Key Points
Posttransplantation, glasdegib maintenance therapy in patients at high risk for relapse did not meaningfully reduce relapse incidence.
Use of glasdegib in the posttransplantation setting was complicated by adverse events requiring drug holds and occasional discontinuation.
Introduction
Allogeneic stem cell transplantation (ASCT) is the only therapy with curative potential for high-risk patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS).1,2 However, disease relapse remains the main cause of treatment failure and death after ASCT,3 suggesting that even highly ablative therapies and the generation of immunological graft-versus-leukemia responses are often unable to achieve cures. At relapse, salvage maneuvers such as secondary transplantation or donor lymphocyte infusion are highly toxic and seldom effective,4-6 highlighting the need for interventions that can prevent relapse.
The presence of measurable residual disease (MRD), as defined by the persistence of cytogenetic, flow cytometric, or molecular evidence of disease before ASCT, is highly predictive for post-ASCT relapse and overall survival (OS).7-11 Development of therapies to prevent post-ASCT relapse in AML and MDS patients at high risk for this outcome is critical. The ideal therapy for this purpose would be a conveniently dosed, orally bioavailable, well-tolerated therapy with activity against the leukemic stem cell (LSC) population, a reservoir from which disease relapse may occur.12 The hedgehog (Hh) signaling pathway, which regulates essential elements of embryonic development, homeostasis, and tissue regeneration,13 is now also recognized for its involvement in the maintenance, growth, and drug resistance of hematological malignancies.14 Hh pathway signaling is preferentially activated in MDS-derived cells and CD34+ AML blasts and cell lines,15-17 including chemotherapy-resistant cell lines,18 suggesting aberrant Hh signaling may contribute to the survival and expansion of LSCs.19 Given the central role that Hh signaling plays in cell differentiation, Hh inhibition represents a mechanistically novel approach to eliminate the LSC population and thus abrogate tumor proliferation in at least a subset of stem cell–driven hematopoietic malignancies, making this pathway an attractive target for residual, treatment-resistant disease. Glasdegib is a novel small-molecule inhibitor of SMO, a key regulatory element of the Hh pathway.20 Early-phase clinical trials using glasdegib showed it to be well tolerated, with activity in patients with hematological malignancies21 ; because of its ability to prolong OS compared with a standard-of-care therapy, glasdegib was approved by the US Food and Drug Administration for older, newly diagnosed patients with AML unfit for intensive chemotherapy.22,23 In this dual-center pilot study, we tested the ability of glasdegib to prevent relapse in a post-ASCT population at high risk for this outcome.
Methods
Study population
Thirty-one patients were enrolled. The complete protocol is available in the supplemental Materials. All eligible patients were age ≥18 years and signed consent within 28 days of ASCT for AML or MDS. To enroll, patients had to have achieved stable engraftment, as defined by absolute neutrophil count ≥1000/mm3 and platelets ≥25× 109/L, and morphological remission (<5% marrow blasts) based on bone marrow biopsy performed ±5 days of day 28 posttransplantation. Patients were enrolled at University of Colorado and Ohio State University. Inclusion criteria required high-risk status for post-ASCT relapse. This was defined for patients with AML who underwent myeloablative conditioning regimens as the presence of MRD at the time of transplantation, as measured by multiparameter flow cytometry (MPFC; Hematologics),7-9 cytogenetics, or fluorescence in situ hybridization. In addition to these criteria, patients undergoing nonmyeloablative transplantation could be enrolled if they had a relapse risk score >0, as defined by the Fred Hutchinson Cancer Research Center scoring system.24 For MDS patients, high risk was defined as persistent disease at the time of ASCT with intermediate-, poor-, or very poor–risk cytogenetics by the revised International Prognostic Scoring System.25 Exclusion criteria included concomitant treatment with other antineoplastic agents or active grade 3 or 4 acute graft-versus-host disease (GVHD). The study was approved by the institutional review boards at both institutions. All patients provided written informed consent before any procedures, and the study was performed in accordance with the Declaration of Helsinki.
Study schema and end points
Patients received 100 mg of glasdegib orally daily and were continuously dosed for 28-day cycles, beginning no sooner than post-ASCT day 28 and no later than day 100 and continuing for 1 year in the absence of relapse or intolerance. Bone marrow biopsies with MRD assessments were performed on or within 10 days of post-ASCT day 80, within 15 days of post-ASCT day 175, and within 30 days of post-ASCT day 365. The primary end point was 1-year relapse-free survival (RFS), defined as the absence of relapse (as detected by recrudescent MRD or morphological disease) or death resulting from any cause. Secondary end points included incidence and severity of adverse events (AEs) and 1- and 2-year OS. AEs were defined using Common Terminology Criteria for Adverse Events (version 5), and attributions were made by the investigators. Glasdegib was discontinued in patients who experienced grade ≥3 AEs.
