Key Points:
In older alloHCT recipients, cognitive impairment is associated with inferior OS via increased NRM.
Cognitive impairment is identified as a novel risk factor and should be considered prior to alloHCT.
Abstract
Use of allogeneic hematopoietic cell transplantation (alloHCT) is increasing in older patients with hematologic malignancies. Studies suggest that geriatric assessment (GA), incorporating functional measures such as instrumental activities of daily living (IADL), delineates subtle age-related impairments that enhance risk-stratification. The objective of this multi-institutional retrospective study was to evaluate the prognostic utility of GA metrics collected pre-alloHCT. Eligibility criteria included age ≥50 and pre-alloHCT GA inclusive of at least IADL. Beyond IADL, additional geriatric metrics were collected where available and included Medical Outcomes Study Physical Health score (MOS-PH), Timed Up and Go (TUG), and cognition by Blessed Orientation Memory Concentration (BOMC). Three hundred thirty subjects were included, with a median age of 63 (range 50 to 77). Impairments were frequent: 36% had at least 1 IADL impairment; 14% had TUG ≥13.5 seconds; and 17% had cognitive impairment (BOMC ≥ 7). Median MOS-PH score was 80. IADL and age were not significantly associated with nonrelapse mortality (NRM) or overall survival (OS). In multivariate analysis, only impaired cognition and Hematopoietic Cell Transplant-Comorbidity Index score ≥3 showed an independent association with 1-year NRM (subdistribution hazard ratio [SHR], 2.36; P = .01; and SHR, 2.19; P = .009, respectively). Cognitive impairment independently conferred inferior 1-year OS (hazard ratio, 1.94; P = .01). In a preplanned subgroup analysis in 224 patients aged ≥60 years, cognitive impairment remained the sole GA metric predictive of NRM (2-year NRM: SHR, 2.72; P = .007). These data suggest that cognitive impairment elevates risk of post-alloHCT NRM in older patients.
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) is increasingly being offered to older patients with hematologic malignancies.1,2 This trend reflects both the aging of the general population and improvements in nonmyeloablative transplant methodology and supportive care. However, alloHCT remains a treatment with significant morbidity and mortality,3 and determination of which older patients are good candidates for transplant is crucial. More accurate risk-stratification of nonrelapse mortality (NRM) or morbidity based on novel health determinants rather than chronologic age alone could encourage offering alloHCT to fit older patients, allow appropriate counseling of higher-risk patients, and facilitate the design of studies to mitigate risks. Although the association of comorbidities and performance status with NRM has been validated,4,5 individualizing treatment requires enhanced discrimination beyond age, comorbidity, and performance status.6
Geriatric assessment (GA) entails a formal battery of testing designed to assess key domains often impaired in older age, many of which have been shown to predict morbidity and/or mortality in an oncogeriatric population.7–11 Even among patients deemed fit for alloHCT, functional measures within the GA have been shown to predict post-alloHCT outcomes independent of clinical factors in a number of single-institution studies.12–15 In the largest prospective study by Muffly et al,12 functional compromise defined by impairment in instrumental activities of daily living (IADL) predicted higher rates of NRM and inferior overall survival (OS) in allogeneic transplant patients age ≥50 years. A simple scoring system combining the Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI) and IADL strongly stratified patients for OS; this was validated in retrospective fashion by Lin et al,14 although not by other prospective studies.13,15 Based on these emerging data and the wider use of alloHCT among older adults, including those age ≥70 years,2 an increasing number of transplant centers now collect GA data as part of standard care.16 A prospective trial of GA prior to alloHCT is currently underway through the Bone Marrow Transplant Clinical Trials Network (BMT CTN 1704, NCT03992352) to better stratify 1-year NRM in older patients.
Many different GAs exist, with numerous patient-reported and/or provider performed instruments for each health domain (eg, function, nutrition, cognition). IADL represents one of the most universally administered patient-reported tools to gauge function. Recently, a GA designed specifically for cancer patients has been developed and validated as a predictor of chemotherapy toxicity.8,17 Although primarily studied in solid tumor patients, this tool has gained acceptance in much of the hematology and oncology community desiring standardization.11
In this study, we aimed to analyze for the first time in multicenter fashion the association of specific geriatric metrics with post-alloHCT outcomes, with a goal of validating the prognostic ability of IADL and exploring the utility of other core functional tools, such as Medical Outcomes Study Physical Health score (MOS-PH), Timed Up and Go (TUG), and cognition by Blessed Orientation Memory Concentration (BOMC).18–21
Methods
CIBMTR
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of >500 transplant centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin in Milwaukee, Wisconsin and the National Marrow Donor Program/Be the Match in Minneapolis, Minnesota. Participating centers are required to report all consecutive transplants, and compliance is monitored by on-site audits. Computerized checks for discrepancies and physicians' review of submitted data ensure compliance and data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The National Marrow Donor Program Central Institutional Review Board and the University of Chicago Institutional Review Board reviewed and approved this study. All patients provided written informed consent for the CIBMTR registry.
