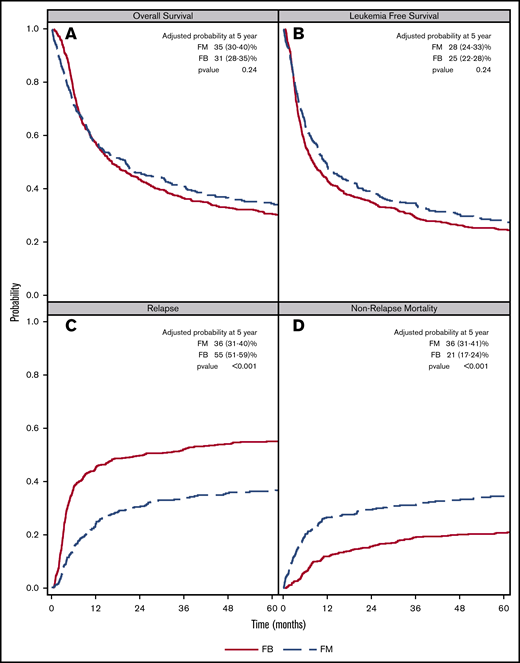
There was a lower OS associated with FM group compared with FB group within 3 months posttransplant (HR = 1.82; 95% confidence interval [CI], 1.36-2.45; P < .001). OS was marginally superior with FM compared with FB beyond 3 months (HR = 0.87; 95% CI, 0.76-1.00; P = .05). At 5 years posttransplant, the survival rate was 29% (95% CI, 25-32) for FM and 27% (95% CI, 24-30) for FB (P = .57) (Figure 1). The results were shown to be consistent in patients with CR pretransplant (Figure 2; Table 2) for an increased early mortality in the FM group within 3 months posttransplant (HR = 2.70; 95% CI, 1.80-4.07; P < .001), and a better OS beyond 3 months compared with the FB group (HR = 0.81; 95% CI, 0.68-0.95; P = .01) (Table 2). Regarding the LFS, again there was a marginal advantage with FM compared with FB (entire cohort: HR = 0.89; 95% CI, 0.78-1.00; P = .05; and CR cohort: HR = 0.87; 95% CI, 0.75-1.01; P = .06) (Table 2). Adjusted probabilities (at specific time points) among patients in CR at transplant showed comparable OS at 1, 3, and 5 years posttransplant. Similarly, LFS was similar between the FM and FB groups (Figure 2; supplemental Table 2).
FM vs FB on AML transplant outcomes. Adjusted survival: OS (A) and LFS (B). CIF curves: relapse (C) and NRM (D).
FM vs FB on AML transplant outcomes. Adjusted survival: OS (A) and LFS (B). CIF curves: relapse (C) and NRM (D).
Transplant outcomes in AML patients receiving FM vs FB RIC for allogeneic HCT
| . | FM vs FB (entire cohort), HR (95% CI) . | FM vs FB (CR only cohort), HR (95% CI) . |
|---|---|---|
| OS | ||
| ≤3 mo | 1.82 (1.36-2.45), P < .001* | 2.70 (1.80-4.07), P < .001* |
| >3 mo | 0.87 (0.76-1.00), P = .05 | 0.81 (0.68-0.95), P = .01* |
| LFS | 0.89 (0.78-1.00), P = .05 | 0.87 (0.75-1.01), P = .06 |
| NRM | ||
| ≤3 mo | 3.85 (2.46-6.03), P < .001* | 5.09 (2.83-9.14), P < .001* |
| >3 mo | 1.14 (0.88-1.47), P = .32 | 1.17 (0.88-1.57), P = .28 |
| Relapse | 0.65 (0.55-0.76), P < .001* | 0.60 (0.49-0.73), P < .001* |
| GRFS | 1.03 (0.91-1.15), P = .66 | 1.01 (0.87-1.16), P = .92 |
| . | FM vs FB (entire cohort), HR (95% CI) . | FM vs FB (CR only cohort), HR (95% CI) . |
|---|---|---|
| OS | ||
| ≤3 mo | 1.82 (1.36-2.45), P < .001* | 2.70 (1.80-4.07), P < .001* |
| >3 mo | 0.87 (0.76-1.00), P = .05 | 0.81 (0.68-0.95), P = .01* |
| LFS | 0.89 (0.78-1.00), P = .05 | 0.87 (0.75-1.01), P = .06 |
| NRM | ||
| ≤3 mo | 3.85 (2.46-6.03), P < .001* | 5.09 (2.83-9.14), P < .001* |
| >3 mo | 1.14 (0.88-1.47), P = .32 | 1.17 (0.88-1.57), P = .28 |
| Relapse | 0.65 (0.55-0.76), P < .001* | 0.60 (0.49-0.73), P < .001* |
| GRFS | 1.03 (0.91-1.15), P = .66 | 1.01 (0.87-1.16), P = .92 |
Significant P value.