Statistical analysis
Although a range of historical data exist, we conservatively estimated 1-year probability of RFS for high-risk patients to be 30%.11,26-28 An improvement to 50% 1-year RFS with the addition of glasdegib was felt to be a clinically meaningful difference and required the enrollment of 30 patients to allow for 80% power.11 Cumulative incidence estimates were used to calculate relapse incidence, with transplantation-related mortality as the competing risk.29 OS was calculated according to the Kaplan-Meier method. Kaplan-Meier survival curves and cumulative incidence curves were derived using R software.30
Results and discussion
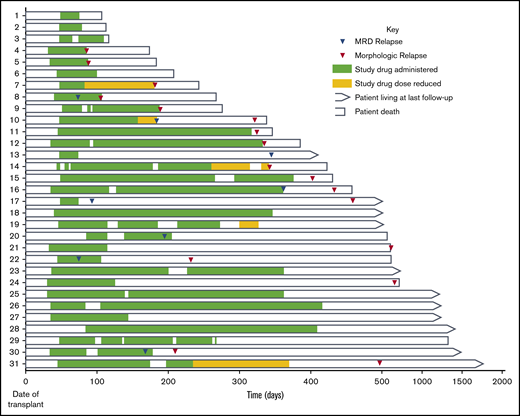
A total of 31 AML and MDS patients were enrolled. Table 1 summarizes the baseline characteristics of our study population. Figure 1 depicts the overall treatment course for each patient; each row represents an individual. Median time to starting glasdegib post-ASCT was 46 days (range, 31-86 days); 8 (25.8%) of 31 patients completed the entire 1 year of glasdegib. All patients had >95% donor-derived chimerism at day 28 post-ASCT assessment, except 1 patient who was 53% donor and had persistent mixed chimerism throughout the posttransplantation course (supplemental Table). At day 80, only 1 patient exhibited an abnormal karyotype, and no patients had detectable MRD. Median time on treatment before permanent discontinuation, ignoring interim holds and dose reductions, was 142 days (range, 28-336 days). Post-ASCT, all patients were followed for a median of 471 days, and survivors were followed for a median of 1268 days (42.3 months).
Patient characteristics (N = 31)
| Characteristic . | n . | % . |
|---|---|---|
| Age, y | ||
| Median | 58 | |
| Range | 20-71 | |
| Disease | ||
| AML | 28 | 90.3 |
| MDS | 3 | 9.7 |
| Donor source | ||
| Matched sibling | 13 | 41.9 |
| Matched unrelated | 7 | 22.6 |
| Cord blood | 11 | 35.5 |
| Enrollment criteria for AML | ||
| Myeloablative conditioning regimen (n = 19) | 51.6 | |
| MRD by MPFC | 16 | |
| MRD by FISH | 7 | |
| FHCC score >0 | — | |
| Nonmyeloablative conditioning regimen (n = 9) | 29.0 | |
| MRD by MPFC | 4 | |
| MRD by FISH | 2 | |
| FHCC score >0 | 4 | |
| Enrollment criteria for MDS (R-IPSS) | ||
| Myeloablative conditioning regimen | 3 | 9.7 |
| Nonmyeloablative conditioning regimen | — | — |
| Myeloablative conditioning regimen | ||
| Fludarabine/busulfan/ATG | 5 | 16.1 |
| Fludarabine/cyclophosphamide/thiotepa/TBI | 7 | 22.6 |
| Busulfan/cyclophosphamide | 10 | 32.3 |
| Nonmyeloablative conditioning regimen | ||
| Fludarabine/melphalan | 4 | 12.9 |
| Fludarabine/TBI | 1 | 3.2 |
| Fludarabine/cyclophosphamide/TBI | 4 | 12.9 |
| Treating facility | ||
| University of Colorado | 26 | 83.9 |
| Ohio State University | 5 | 16.1 |
| Follow-up for all patients, d | ||
| Median | 371 | |
| Range | 75-1825 | |
| Follow-up for survivors, d | ||
| Median | 1268 | |
| Range | 411-1825 |
| Characteristic . | n . | % . |
|---|---|---|
| Age, y | ||
| Median | 58 | |
| Range | 20-71 | |
| Disease | ||
| AML | 28 | 90.3 |
| MDS | 3 | 9.7 |
| Donor source | ||
| Matched sibling | 13 | 41.9 |
| Matched unrelated | 7 | 22.6 |
| Cord blood | 11 | 35.5 |
| Enrollment criteria for AML | ||
| Myeloablative conditioning regimen (n = 19) | 51.6 | |
| MRD by MPFC | 16 | |