Subjects
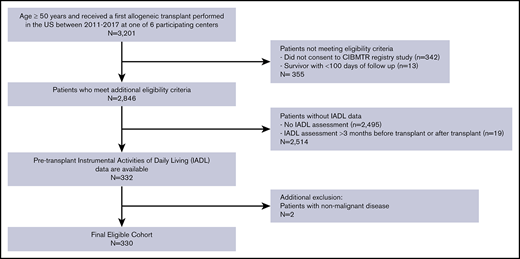
Subjects were identified from 6 transplant centers based on availability of GA data prior to transplant. Inclusion criteria consisted of (1) first allogeneic transplant performed in the United States from 2011 to 2017; (2) age ≥50 years at the time of transplant; and (3) previously consented to the CIBMTR registry. Subjects were excluded if IADL was incomplete or had not been collected within 3 months prior to transplant; other core GA metrics were collected where available but were not required. Additional exclusion criteria included <100 days of follow-up posttransplant or transplant performed for a nonmalignant disorder, as NRM cannot be easily defined in such patients.
Study design
Eligible subjects’ GA data were compiled by individual participating sites. Across sites, GA data were collected by variable staff members (provider, nurse, research staff) and by variable methods (electronically vs on paper). The indication for GA by site was categorized into 3 groups: collection as part of research, systematic standard of care due to age, or nonsystematic standard of care with GA based on individual patient need. This categorization allowed for potential sensitivity analysis excluding the latter category, enabling minimization of bias associated with nonsystematic GA collection. Some subjects from 3 centers were included in previously published reports related to the prognostic value of specific GA metrics (n = 191).15,22,23
The GA data included IADL for each subject as well as MOS-PH, BOMC, and TUG scores where available; data were submitted by each site and collated centrally by the CIBMTR. Pretransplant data provided by the CIBMTR included demographic, disease-related, and transplant-related variables. The primary outcome measure was defined as NRM at 1-year post-alloHCT, mirroring the BMT CTN 1704 primary endpoint. Secondary outcome measures included 2-year NRM, OS, and progression-free survival (PFS). For symmetry to 1-year NRM, 1-year OS was also explored.
GA metrics
The OARS IADL is a 7-question tool assessing the following abilities: using the telephone, transportation, shopping, preparing meals, housework, taking medication, and managing money.18 Each question is scored as no, moderate, or complete dependence, and impairment was defined as the presence of any deficit. MOS-PH is a 10-question tool assessing a range of physical abilities, from bathing and dressing to vigorous activities such as running or lifting heavy objects.19 Each ability is scored as not limited, limited a little, or limited a lot, and impairment was defined as a score at or below the sample median. TUG is an objective test of physical function in which subjects are asked to rise from a seated position, walk 3 meters, turn around, walk back and sit down; impairment was defined as a score of ≥13.5 seconds.20 BOMC is a 6-question test of cognitive abilities, including memory, orientation, and concentration; impairment was defined as a score of ≥7.21
Statistical analysis
The CIBMTR provided the deidentified dataset to 1 participating site for statistical analysis. Testing of association of baseline variables was performed using Pearson’s χ2 test for categorical variables and equality-of-medians test for continuous nonnormally distributed variables. Cumulative incidence curves were generated to estimate the cumulative incidence rates of NRM, with death in the absence of relapse as a competing event. The Kaplan-Meier method was used to estimate OS and PFS rates. Prognostic factors, including demographic, clinical, and geriatric variables, were evaluated with the use of Fine-Gray subdistribution hazard models for NRM and Cox proportional-hazards model for OS and PFS. We first conducted univariate analysis for each prognostic factor, and multivariate analysis included variables showing an association with outcomes at P ≤ .1. A preplanned subgroup analysis examined subjects age ≥60 years, to focus on this older population and align with prior reports.12–14 Two-sided values of P <.05 were considered to indicate statistical significance. All analyses were performed using R software version 3.0.1 and SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics
The consort diagram displayed in Figure 1 depicts the application of inclusion/exclusion criteria to reach a final study sample of 330 patients, as well as the number of patients undergoing alloHCT at participating centers during this time period. Table 1 summarizes baseline characteristics of the study population. Median age was 63 years (range 50 to 77), and the most common disease types were acute myeloid leukemia (AML; 46%) and myelodysplastic syndrome (33%). Karnofsky performance status (KPS) was 90 to 100 in 73% of subjects, and 51% had HCT-CI score of ≥3. Reduced intensity conditioning preceded alloHCT in 68% of patients. Median follow-up of survivors was 24 months (range 3 to 61 months).