NRM
NRM was significantly higher in the FM compared with the FB group during the early period (<3 months) posttransplant. This was found in both the entire cohort (HR = 3.85; 95% CI, 2.46-6.03; P < .001) and CR cohort (HR = 5.09; 95% CI, 2.83-9.14; P < .001). In the multivariate survival analysis, excess in NRM in the FM group did not persist beyond 3 months (entire cohort: HR = 1.14; 95% CI, 0.88-1.47; P = .32; CR cohort only: HR = 1.17; 95% CI, 0.88-1.57; P = .28) (Table 2). CIF comparing FM vs FB at 1-, 3-, and 5-year time points in patients with pretransplant CR showed higher NRM in the FM group, largely owing to increased early NRM occurring within the first 3 months posttransplant (Figure 2; supplemental Table 2).
Relapse and GRFS
In both the full and the CR cohorts, there were an overall significant decrease in relapse associated with the FM group compared with the FB group (entire cohort: HR = 0.65; 95% CI, 0.55-0.76; P < .001; and CR cohort: HR = 0.60, 95% CI, 0.49-0.73; P < .001). CIF at respective time points posttransplant showed a consistent lower relapse rate in the FM compared with the FB group, P < .001 (Figure 2; supplemental Table 2). There was no detectable difference with regard to GRFS when comparing the 2 conditioning regimens, either in the entire cohort or the CR cohort (Table 2).
Multivariable analysis comparing FM with FB based on patient, leukemia, and transplant-related characteristics
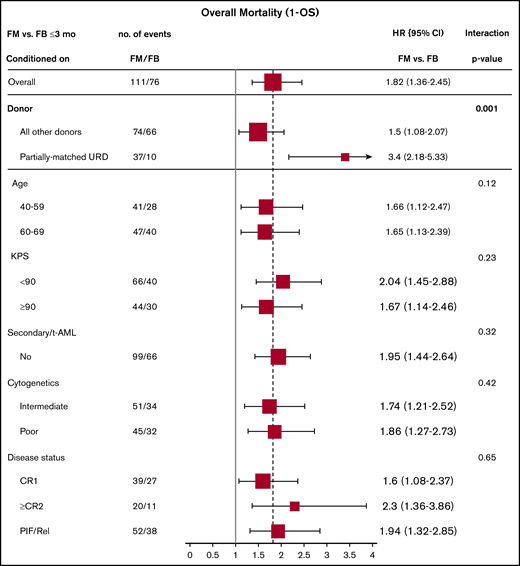
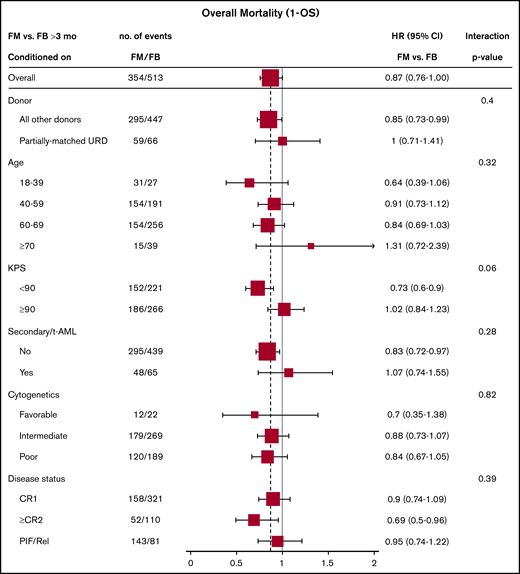
Effect of baseline characteristics on overall mortality comparing the 2 treatment groups were reported in forest plots according to the early (<3 months) and late (>3 months) time periods (Figures 3 and 4), as well as with the multivariate models (supplemental Tables 3 and 4). The results showed a uniformly increased early mortality associated with FM group irrespective of patient-, disease-, and transplant-specific factors. These included age, Karnofsky Performance Scale, cytogenetics risk, secondary AML, or remission status. Differences in mortality between the 2 regimens diminished beyond 3 months posttransplant without influence from specific baseline characteristics. Those included well-established adverse risk groups. In other words, there was consistency of the results within all subgroups (all P values for interaction >.05).