| MRD by FISH | 7 | |
| FHCC score >0 | — | |
| Nonmyeloablative conditioning regimen (n = 9) | 29.0 | |
| MRD by MPFC | 4 | |
| MRD by FISH | 2 | |
| FHCC score >0 | 4 | |
| Enrollment criteria for MDS (R-IPSS) | ||
| Myeloablative conditioning regimen | 3 | 9.7 |
| Nonmyeloablative conditioning regimen | — | — |
| Myeloablative conditioning regimen | ||
| Fludarabine/busulfan/ATG | 5 | 16.1 |
| Fludarabine/cyclophosphamide/thiotepa/TBI | 7 | 22.6 |
| Busulfan/cyclophosphamide | 10 | 32.3 |
| Nonmyeloablative conditioning regimen | ||
| Fludarabine/melphalan | 4 | 12.9 |
| Fludarabine/TBI | 1 | 3.2 |
| Fludarabine/cyclophosphamide/TBI | 4 | 12.9 |
| Treating facility | ||
| University of Colorado | 26 | 83.9 |
| Ohio State University | 5 | 16.1 |
| Follow-up for all patients, d | ||
| Median | 371 | |
| Range | 75-1825 | |
| Follow-up for survivors, d | ||
| Median | 1268 | |
| Range | 411-1825 |
Five patients with AML met multiple enrollment criteria.
ATG, antithymocyte globulin; FHCC, Fred Hutchinson Cancer Center; FISH, fluorescence in situ hybridization; R-IPSS, revised International Prognostic Scoring System; TBI, total body irradiation.
Detailed treatment timeline and outcomes for each enrolled patient (by row).
Twenty-eight (90.3%) of 31 patients experienced at least 1 AE attributable to glasdegib. A majority (16 [51.6%] of 31) experienced at least 1 grade ≥2 AE attributable to glasdegib, for a total of 34 grade ≥2 AEs attributable to glasdegib (Table 2). Cramping or myalgias were the most common AEs (23.5%), and dysgeusia and other gastroenterological complaints (eg, anorexia, nausea, diarrhea) accounted for 38.2%. Nineteen (61.3%) of 31 patients had glasdegib interruptions as a result of AEs, and 5 (16.1%) had dose reductions to mitigate AEs (Figure 1). One patient permanently discontinued the study drug after 4 cycles because of grade 1 myalgias.
Frequency of grade 2 to 4 AEs related or possibly related to glasdegib
| AE . | n . | Grade . | ||
|---|---|---|---|---|
| 2 . | 3 . | 4 . | ||
| Related or possibly related to study drug | 34 | |||
| Cramping/myalgia | 8 | 5 | 3 | 0 |
| Dysgeusia | 4 | 3 | 1 | 0 |
| Anorexia/weight loss | 3 | 1 | 2 | 0 |
| Nausea | 3 | 2 | 1 | 0 |
| Diarrhea | 3 | 0 | 3 | 0 |
| Thrombocytopenia | 2 | 0 | 1 | 1 |
| Alopecia, arthralgia, lymphopenia, neutropenia, hypophosphatemia, hypocalcemia, hypoalbuminemia, hyperbilirubinemia, hypertriglyceridemia, LFT increase, hyponatremia | 1 each | Alopecia, arthralgia, hypophosphatemia, hypocalcemia, hypoalbuminemia, hyperbilirubinemia, hypertriglyceridemia | Hyponatremia, LFT increase, lymphopenia | Neutropenia |
| AE . | n . | Grade . | ||
|---|---|---|---|---|
| 2 . | 3 . | 4 . | ||
| Related or possibly related to study drug | 34 | |||
| Cramping/myalgia | 8 | 5 | 3 | 0 |
| Dysgeusia | 4 | 3 | 1 | 0 |
| Anorexia/weight loss | 3 | 1 | 2 | 0 |
| Nausea | 3 | 2 | 1 | 0 |
| Diarrhea | 3 | 0 | 3 | 0 |
| Thrombocytopenia | 2 | 0 | 1 | 1 |
| Alopecia, arthralgia, lymphopenia, neutropenia, hypophosphatemia, hypocalcemia, hypoalbuminemia, hyperbilirubinemia, hypertriglyceridemia, LFT increase, hyponatremia | 1 each | Alopecia, arthralgia, hypophosphatemia, hypocalcemia, hypoalbuminemia, hyperbilirubinemia, hypertriglyceridemia | Hyponatremia, LFT increase, lymphopenia | Neutropenia |
LFT, liver function test.