Consort diagram. Derivation of study cohort based on inclusion/exclusion criteria.
Consort diagram. Derivation of study cohort based on inclusion/exclusion criteria.
Subjects’ baseline characteristics
| Characteristic . | n (%) or median (range) . |
|---|---|
| Age, y | 63 (50 to 77) |
| Age by decade, y | |
| 50 to 59 | 106 (32) |
| 60 to 69 | 166 (50) |
| 70+ | 58 (18) |
| Female sex | 132 (40) |
| Race | |
| White | 294 (89) |
| African American | 13 (4) |
| Asian | 11 (3) |
| >1 race | 1 (<1) |
| Missing | 11 (3) |
| Non-Hispanic ethnicity | 306 (93) |
| Disease type | |
| AML | 151 (46) |
| MDS/MPN | 109 (33) |
| NHL | 23 (7) |
| ALL | 21 (6) |
| Other leukemia | 12 (4) |
| CML | 9 (3) |
| PCD/MM | 5 (2) |
| DRI25 | |
| Low | 21 (6) |
| Intermediate | 189 (57) |
| High | 84 (25) |
| Very high | 5 (2) |
| Unknown | 31 (9) |
| Karnofsky performance score | |
| 90 to 100 | 241 (73) |
| <90 | 83 (25) |
| Missing | 6 (2) |
| HCT-CI score | |
| 0 | 66 (20) |
| 1 | 44 (13) |
| 2 | 52 (16) |
| 3+ | 167 (51) |
| Missing | 1 (<1) |
| Donor type | |
| Matched sibling | 110 (33) |
| Other related* | 13 (4) |
| Well-matched unrelated; 8/8 match | 139 (42) |
| Partially matched unrelated; 7/8 match | 29 (9) |
| Cord blood | 39 (12) |
| Conditioning intensity | |
| Myeloablative conditioning | 105 (32) |
| Reduced intensity conditioning | 224 (68) |
| Missing | 1 (<1) |
| Conditioning regimen | |
| Fludarabine/busulfan | 150 (45) |
| Fludarabine/melphalan | 103 (31) |
| TBI/cyclophosphamide based | 64 (19) |
| Other | 13 (4) |
| GVHD prophylaxis | |
| Tacrolimus/methotrexate based | 116 (35) |
| Tacrolimus alone | 85 (26) |
| Tacrolimus/MMF based | 49 (15) |
| Cyclosporine based | 28 (8) |
| CD34 selection | 12 (4) |
| Posttransplant cyclophosphamide based | 12 (4) |
| Other | 28 (8) |
| ATG/alemtuzumab | |
| ATG | 135 (41) |
| Alemtuzumab | 80 (24) |
| Neither | 113 (34) |
| Missing | 2 (<1) |
| Year of transplant | |
| 2011 | 5 (2) |
| 2012 | 16 (5) |
| 2013 | 45 (14) |
| 2014 | 85 (26) |
| 2015 | 68 (21) |
| 2016 | 51 (15) |
| 2017 | 60 (18) |
| Transplant center | |
| MD Anderson | 23 (7) |
| Memorial Sloan Kettering Cancer Center | 14 (4) |
| University of California San Francisco | 136 (41) |
| University of Chicago | 105 (32) |
| University of Minnesota | 41 (12) |
| University of Nebraska | 11 (3) |
| Indication for GA | |
| Research | 177 (54) |
| Systematic standard of care | 116 (35) |
| Nonsystematic standard of care | 37 (11) |
| Characteristic . | n (%) or median (range) . |
|---|---|
| Age, y | 63 (50 to 77) |
| Age by decade, y | |
| 50 to 59 | 106 (32) |
| 60 to 69 | 166 (50) |
| 70+ | 58 (18) |
| Female sex | 132 (40) |
| Race | |
| White | 294 (89) |
| African American | 13 (4) |
| Asian | 11 (3) |
| >1 race | 1 (<1) |
| Missing | 11 (3) |
| Non-Hispanic ethnicity | 306 (93) |
| Disease type | |
| AML | 151 (46) |
| MDS/MPN | 109 (33) |
| NHL | 23 (7) |
| ALL | 21 (6) |
| Other leukemia | 12 (4) |
| CML | 9 (3) |
| PCD/MM | 5 (2) |
| DRI25 | |
| Low | 21 (6) |