Early overall mortality (1 – OS) (≤3 months) comparing FM vs FB based on patient-, disease-, and transplant-related characteristics (full cohort).
Early overall mortality (1 – OS) (≤3 months) comparing FM vs FB based on patient-, disease-, and transplant-related characteristics (full cohort).
Late overall mortality (1 – OS) (>3 months) comparing FM vs FB based on patient-, disease-, and transplant-related characteristics (full cohort).
Late overall mortality (1 – OS) (>3 months) comparing FM vs FB based on patient-, disease-, and transplant-related characteristics (full cohort).
In sensitivity analyses, there was no evidence showing superior outcome associated with either the FM or FB group, especially when restricted to patients with HCT-CI available for multivariate adjustment. There was also no difference in outcomes when limiting analysis to patients who received sibling or well-matched unrelated donors (supplemental Tables 5 and 6).
GVHD
Based on the multivariable model of the entire cohort, after adjusting for all other characteristics, FM was associated with higher grade 2-4 and grade 3-4 aGVHD (supplemental Table 3). This was true both in the full and CR cohorts (supplemental Tables 3 and 4). With regard to cGVHD, for the full cohort, FM was associated with higher cGVHD in absence of in vivo T-cell depletion, but effect was not seen in its presence, suggesting presence of interaction. For the CR cohort, this effect modification with in vivo T-cell depletion on the outcome of cGVHD was not seen.
Compared with tacrolimus and mycophenolate (TAC/MMF), GVHD prophylaxis using calcineurin inhibitor (TAC or cyclosporine [CsA]) and methotrexate (MTX) led to a lower incidence of grade 2-4 aGVHD (TAC/MTX vs TAC/MMF: HR = 0.65; 95% CI, 0.52-0.80; P < .001; CsA/MTX vs TAC/MMF: HR = 0.59; 95% CI, 0.37-0.93; P = .02). Notably, in vivo T-cell depletion by either ATG or alemtuzumab consistently showed a benefit in reducing both grade II-IV aGVHD (HR = 0.80; 95% CI, 0.66-0.96; P = .02) and cGVHD (HR = 0.65; 95% CI. 0.51-0.81; P < .001), as well as a lower NRM (HR = 0.78; 95% CI, 0.63-0.97; P = .02).
Engraftment
Data on neutrophil (ANC) and platelet engraftment of the 2 regimens is shown in supplemental Table 7. There appeared to be notably faster engraftment of counts in the FB compared with the FM group, but with both groups achieving at least 95% probability of ANC recovery by day 100.
Cause of death
Finally, details on cause of death between the FM and FB group were shown in supplemental Table 8. Notably, in comparing to FB, patients who received FM condition experienced relatively higher rates of infection and organ failure as their cause of death.
