TO THE EDITOR:
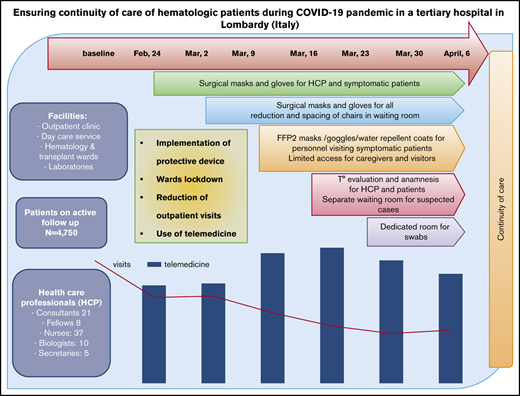
The abrupt outbreak of the COVID-19 pandemic in Italy began on 20 February 2020, with the first patient developing severe acute respiratory syndrome in Lombardy (northern Italy). On 11 March 2020, the World Health Organization declared the pandemic state,1 and Italy was the third most affected country, after the United States and Spain, at the time of writing.2,3 Lombardy was particularly affected, with 984 positive cases in the first week and 50 455 cases after 6 weeks, whereas the rest of Italy, particularly the central and southern regions, faced about one tenth of the number of cases.4 The rapidly evolving situation left only a short time to adopt effective measures aimed at limiting the spread of COVID-19, particularly in hospitals among patients and health care professionals (HCPs). Hospitals started to reorganize into intensive care units (ICUs) to manage COVID-19+ cases, and most decisions were made at a single-center level and in a “day-by-day” manner. Hematology care is particularly complex because it deals with malignant and benign diseases, rare conditions, highly expensive drugs, and life-saving nondeferrable therapies. Hematological patients are often elderly, highly comorbid, and immunocompromised,5 and they experience an annual mortality peak that is caused by seasonal influenza.6 In this setting, knowledge of the best preventive measures to adopt during a pandemic is crucial, because hematologic patients may be affected more by COVID-19 and, at the same time, be a focus of further spread.5 This article reports the strategies of progressive systematic reorganization of hematologic care in a large public university hospital in Milan (Lombardy) during the first 6 weeks of the COVID-19 pandemic. At this site, 4750 adult patients (median age, 67 years; range, 28%-96; 56% males), almost all of whom were diagnosed with hematological diseases, including malignant conditions (n = 3900), benign conditions (n = 650), and rare diseases (n = 550), are actively followed. Typically, 18 831 routine visits, 9930 day-hospital care services, and 436 admissions to the wards occur annually. The health care staff includes physicians (21 consulting hematologists and 8 fellows), 36 nurses, 14 biologists, and 5 technicians operating at 4 main facilities: outpatient clinics (divided into 8 disease-specific groups, all open Monday through Friday), day-hospital care services, 1 hematology ward, 1 transplant ward, and 2 laboratories (1 for diagnosis and research on malignant conditions and 1 for rare anemias). There is also a trial office, including 4 data managers (biologists), a pharmacologist, and a trial nurse; a total of 76 trials (observational and interventional) are ongoing. This study was conducted in accordance with the Declaration of Helsinki, and each participant provided written informed consent for data collection during routine evaluation/follow-up.
As shown in Table 1, the number of clinical services decreased by ∼10% during the first 2 weeks of the pandemic, stabilized during weeks 3 and 4, and decreased by 17% during the last 2 weeks. Specifically, outpatient visits decreased by a median of 48% (range, 28.9%-59.4%) from baseline to week 6, exceeding a 50% decrease by week 4. Scheduled, but deferrable, first hematological consultations were cancelled; only urgent appointments were accepted. The criteria for deferring routine outpatient visits were malignancies off treatment or on maintenance, autoimmune cytopenias on steroid tapering/stable immunosuppressive therapy, and benign conditions not requiring treatment. Moreover, some patients decided on their own to cancel/postpone their appointments. With regard to day-care service, a progressive reduction in global activity occurred (median, 18%; range, 4.4%-31.4%), with the greatest decrease by week 5. A total of 44 nonurgent therapies/procedures were deferred, whereas therapies for acute leukemia (AL), aggressive Hodgkin lymphomas (NHL), and Hodgkin lymphomas were not delayed. Of note, an initial decrease was observed in the first week as a result of the chaotic situation and the fear experienced by patients and HCPs; activities temporarily recovered during the second and third weeks. Seventeen nonurgent bone marrow samplings were postponed. Urgent transfusions were guaranteed, and transfusion-dependent patients adapted their schedules to fit with donor availability (1 unit per week instead of 2 units per 14 days), because the transfusion center experienced a marked reduction in donors. With regard to hospitalization, admissions to the hematology and transplant wards showed a decrease at week 1; thereafter, the limited number of admissions did not allow for definitive conclusions to be made (paradoxical increase, as shown in Table 1). With regard to reallocation of HCPs, from 24 February 2020, hematology/transplant wards were separated from the internal medicine wards, where potentially COVID-19+ patients could be admitted from the Emergency Department. In the general reorganization of the hospital, some personnel from the hematology unit were reasssigned to COVID-19+ wards. From 2 March 2020, laboratory and trial office on-site personnel were reduced, and telecommuting was instituted.