| Intermediate | 189 (57) |
| High | 84 (25) |
| Very high | 5 (2) |
| Unknown | 31 (9) |
| Karnofsky performance score | |
| 90 to 100 | 241 (73) |
| <90 | 83 (25) |
| Missing | 6 (2) |
| HCT-CI score | |
| 0 | 66 (20) |
| 1 | 44 (13) |
| 2 | 52 (16) |
| 3+ | 167 (51) |
| Missing | 1 (<1) |
| Donor type | |
| Matched sibling | 110 (33) |
| Other related* | 13 (4) |
| Well-matched unrelated; 8/8 match | 139 (42) |
| Partially matched unrelated; 7/8 match | 29 (9) |
| Cord blood | 39 (12) |
| Conditioning intensity | |
| Myeloablative conditioning | 105 (32) |
| Reduced intensity conditioning | 224 (68) |
| Missing | 1 (<1) |
| Conditioning regimen | |
| Fludarabine/busulfan | 150 (45) |
| Fludarabine/melphalan | 103 (31) |
| TBI/cyclophosphamide based | 64 (19) |
| Other | 13 (4) |
| GVHD prophylaxis | |
| Tacrolimus/methotrexate based | 116 (35) |
| Tacrolimus alone | 85 (26) |
| Tacrolimus/MMF based | 49 (15) |
| Cyclosporine based | 28 (8) |
| CD34 selection | 12 (4) |
| Posttransplant cyclophosphamide based | 12 (4) |
| Other | 28 (8) |
| ATG/alemtuzumab | |
| ATG | 135 (41) |
| Alemtuzumab | 80 (24) |
| Neither | 113 (34) |
| Missing | 2 (<1) |
| Year of transplant | |
| 2011 | 5 (2) |
| 2012 | 16 (5) |
| 2013 | 45 (14) |
| 2014 | 85 (26) |
| 2015 | 68 (21) |
| 2016 | 51 (15) |
| 2017 | 60 (18) |
| Transplant center | |
| MD Anderson | 23 (7) |
| Memorial Sloan Kettering Cancer Center | 14 (4) |
| University of California San Francisco | 136 (41) |
| University of Chicago | 105 (32) |
| University of Minnesota | 41 (12) |
| University of Nebraska | 11 (3) |
| Indication for GA | |
| Research | 177 (54) |
| Systematic standard of care | 116 (35) |
| Nonsystematic standard of care | 37 (11) |
ALL, acute lymphoid leukemia; CML, chronic myelogenous leukemia; GVHD, graft-versus-host disease; HCT-CI, Hematopoietic Cell Transplant-Comorbidity Index; MDS, myelodysplastic syndrome; MM, multiple myeloma; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; PCD, plasma cell dyscrasia; TBI, total body irradiation.
Other related: this includes 9 haploidentical transplants.
GA data for the entire cohort are displayed in Table 2. In brief, 36% (120/330) presented with at least 1 IADL impairment; the median MOS-PH score was 80 (range 0 to 100; n = 230), and 14% (38/273) had a slow TUG score at ≥13.5 seconds, a threshold associated with increased risk of falls in other settings.24 BOMC score ≥7 defined cognitive impairment (based on original validation of the BOMC instrument21 ) and was present in 40 of 235 subjects (17%). Impairment in TUG and BOMC scores did not differ by age group; rates of cognitive impairment were 20% for age 50 to 59, 15% for age 60 to 69 and 18% for age ≥70 (P = .57). Conversely, IADL impairment was less common in subjects ≥70 years (38% for age 50 to 59, 41% for age 60 to 69, and 21% for age ≥70; P = .02), and MOS-PH scores were more commonly above the median in subjects ≥70 years (P = .04), suggesting patient selection for better functional status in the oldest patients.