Discussion
The current CIBMTR study is the largest cohort to date comparing the 2 most common RIC regimens for AML allogeneic transplantation. The initial CIBMTR report by Luger et al comparing RIC and MAC regimens for myelodysplastic syndrome and AML showed similar survival.19 A more recent randomized Clinical Trials Network study 0901 comparing RIC (FB and FM) vs MAC regimens for AML was stopped early because of significant increase in relapse in the RIC arm (48.3% vs 13.5%)18,20 ; however, only 19% of the RIC transplants in the study were FM. Although there is no prospective trial comparing the utility of FB- and FM-based RIC regimens, several observational studies have looked at the outcomes of using the 2 RIC regimens, including single-center retrospective analyses,7,10,14,15 1 Blood and Marrow Transplantation registry analysis,21 and 1 meta-analysis of ∼2000 patients pooled from various studies and disease settings.16 The study by Shimoni et al15 of 151 patients included several hematological malignancies signified a higher NRM for FM (40% vs 16%). Patients transplanted in remission had a better OS with FM compared with FB (72% vs 36%) because of less relapse in the FM group, whereas patients transplanted with active disease showed a similar survival where increased NRM in the FM group was compensated by a lower relapse rate. Registry study from the Blood and Marrow Transplantation Clinical Trials Network restricted to AML patients transplanted from MSD without T-cell-depleting agents and with oral and intravenous FB RIC regimen also showed similar OS between the 2 RIC regimens, where higher NRM with FM was compensated by better disease control. Both groups experienced similar rate of acute and chronic GVHD. For the patients in CR, FM had a better OS (54% vs 32%, P = .02) and LFS (46% vs 30%, P = .03). Similar outcomes were found in a French study of myelofibrosis12 and another from the Mayo Clinic using only IV busulfan, suggesting a better disease control with FM at the expense of increased NRM. The Mayo study also suggested a better OS in the FM group with Karnofsky ≥90 (78.3% vs 48.3%; P = .03) but a threefold increase in 2-year NRM among patients with Karnofsky <90, leading the authors to speculate the need of individualization of RIC regimens for HCT.14
The results of current study indicate a marginal long-term OS advantage beyond 3 months as well as LFS advantage in association with the FM regimen group, especially in patients with CR and well-matched sibling and unrelated donor recipient. There was yet a statistically significant lower survival during the early period posttransplant resulting from excess NRM of the FM regimen. Long-term OS and LFS advantage with FM were driven by a marked reduction in leukemia relapse with the FM regimen. This held true in younger patients with high-risk disease in adjusted analysis.
There are several notable findings, in particular, the strategy to reduce GVHD using calcineurin inhibitors combined with methotrexate, as well as approaches for in vivo T-cell depletion using ATG or alemtuzumab have led to significant reduction of both acute and chronic GVHD, but no significant improvement in GRFS when comparing the 2 RIC regimens. There was not enough data to study prophylaxis regimen such as posttransplant Cytoxan and effect on the study outcomes.
Our results based on rather comprehensive analyses endorsed findings by the previous reports. Using this large study cohort, we were able to apply additional approaches to reduce confounding. These included analyses limited to only patients in CR, and with matched sibling and full-matched unrelated donor, as well as in a subcohort (2007 and beyond) in which HCT-CI was available for adjustment. In addition, we were able to examine the effect of leukemia and transplant specific factors on the main comparison (FM vs FB) with respect to the study outcomes. Using a multivariate regression survival model, factors such as AML disease risk, degree of donor matching and regimens for GVHD prophylaxis were studied. There are several limitations that are intrinsic to the retrospective cohort design and database-based study, such as missing data and uncollected information that could introduce residual confounding. Information related to more recent transplant strategies is limited or not available, such as knowledge related to minimal residual disease, pre- or posttransplant utilization of novel targeted therapy, as well as haploidentical donor transplantation.
In conclusion, the results of this large comparative study from CIBMTR suggest that long-term OS and LFS are marginally superior with FM compared with FB regimen but at the expense of higher early NRM with FM. It is reasonable to postulate in those with high HCT-CI for NRM, FB is likely a preferred regimen, whereas in younger patients with higher risk of relapse, FM could be a better option. Our analysis did not identify strong evidence in support of using FM over FB to prevent relapse in higher risk group, such as in those with later remission, worse cytogenetics, or treatment-related AML. On the other hand, it is possible that the observed lower risk of relapse associated with FM could be also due to patient assignment driven by unmeasured confounding factors or extreme competing risk from NRM associated with FM, both early and late. The choice of FM over FB remains an individualized decision based on perceived risk and benefit of an AML patient undergoing transplant.