Clinical activities of the hematology unit before and during the COVID-19 pandemic
| Services . | Baseline, n . | Week 1 (24 Feb) . | Week 2 (2 Mar) . | Week 3 (9 Mar) . | Week 4 (16 Mar) . | Week 5 (23 Mar) . | Week 6 (30 Mar) . |
|---|---|---|---|---|---|---|---|
| Total services | 743.8 | 672 (−10) | 686 (−8) | 738 (−1) | 712 (−4) | 634 (−15) | 599 (−19) |
| Outpatients visits | 446.2 | 312 (−30) | 317 (−29) | 256 (−43) | 208 (−53) | 181 (−59) | 193 (−57) |
| Day-hospital care accesses | 288.6 | 239 (−17) | 270 (−6) | 276 (−4) | 236 (−18) | 198 (−31) | 201 (−30) |
| Transfusions* | 70.3 | 60 (−15) | 55 (−22) | 63 (−10) | 54 (−23) | 53 (−25) | 49 (−30) |
| Therapies† | 136.6 | 102 (−25) | 174 (+27) | 157 (+15) | 123 (−10) | 107 (−22) | 102 (−25) |
| Bone marrow‡ | 32.6 | 23 (−29) | 22 (−32) | 25 (−23) | 25 (−23) | 15 (−54) | 15 (−54) |
| Other§ | 40.3 | 54 (+34) | 19 (−53) | 31 (−23) | 34 (−16) | 23 (−43) | 35 (−13) |
| New admissions to hematology ward | 5 | 5 (0) | 4 (−20) | 8 (+60) | 6 (+20) | 6 (+20) | 6 (+20) |
| New admissions to transplant ward¶ | 4 | 3 (−25) | 5 (+25) | 2 (−50) | 3 (−25) | 2 (−50) | 4 (0) |
| Services . | Baseline, n . | Week 1 (24 Feb) . | Week 2 (2 Mar) . | Week 3 (9 Mar) . | Week 4 (16 Mar) . | Week 5 (23 Mar) . | Week 6 (30 Mar) . |
|---|---|---|---|---|---|---|---|
| Total services | 743.8 | 672 (−10) | 686 (−8) | 738 (−1) | 712 (−4) | 634 (−15) | 599 (−19) |
| Outpatients visits | 446.2 | 312 (−30) | 317 (−29) | 256 (−43) | 208 (−53) | 181 (−59) | 193 (−57) |
| Day-hospital care accesses | 288.6 | 239 (−17) | 270 (−6) | 276 (−4) | 236 (−18) | 198 (−31) | 201 (−30) |
| Transfusions* | 70.3 | 60 (−15) | 55 (−22) | 63 (−10) | 54 (−23) | 53 (−25) | 49 (−30) |
| Therapies† | 136.6 | 102 (−25) | 174 (+27) | 157 (+15) | 123 (−10) | 107 (−22) | 102 (−25) |
| Bone marrow‡ | 32.6 | 23 (−29) | 22 (−32) | 25 (−23) | 25 (−23) | 15 (−54) | 15 (−54) |
| Other§ | 40.3 | 54 (+34) | 19 (−53) | 31 (−23) | 34 (−16) | 23 (−43) | 35 (−13) |
| New admissions to hematology ward | 5 | 5 (0) | 4 (−20) | 8 (+60) | 6 (+20) | 6 (+20) | 6 (+20) |
| New admissions to transplant ward¶ | 4 | 3 (−25) | 5 (+25) | 2 (−50) | 3 (−25) | 2 (−50) | 4 (0) |
Unless otherwise noted, all data are n (percentage change from baseline). The mean for the 3 weeks preceding the COVID-19 pandemic was used as the baseline value.