GA metrics
| Geriatric metric . | Possible score range and impairment threshold . | n (%) with available data . | Median (range) . | Median (range) by age group . | Impaired, n (%) . | Impairment by age group, n (%) . |
|---|---|---|---|---|---|---|
| Patient-reported function | ||||||
| OARS IADL | 0 to 14 with higher score indicating better function | 330 (100) | 14 (4 to 14) | 50 to 59: 14 (5 to 14) | 120 (36) | 50 to 59: 40 (38) |
| Impairment: <14 | 60 to 69: 14 (4 to 14) | 60 to 69: 68 (41) | ||||
| 70+: 14 (11 to 14) | 70+: 12 (21) | |||||
| MOS-PH | 0 to 100 with higher score indicating better function | 230 (70) | 80 (0 to 100) | 50 to 59: 72.5 (0 to 100) | N/A | N/A |
| Impairment: N/A | 60 to 69: 80 (0 to 100) | |||||
| 70+: 87.5 (55 to 100) | ||||||
| Performance-based function | ||||||
| TUG | Measured in seconds with higher number indicating worse function | 273 (83) | 9 (4 to 50) | 50 to 59: 9 (4 to 34) | 38 (14) | 50 to 59: 16 (16) |
| Impairment ≥13.5 | 60 to 69: 9 (4 to 50) | 60 to 69: 19 (14) | ||||
| 70+: 10 (4 to 15) | 70+: 3 (9) | |||||
| Cognition | ||||||
| BOMC | 0 to 28 with higher score indicating worse cognition | 235 (71) | 2 (0 to 16) | 50 to 59: 2 (0 to 16) | 40 (17) | 50 to 59: 17 (20) |
| Impairment ≥7 | 60 to 69: 2 (0 to 14) | 60 to 69: 18 (15) | ||||
| 70+: 4 (0 to 10) | 70+: 5 (18) | |||||
| Geriatric metric . | Possible score range and impairment threshold . | n (%) with available data . | Median (range) . | Median (range) by age group . | Impaired, n (%) . | Impairment by age group, n (%) . |
|---|---|---|---|---|---|---|
| Patient-reported function | ||||||
| OARS IADL | 0 to 14 with higher score indicating better function | 330 (100) | 14 (4 to 14) | 50 to 59: 14 (5 to 14) | 120 (36) | 50 to 59: 40 (38) |
| Impairment: <14 | 60 to 69: 14 (4 to 14) | 60 to 69: 68 (41) | ||||
| 70+: 14 (11 to 14) | 70+: 12 (21) | |||||
| MOS-PH | 0 to 100 with higher score indicating better function | 230 (70) | 80 (0 to 100) | 50 to 59: 72.5 (0 to 100) | N/A | N/A |
| Impairment: N/A | 60 to 69: 80 (0 to 100) | |||||
| 70+: 87.5 (55 to 100) | ||||||
| Performance-based function | ||||||
| TUG | Measured in seconds with higher number indicating worse function | 273 (83) | 9 (4 to 50) | 50 to 59: 9 (4 to 34) | 38 (14) | 50 to 59: 16 (16) |
| Impairment ≥13.5 | 60 to 69: 9 (4 to 50) | 60 to 69: 19 (14) | ||||
| 70+: 10 (4 to 15) | 70+: 3 (9) | |||||
| Cognition | ||||||
| BOMC | 0 to 28 with higher score indicating worse cognition | 235 (71) | 2 (0 to 16) | 50 to 59: 2 (0 to 16) | 40 (17) | 50 to 59: 17 (20) |
| Impairment ≥7 | 60 to 69: 2 (0 to 14) | 60 to 69: 18 (15) | ||||
| 70+: 4 (0 to 10) | 70+: 5 (18) | |||||
N/A, not applicable; OARS, Older Americans Resources and Services.
Transplant outcomes
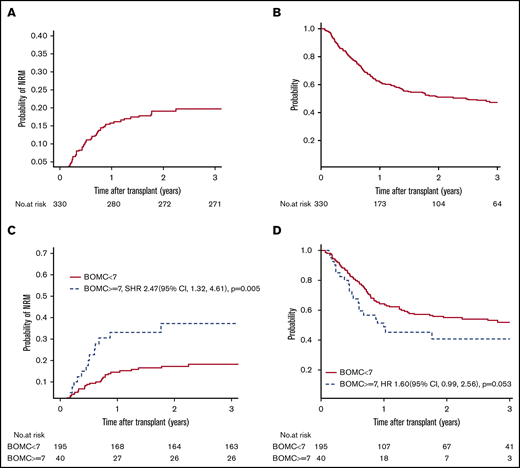
For the entire cohort, NRM at 1 year was 16% (95% confidence interval [CI], 12% to 20%), and at 2 years was 19% (95% CI, 15% to 24%). Cumulative incidence of relapse at 2 years was 41% (95% CI, 35% to 46%). PFS was 40% at 2 years (95% CI 35% to 46%) and 2-year OS was 51% (95% CI, 45% to 57%). Figure 2A-B displays the NRM and OS for the entire cohort, respectively. At the time of analysis, 160 of 330 patients had died; cause of death as reported to the CIBMTR was primary disease in 51%, graft-versus-host disease in 14%, infection in 10%, organ failure in 8%, and other/unknown in 17%. OS did not differ between this cohort and patients age ≥50 years not undergoing GA at the same institutions over the same time period (supplemental Table 1).
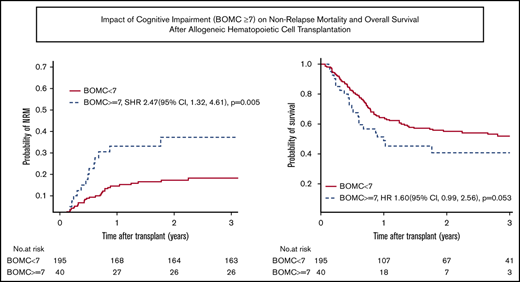
NRM and OS. (A-B) NRM and OS for the entire cohort. (C-D) Unadjusted NRM and OS by presence of cognitive impairment (BOMC score ≥7).