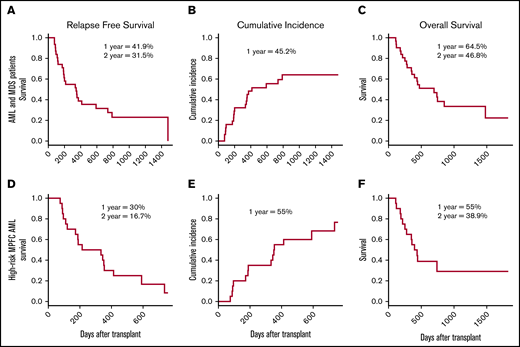
Among all AML and MDS patients, 1- and 2-year RFS rates were 41.9% and 31.5% (Figure 2A). The cumulative incidence of relapse (MRD and morphological) for AML/MDS patients at 1 year was 45.2% (Figure 2B). Eight patients had MRD relapse, at a median time posttransplantation of 180.5 days (range, 75-371 days). Six of these 8 patients ultimately experienced a morphological relapse, which occurred a median of 284 days (range, 104-473 days) after ASCT; 5 of these 6 subsequently died, and 1 remained alive at last follow-up 535 days post-ASCT. Of the 2 who did not experience a morphological relapse, 1 died as a result of treatment-related mortality, and 1 remained alive without morphological relapse at last follow-up (411 days from ASCT). A total of 17 patients had a morphological relapse at a median time post-ASCT of 333 days (range, 87-787 days). One- and 2-year OS were 64.5% and 46.8%, respectively (Figure 2C). Seven patients (22.6%) remained alive and in remission at the end of the study. Twelve patients (38.7%) experienced grade 2 to 4 acute GVHD (grade 2, n = 10; grade 3, n = 1; grade 4, n = 1), and 13 patients (41.9%) experienced chronic GVHD (mild, n = 7; moderate, n = 2; severe, n = 4). Twenty-one (67.6%) of 31 patients experienced at least 1 infectious complication. Four patients experienced treatment-related mortality resulting from acute GVHD (n = 2), sepsis (n = 1), and failure of engraftment of a second transplant after relapse (n = 1). When analyzing the cohort of patients with AML who were MRD+ by MPFC (n = 20), 1- and 2-year RFS rates were 30% and 16.7%, respectively (Figure 2D). Cumulative incidence of relapse at 1 year for this subgroup was 55% (Figure 2E), and 1- and 2-year OS rates were 55% and 38.9%, respectively (Figure 2F). There was no difference in RFS when comparing patients who underwent myeloablative vs nonmyeloablative conditioning regimens (Mantel-Cox, P = .34).
Survival and incidence of relapse. RFS (A,D), cumulative incidence of relapse (B,E), and OS (C,F) for AML and MDS patients (A-C) and high-risk patients with AML defined by MPFC (D-F).
Survival and incidence of relapse. RFS (A,D), cumulative incidence of relapse (B,E), and OS (C,F) for AML and MDS patients (A-C) and high-risk patients with AML defined by MPFC (D-F).
This pilot study demonstrated limited ability for glasdegib to prevent relapse in the high-risk, post-ASCT setting. One-year probability of RFS for high-risk patients with AML without maintenance therapy reported in the literature ranges from ∼30% to 50%.11,26-28 Acknowledging discrepancies in study design and definition of high-risk that render historical comparisons imprecise, glasdegib did not seem to meaningfully reduce the risk of relapse after ASCT in our population. Furthermore, it did not affect chimerism or MRD elimination post-ASCT, nor did it affect the incidence of GVHD or infectious complications, compared with historical data.31-34 There are many potential explanations for these findings. From a mechanistic standpoint, there is heterogeneity in activation of the Hh pathway in various leukemic cell types and differential expression among patients with the same type of leukemia.17 Prestratification of patients based on expression and activation of the Hh pathway may help define a population of patients deriving greater benefit from glasdegib. It is also possible that the biology of Hh signaling is more complex than models suggest. Silencing SMO alone may not lead to the desired antiproliferative effects hypothesized. Indeed, 1 study in chronic myeloid leukemia found that glasdegib decreased LSC dormancy and enhanced cell-cycle progression.35 In addition, targeting the Hh pathway alone may not be sufficient to prevent relapse, but combining glasdegib with additional agents that target other pathways active in LSCs and/or other nonstem leukemic cells could be more efficacious.