Acknowledgments
The CIBMTR is supported primarily by grants from the National Institutes of Health, National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID) via a Public Health Service grant/cooperative agreement (U24CA076518); NHLBI and NCI (U24HL138660); NHLBI (R21HL140314, U01HL128568); Health Resources and Services Administration (HHSH250201700006C); Office of Naval Research (N00014-18-1-2888, N00014-17-1-2850); HRSA subaward (prime contract award SC1MC31881-01-00); NHLBI subawards (prime grant awards R01HL131731, R01HL126589); and subawards from NCI (prime grant awards 5P01CA111412, R01CA152108, 1R01CA231141), NHLBI (prime grant awards 5R01HL129472, 1R01HL131731), and National Institute of Allergy and Infectious Diseases (prime grant award 1U01AI126612). The CIBMTR was also supported by commercial funds from Actinium Pharmaceuticals Inc., Adaptive Biotechnologies, Allovir Inc., Amgen Inc., an anonymous donation to the Medical College of Wisconsin, Anthem Inc., Astellas Pharma US, Atara Biotherapeutics Inc., BARDA, Be the Match Foundation, bluebird bio Inc., Boston Children’s Hospital, Bristol Myers Squibb Co., Celgene Corp., Children’s Hospital of Los Angeles, Chimerix Inc., City of Hope Medical Center, CSL Behring, CytoSen Therapeutics Inc., Daiichi Sankyo Co. Ltd., Dana Farber Cancer Institute, Enterprise Science and Computing Inc., Fred Hutchinson Cancer Research Center, Gamida-Cell Ltd., Genzyme, Gilead Sciences Inc., GlaxoSmithKline, HistoGenetics Inc., Immucor, Incyte Corporation, Janssen Biotech Inc., Janssen Pharmaceuticals Inc., Janssen Research & Development LLC, Janssen Scientific Affairs LLC, Japan Hematopoietic Cell Transplantation Data Center, Jazz Pharmaceuticals Inc., Karius Inc., Karyopharm Therapeutics Inc., Kite (a Gilead Company), Kyowa Kirin, Magenta Therapeutics, Mayo Clinic and Foundation Rochester, Medac GmbH, Mediware, Memorial Sloan-Kettering Cancer Center, Merck & Company Inc., Mesoblast, MesoScale Diagnostics Inc., Millennium, the Takeda Oncology Co., Miltenyi Biotec Inc., Mundipharma EDO, National Marrow Donor Program, Novartis Oncology, Novartis Pharmaceuticals Corporation, Omeros Corporation, Oncoimmune Inc., OptumHealth, Orca Biosystems Inc., PCORI, Pfizer Inc., Phamacyclics LLC, PIRCHE AG, Regeneron Pharmaceuticals Inc., REGiMMUNE Corp., Sanofi Genzyme, Seattle Genetics, Shire, Sobi Inc., Spectrum Pharmaceuticals Inc., St. Baldrick's Foundation, Swedish Orphan Biovitrum Inc., Takeda Oncology, The Medical College of Wisconsin, University of Minnesota, University of Pittsburgh, University of Texas–MD Anderson, University of Wisconsin–Madison, Viracor Eurofins, and Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government. The CIBMTR database project has been reviewed by IRB and received approval at each participating institution.