NRM and OS. (A-B) NRM and OS for the entire cohort. (C-D) Unadjusted NRM and OS by presence of cognitive impairment (BOMC score ≥7).
Prognostic factors for NRM
Factors associated with the primary outcome of 1-year NRM in univariate analysis at P ≤ .1 were comorbidity (HCT-CI score ≥3), patient-reported physical function by MOS-PH score below the median of 80, and cognitive impairment (BOMC ≥7) (Table 3). Clinical variables not associated with 1-year NRM included KPS, age by decade, refined Disease Risk Index (DRI) score,25 donor type, conditioning intensity, and graft source. Neither patient-reported functional limitations by IADL nor performance-based function by slow TUG influenced 1-year NRM. In multivariate analysis, high HCT-CI comorbidity (SHR, 2.19; 95% CI, 1.22 to 3.94; P = .009) and cognitive impairment (SHR, 2.36; 95% CI, 1.21 to 4.60; P = .01) remained independently prognostic (Table 4). Results for 2-year NRM recapitulated the 1-year NRM results, with high comorbidity and cognitive impairment being the only significant predictors in multivariate analysis (SHR, 1.81; 95% CI, 1.07 to 3.09; P = .03 for HCT-CI ≥ 3; and SHR, 2.41; 95% CI, 1.29 to 4.53; P = .006 for BOMC ≥7). For subjects without cognitive impairment (ie, BOMC <7), NRM was low at 1 and 2 years at 14% and 17%, respectively, whereas pre-alloHCT cognitive impairment posed a high risk of NRM at 1 and 2 years at 33% and 37%, respectively.
Univariate analysis of 1-y NRM and 1-y OS
| Variable . | 1-y NRM SHR (95% CI) . | 1-y OS HR (95% CI) . |
|---|---|---|
| GA | ||
| Cognitive impairment by BOMC | ||
| <7 | Reference | Reference |
| ≥7 | 2.57 (1.34 to 4.95), P = .005* | 1.56 (0.94 to 2.60), P = .09* |
| IADL | ||
| Normal (14) | Reference | Reference |
| Impaired (<14) | 1.17 (0.69 to 2.06), P = .58 | 1.37 (0.95 to 1.97), P = .09* |
| Physical function by MOS-PH score | ||
| >80 (median score) | Reference | Reference |
| ≤80 | 1.85 (0.94 to 3.61), P = .08* | 1.26 (0.81 to 1.94), P = .30 |
| TUG, s | ||
| <13.5 | Reference | Reference |
| ≥13.5 | 1.38 (0.64 to 2.99), P = .41 | 1.43 (0.86 to 2.38), P = .17 |
| Clinical variables | ||
| Age, y | ||
| 50 to 59 | Reference | Reference |
| 60 to 69 | 1.09 (0.56 to 2.10), P = .80 | 1.27 (0.83 to 1.93), P = .27 |
| ≥70 | 1.66 (0.77 to 3.59), P = .20 | 1.29 (0.75 to 2.22), P = .35 |
| Karnofsky performance status | ||
| 90 to 100 | Reference | Reference |
| <90 | 1.31 (0.72 to 2.41), P = .38 | 1.40 (0.94 to 2.07), P = .1* |
| Comorbidity by HCT-CI | ||
| 0 to 2 | Reference | Reference |
| ≥3 | 2.20 (1.22 to 3.97), P = .009* | 1.28 (0.89 to 1.84), P = .18 |
| DRI | ||
| Low, intermediate | Reference | Reference |
| High, very high | 1.60 (0.87 to 2.93), P = .13 | 1.44 (0.97 to 2.13), P = .07* |
| Conditioning intensity | ||
| Myeloablative conditioning | Reference | Reference |
| Reduced intensity conditioning | 1.48 (0.77 to 2.84), P = .24 | 1.43 (0.95 to 2.15), P = .09* |
| Donor type | ||