In a phase 1 study of predominately relapsed patients with AML, in which several different dosing cohorts were studied, the nonhematological AEs associated with single-agent glasdegib were primarily gastrointestinal in nature and mainly grade <3.36 Implementation of glasdegib for post-ASCT maintenance therapy was complicated by the AE profile. Although there were no glasdegib-related deaths, the AEs experienced by patients in this study did result in frequent dose interruptions and reductions because of their impact on quality-of-life issues. These AEs, such as myalgias, dysgeusia, and anorexia, were also observed in the relapsed single-agent setting,36 but they were more limiting in the post-ASCT setting, possibly because of extended duration of treatment. In addition to the chronicity of treatment, tolerability issues in this study may have been due to interactions with the multitude of concomitant medications routinely administered in the post-ASCT setting or additional medical issues, including GVHD, in the posttransplantation population. This experience reinforces our hypothesis that an effective maintenance therapy in the post-ASCT setting must have a benign AE profile and limited drug interactions if patients in this vulnerable position are to be expected to add it to their already extensive medication regimens. It is possible that a lower dose of glasdegib would have been better tolerated in the post-ASCT setting, thus improving adherence and thereby efficacy. Future consideration may be necessary for drugs with an established maximum tolerated dose in a particular disease to repeat a dose-escalation study in the post-ASCT setting.
The study had several limitations. First, as a phase 2 design, the trial was not randomized. Second, there is currently no standard of practice for identifying patients who are at high risk for relapse. In our cohort, MPFC seemed to identify a subpopulation of patients with on average worse outcomes than the cohort as a whole, suggesting current definitions are heterogeneous. Future studies must acknowledge that the approach to defining high-risk patients will influence outcomes and may also influence our ability to identify patients who would benefit from various therapies. Inclusion of intrinsic controls would be essential for truly defining any clinical benefit of glasdegib maintenance therapy. Finally, this study had no direct readout for the impact on LSCs. Future studies could incorporate correlative readouts to track LSC dynamics, coupled with a dose-escalation design for maximizing therapy benefit while minimizing adverse effects.
Maintenance strategies are appealing in patients with AML. In nontransplantation patients who achieve remission, the use of midostaurin for FLT3+ patients has shown benefit37 ; for other patients, there may be some benefit from azacitidine,38 and there was a significant OS benefit with the use of CC-486 in this setting.39 There is similar rationale to consider these and other newly approved targeted therapies in the post-ASCT population. Preliminary studies with FLT3 inhibitors, azacitdine, and low-dose cytarabine have had mixed results.40,41 Because any maintenance therapy has the potential to increase toxicity, this strategy would have the greatest potential benefit/risk ratio for patients at the highest risk of post-ASCT relapse. Although our study does not seem to provide an effective therapy to prevent this outcome, it does establish a clear framework for how to define and identify these patients as well as identify the key primary end point in question for other pilot clinical trials using other promising therapies.
In summary, glasdegib did not seem to affect 1-year RFS in the post-ASCT setting for patients at high risk of relapse, although a controlled study would be necessary to establish this finding definitively. Adherence was limited by frequent AEs that were not life threatening but limited quality of life in this population. Future studies could be used to identify a subpopulation of patients who may respond to glasdegib therapy, and combinatorial approaches with other maintenance therapies could be used to enhance elimination of residual LSCs that form the basis for disease relapse.
Data sharing requests can be e-mailed to the corresponding author, Daniel A. Pollyea (daniel.pollyea@cuanschutz.edu).
Acknowledgments
This study was supported by the Leukemia and Lymphoma Society’s Therapy Acceleration Program and Pfizer.
Authorship
Contribution: D.A.P. and J.A.G. devised this study, wrote the protocol, supervised implementation of the research, and edited the manuscript; D.A.P., J.A.G., S.V., D.S., N.M., S.D., and C.S. recruited and treated patients and recorded data; and A.K. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: D.A.P. has served as a consultant for Karyopharm, Pfizer, Alexion, Celgene, Gilead, Jazz, Takeda, Curis, Agios, AbbVie, Forty-Seven, Daiichi Sankyo, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Daniel A. Pollyea, Division of Hematology, School of Medicine, University of Colorado, 1665 Aurora Court, F754, Aurora, CO 80045; e-mail: daniel.pollyea@cuanschutz.edu.
References
Author notes
J.A.G. and D.A.P. contributed equally to this study.
The full-text version of this article contains a data supplement.