Transfusions are intended as the number of patients receiving transfusions.
Therapies performed included the following drugs or schemes: adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), bendamustine, gemcitabine, and vinorelbine (BEGEV), cyclophosphamide, doxorubicine, etoposide, vincristine, and prednisolone (CHEOP), rituximab, cyclophosphamide, doxorubicine, vincristine, and prednisolone (R-CHOP), rituximab, cyclophosphamide, lyposomial doxorubicine, vincristine, and prednisolone (R-COMP), gemcitabine, oxalyplatinum, and prednisolone (GDP), carfilzomib, lenalidomide, dexamethazone (KRD), rituximab, bendamustine, blinatumomab, bortezomib, brentuximab, daratumumab, decitabine, azacitidine, obinutuzumab, cyclophosphamide, elotuzumab, and arsenic trioxide (TRISENOX), and eculizumab. The following therapies were deferred: 3 chemoimmunotherapy cycles for indolent NHL, 17 biologic drugs (4 bortezomib, 12 carfilzomib, 1 daratumumab) for MM and amyloidosis, and 2 hypomethylating agents (azacitidine) for high-risk myelodysplastic syndrome (MDS). Lenalidomide maintenance was interrupted in 5 MM patients, all in long-term remission.
Reasons for bone marrow deferral were routine follow-up of AL (n = 2) and myeloproliferative neoplasms (n = 4), staging of indolent NHL (n = 5), evaluation of monoclonal gammopathy of undetermined significance (n = 3), and reevaluation of immune thrombocytopenia, aplastic anemia, and low-risk MDS (n = 1 each).
Other procedures included IV hydration, phlebotomy, IV immunoglobulin, albumin infusions, lumbar punctures, and venetoclax ramp-up.
Two allogeneic nonurgent transplants for cutaneous NHL were postponed (1 because of the inability to receive the donor’s stem cells from Australia, and 1 because of the temporary unavailability of ICU support). However, 4 allogeneic transplants for acute myeloid leukemia (3 haploidentical and 1 matched unrelated donor) and 5 autologous transplants for MM were performed during the study period.
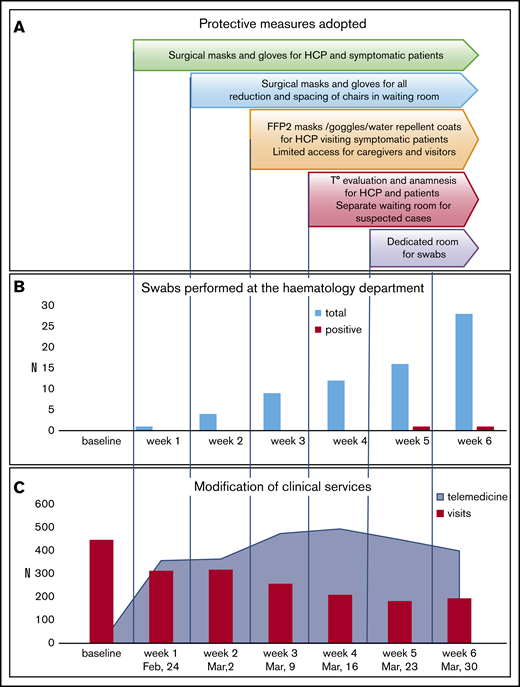
Figure 1A shows the progressive adoption of personal protective equipment (PPE) starting with surgical masks and disposable gloves and proceeding to filtering face piece-2 masks, protective eye goggles, and water-repellent disposable coats. The waiting room of the outpatient clinic was reorganized to guarantee social distancing (2 m), with limited access for relatives/caregivers. From 16 March 2020, body temperature and history of symptoms were recorded every day for anyone (HCPs and visitors) at the entrance of the clinic/day service. Suspected cases were isolated in a protected area before medical evaluation and the naso-pharingeal swab results (performed in a dedicated room that was sanitized every day). Outpatients were tested if symptomatic for COVID-19 or if they had to be admitted to the hematology/transplant ward. The procedure allowed us to identify 2 positive cases among symptomatic outpatients; all patients to be admitted were negative (Figure 1B). All HCPs were tested at week 6, with negative results. Overall, the progressive adoption of protective measures and careful anamnesis of symptoms and contacts appeared to be useful to identify potential positive cases, whereas the systematic use of nasal-pharyngeal swabs, although useful, is affected by low sensitivity.7
Modification of clinical activities during COVID-19 pandemic. (A) Protective measures adopted during the study period. (B) Number of tests performed on patients in the Hematology Department and rate of positivity during the study. (C) Outpatients visits and telemedicine trends during the study. All evaluations were calculated as “total observations per week” and compared with the routine numbers preceding COVID-19 infection. The latter were calculated as mean number per week considering the 3 weeks preceding February 24 2020. With regard to telemedicine before the pandemics, e-mails and phone calls received for medical reasons represented ∼3% of our practice. FFP2, filtering facepiece 2; T°, temperature.