| Matched sibling | Reference | Reference |
| Other related | 2.96 (1.02 to 8.59), P = .05 | 1.93 (0.85 to 4.35), P = .11 |
| Well-matched unrelated; 8/8 match | 1.29 (0.63 to 2.68), P = .49 | 1.18 (0.76 to 1.83), P = .46 |
| Partially matched unrelated; 7/8 match | 1.62 (0.57 to 4.63), P = .37 | 1.29 (0.65 to 2.54), P = .47 |
| Cord blood | 2.49 (1.08 to 5.76), P = .03 | 1.80 (1.03 to 3.15), P = .04 |
| Across all donor types, by log rank | P = .14 | P = .22 |
| Graft source | ||
| Bone marrow | Reference | Reference |
| Peripheral blood | 0.68 (0.27 to 1.72), P = .41 | 1.23 (0.60 to 2.53), P = .58 |
| Umbilical cord blood | 1.37 (0.47 to 3.97), P = .57 | 1.88 (0.82 to 4.30), P = .13 |
| Variable . | 1-y NRM SHR (95% CI) . | 1-y OS HR (95% CI) . |
|---|---|---|
| GA | ||
| Cognitive impairment by BOMC | ||
| <7 | Reference | Reference |
| ≥7 | 2.57 (1.34 to 4.95), P = .005* | 1.56 (0.94 to 2.60), P = .09* |
| IADL | ||
| Normal (14) | Reference | Reference |
| Impaired (<14) | 1.17 (0.69 to 2.06), P = .58 | 1.37 (0.95 to 1.97), P = .09* |
| Physical function by MOS-PH score | ||
| >80 (median score) | Reference | Reference |
| ≤80 | 1.85 (0.94 to 3.61), P = .08* | 1.26 (0.81 to 1.94), P = .30 |
| TUG, s | ||
| <13.5 | Reference | Reference |
| ≥13.5 | 1.38 (0.64 to 2.99), P = .41 | 1.43 (0.86 to 2.38), P = .17 |
| Clinical variables | ||
| Age, y | ||
| 50 to 59 | Reference | Reference |
| 60 to 69 | 1.09 (0.56 to 2.10), P = .80 | 1.27 (0.83 to 1.93), P = .27 |
| ≥70 | 1.66 (0.77 to 3.59), P = .20 | 1.29 (0.75 to 2.22), P = .35 |
| Karnofsky performance status | ||
| 90 to 100 | Reference | Reference |
| <90 | 1.31 (0.72 to 2.41), P = .38 | 1.40 (0.94 to 2.07), P = .1* |
| Comorbidity by HCT-CI | ||
| 0 to 2 | Reference | Reference |
| ≥3 | 2.20 (1.22 to 3.97), P = .009* | 1.28 (0.89 to 1.84), P = .18 |
| DRI | ||
| Low, intermediate | Reference | Reference |
| High, very high | 1.60 (0.87 to 2.93), P = .13 | 1.44 (0.97 to 2.13), P = .07* |
| Conditioning intensity | ||
| Myeloablative conditioning | Reference | Reference |
| Reduced intensity conditioning | 1.48 (0.77 to 2.84), P = .24 | 1.43 (0.95 to 2.15), P = .09* |
| Donor type | ||
| Matched sibling | Reference | Reference |
| Other related | 2.96 (1.02 to 8.59), P = .05 | 1.93 (0.85 to 4.35), P = .11 |
| Well-matched unrelated; 8/8 match | 1.29 (0.63 to 2.68), P = .49 | 1.18 (0.76 to 1.83), P = .46 |
| Partially matched unrelated; 7/8 match | 1.62 (0.57 to 4.63), P = .37 | 1.29 (0.65 to 2.54), P = .47 |
| Cord blood | 2.49 (1.08 to 5.76), P = .03 | 1.80 (1.03 to 3.15), P = .04 |
| Across all donor types, by log rank | P = .14 | P = .22 |
| Graft source | ||
| Bone marrow | Reference | Reference |
| Peripheral blood | 0.68 (0.27 to 1.72), P = .41 | 1.23 (0.60 to 2.53), P = .58 |
| Umbilical cord blood | 1.37 (0.47 to 3.97), P = .57 | 1.88 (0.82 to 4.30), P = .13 |
HCT-CI, hematopoietic cell transplant-comorbidity index.
Included in multivariate analysis since P ≤ .1.