Authorship
Contribution: Z.Z., R.N., J.C., and W.S. conceived and designed the study; CIBMTR and collaborative transplant centers provided study materials and patients; H.-L.W., M.-J.Z., and W.S. collected and assembled data; Z.Z., R.N., J.C., H.-L.W., M.-J.Z., W.S. analyzed and interpreted the data; Z.Z., R.N., J.C., and W.S. wrote the manuscript; and all coauthors are leading physicians at respective transplant centers that made significant contributions to data collection and submission to the CIBMTR, and participated substantively in the writing of this manuscript
Conflict-of-interest disclosure: R.N. serves on the advisory boards of, was paid travel fees to the advisory board, and is a consultant for Actinum, Astellas, Daiichi Sankyo. N.N.S. has received honoraria and/or travel support from Incyte, Celgene, and Miltenyi Biotec; serves on scientific advisory boards for Kite, Celgene, and Cellectar; has equity ownership in Geron, Exelexis, Oncosec, and Cell Vault;, and has received institutional research support for clinical trials from BMS and Miltenyi Biotec. D.R. is an advisory board member for AbbVie, Agios, Jazz Novartis, Sanofi, Teva; a consultant advisory board member for AROG, Bayer, Pfizer; a speaker, advisory board member for Celgene and Gilead; and a speaker, advisory board member, consultant, and has received research funding from Stemline. I.P. is a data and safety monitoring board member for ExcellThera; and has received an educational grant from Merck. M.-A.P. is an ad hoc advisory board member for AbbVie, Bellicum, Bristol-Myers Squibb; a member of the scientific advisory board for MolMed and Nex-Immune; is a consultant for Merck; and has received research funding from Incyte (clinical trial) and Kite/Gilead (clinical trial). R.F.O. reports personal fees from AstraZeneca, outside the submitted work. M. Hertzberg receives speaker fees from and is on the advisory boards for Takeda and Roche; is on the advisory board for MSD; and receives speaker fees from Celgene. M.R.G. is a consultant for Agios, AbbVie, Amgen, Cardinal Health, Celgene, Incyte, Merck, Pfizer, Travagene, and Daischi Dankyo; owns stock in Medtronic; and receives research funding from Farma Therapeutics, Amgen, Genetech, Incyte, Janssen, and Novartis. U.G. receives speaker bureau and travel expense reimbursements from Incyte; research support, speaker bureau, and travel expense reimbursements from Jazz; speaker bureau and travel expense reimbursements from Astellas; and speaker bureau and travel expense reimbursements from Merck. A.T.G. receives grant/research support from Incyte, CTI Biopharma, and Sierra Oncology; and is on the advisory boards for PharmEssentia, CTI Biopharma, Promedior, Kartos, and Pfizer. M.D.L. received research grants from Pfizer and Celgene and is on the advisory boards for Kadmon, Pfizer, Incyte, and BMS. J.C. serves on the advisory boards for and receives travel expense reimbursements for Jazz Pharmaceuticals, Dallcal-Sankyo, and Incyte Inc. V.R.B. receives consulting fees from Rigel, Agios, Incyte, Omeros, and Takeda; has a partnership for health analytic research, LLC, and AbbVie; receives research funding (institutional) from Incyte, Jazz, Tolero Pharmaceuticals, Inc, and the National Marrow Donor Program; and receives drug support for a trial from Oncoceutics. A.A. is on the advisory board of Boston Biomedical and Incyte; receives research funding from Incyte; and is a consultant for Alpha Insights. B.M.S. is a consultant for Kiadis, Actinium Pharmaceutical, and Bristol-Meyers Squibb; has received research funding for clinical trials from Bellicum for clinical trials they sponsor; is a consultant for and has received funds from Actinium Pharmaceutical for trials they sponsor; and her spouse has equity in Oncoresponse, Inipharm, Blaze Bioscience, and AbbVie. G.L.U. is a consultant to Jaz, Astellas, and Genentech; and receives research funding from Macrogenics. The remaining authors declare no competing financial interests.
Correspondence: Zheng Zhou, Beth Israel Lahey Health, Lahey Hospital and Medical Center, Division of Hematology/Oncology and Bone Marrow Transplant, Department of Medicine, Burlington, MA 01805; e-mail: zheng.x.zhou@lahey.org.
References
Author notes
CIBMTR supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only deidentified datasets that comply with all relevant global regulations regarding privacy and confidentiality. Please send any correspondence to the corresponding author, Zheng Zhou (zheng.x.zhou@lahey.org).
The full-text version of this article contains a data supplement.