Modification of clinical activities during COVID-19 pandemic. (A) Protective measures adopted during the study period. (B) Number of tests performed on patients in the Hematology Department and rate of positivity during the study. (C) Outpatients visits and telemedicine trends during the study. All evaluations were calculated as “total observations per week” and compared with the routine numbers preceding COVID-19 infection. The latter were calculated as mean number per week considering the 3 weeks preceding February 24 2020. With regard to telemedicine before the pandemics, e-mails and phone calls received for medical reasons represented ∼3% of our practice. FFP2, filtering facepiece 2; T°, temperature.
A progressive implementation of telemedicine was adopted. As shown in Figure 1C, the number of e-mails and telephone calls increased, along with a decrease in outpatient visits. A total of 2530 contacts aimed at evaluating patients’ external laboratory/radiology results and/or at giving specific advice/prescriptions on medications/procedures were recorded. Home sampling performed by nurses of the hematology unit was interrupted to prevent patient/HCP exposure, whereas patients were favorably sampled at external local laboratories. Hospital-distributed drugs (proteasome, BCL2, and TK inhibitors; thrombopoietin analogs; and recombinant erythropoietin) were delivered via hospital pharmacy during the first 2 weeks and through a home delivery service thereafter.
As of 5 April 2020, 26 patients on active follow-up tested positive for COVID-19: 6 with myelodysplastic syndrome, 6 with chronic lymphocytic leukemia, 4 with NHL, 3 with myeloproliferative neoplasm, 3 with AL, 2 with MM, and 2 with autoimmune hemolytic anemia. Almost all patients (22/26) required admission to the hospital, 4 of whom were admitted to the ICU. Treatments included oxygen and/or ventilation support, antibiotics, hydroxychloroquine, steroids, and tocilizumab. Six patients had to discontinue their hematological drugs, including 2 subjects enrolled in a clinical trial. Six patients (23%) died; all were males, and 5 of 6 were older than 80 years of age. Mortality was nearly comparable to that of patients admitted to the ICU.8 Although this is beyond the scope of this article, positive cases within the hematology unit (26/4750; 0.5%) were constantly changing, and estimated to be what was observed in Lombardy as of 3 April (46 065/10 019 166; 0.5%). Among health care workers, 5 nurses and 3 biologists tested positive for COVID-19 (all tested and likely infected during shifts outside of the hematology unit).
In summary, the narrative description of our experience shows the safety and feasibility of care for hematological patients during pandemics and advocates for an earlier adoption of the highest level of preventive measures at the pandemic’s beginning. We advise prompt and systematic use of PPE, separation of hematological wards and shifts, anamnesis of symptoms and contacts, time/space and social limitations, and telemedicine practices. This would limit the spread of the infection among patients and workers, ensure the continuity of care, and allow the delivery of life-saving therapies/transfusions to urgent patients with aggressive diseases.
Data sharing requests should be sent to Bruno Fattizzo (bruno.fattizzo@unimi.it).
Acknowledgments:
The authors thank Pietrina Monni, Manuela Moro, Marta Muru, Cristina Sanavio, and all of the staff of the hematology unit, including the laboratory and trial office staff, as well as the Health Directorate, of Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico (Milan, Italy).
Contribution: B.F., J.A.G., and W.B. designed the study, collected data, and wrote the manuscript; C.B., R.C., F.C., A.F., V.M., G.M., M.M., A. Noto, G.N.S., M.S., and L.B. collected data; and all authors followed patients and revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Fattizzo, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Via Francesco Sforza, 35, 20100 Milan, Italy; e-mail: bruno.fattizzo@unimi.it.