Multivariate analysis of 1-y NRM and 1-y OS
| Variable . | 1-y NRM SHR (95% CI) . | 1-y OS HR (95% CI) . |
|---|---|---|
| Cognitive impairment by BOMC | ||
| <7 | Reference | Reference |
| ≥7 | 2.36 (1.21 to 4.60), P = .01 | 1.94 (1.14 to 3.31), P = .01 |
| Comorbidity by HCT-CI | — | |
| 0 to 2 | Reference | |
| ≥3 | 2.19 (1.22 to 3.94), P = .009 | |
| Physical function by MOS-PH score | — | |
| >80 (median score) | Reference | |
| ≤80 | 1.65 (0.84 to 3.27), P = .15 | |
| IADL | — | |
| Normal (14) | Reference | |
| Impaired (<14) | 1.41 (0.96 to 2.08), P = .08 | |
| Karnofsky performance status | — | |
| 90 to 100 | Reference | |
| <90 | 1.25 (0.82 to 1.90), P = .30 | |
| DRI | — | |
| Low/intermediate | Reference | |
| High/very high | 1.39 (0.93 to 2.08), P = .11 | |
| Conditioning intensity | — | |
| Myeloablative conditioning | Reference | |
| Reduced intensity conditioning | 1.31 (0.85 to 2.02), P = .21 |
| Variable . | 1-y NRM SHR (95% CI) . | 1-y OS HR (95% CI) . |
|---|---|---|
| Cognitive impairment by BOMC | ||
| <7 | Reference | Reference |
| ≥7 | 2.36 (1.21 to 4.60), P = .01 | 1.94 (1.14 to 3.31), P = .01 |
| Comorbidity by HCT-CI | — | |
| 0 to 2 | Reference | |
| ≥3 | 2.19 (1.22 to 3.94), P = .009 | |
| Physical function by MOS-PH score | — | |
| >80 (median score) | Reference | |
| ≤80 | 1.65 (0.84 to 3.27), P = .15 | |
| IADL | — | |
| Normal (14) | Reference | |
| Impaired (<14) | 1.41 (0.96 to 2.08), P = .08 | |
| Karnofsky performance status | — | |
| 90 to 100 | Reference | |
| <90 | 1.25 (0.82 to 1.90), P = .30 | |
| DRI | — | |
| Low/intermediate | Reference | |
| High/very high | 1.39 (0.93 to 2.08), P = .11 | |
| Conditioning intensity | — | |
| Myeloablative conditioning | Reference | |
| Reduced intensity conditioning | 1.31 (0.85 to 2.02), P = .21 |
Subjects with missing BOMC or MOS-PH score were included in multivariate analysis; SHR/HR for subjects with missing scores did not differ from reference categories, data not shown. —, not included in multivariate analysis as P > .1 in univariate analysis.
Given the strong association of cognitive impairment with NRM, we explored confounding factors through correlation with GA-rated function or clinical variables. Cognitive impairment (BOMC ≥7) was associated with slower TUG time of ≥13.5 seconds (P = .001), but was not associated with IADL impairment, MOS-PH score above the median, age by decade, KPS <90, HCT-CI score ≥3, high or very high DRI score, or conditioning intensity. To assess for the presence of potential bias caused by missing data, we also evaluated the effect of missing BOMC score. Subjects with missing BOMC score were older (mean 65 vs 62 years, P = .0001) but had similar probability of HCT-CI ≥3 and IADL impairment (P = 1.0 and P = .99, respectively); the 1-year NRM and 1-year OS of subjects with missing BOMC score were not different from those with available BOMC score (SHR, 0.38; 95% CI, 0.30 to 1.24; P = .17; and hazard ratio [HR], 1.01; 95% CI, 0.68 to 1.51; P = .96, respectively).
Causes of death for 109 deceased patients with BOMC scores are summarized in supplemental Table 2. In those for whom cause of death was known, there was a greater proportion of non–disease-related deaths in patients with BOMC ≥7 vs <7 (14/21 vs 40/86, P = .1), although this was not statistically significant; no category of non–disease-related death predominated.
Prognostic factors for OS and progression-free survival
With respect to 1-year OS, significant univariate predictors at P ≤ .1 were KPS <90, DRI high or very high, reduced intensity conditioning, IADL impairment, and cognitive impairment (Table 3). In multivariate analysis, only cognitive impairment maintained a significant association with worse 1-year OS (HR, 1.94; 95% CI, 1.14 to 3.31; P = .01) (Table 4). Functional impairment by IADL was not significantly associated with OS (HR, 1.41; 95% CI, 0.96 to 2.08; P = .08). At 2 years, the findings for cognitive impairment and IADL were similar (cognitive impairment, HR, 1.60; 95% CI, 0.99 to 2.56; P = .05; IADL impairment, HR, 1.33; HR, 0.96 to 1.86; P = .09). For subjects with normal cognitive function, the probability of OS at 1 and 2 years was 64% and 55%, respectively, whereas for subjects with cognitive impairment, OS at 1 and 2 years was 51% and 40%, respectively. Figure 2C-D displays unadjusted NRM and OS by presence of cognitive impairment, respectively. To further confirm these findings, a sensitivity analysis was performed excluding the 37 subjects whose GA data were collected nonsystematically at their site based on individual patient- or provider-identified need, thereby including only those subjects whose GA was collected as systematic standard of care at their site (typically based on age) or as part of research. Similar effects of IADL impairment and cognitive impairment on 2-year NRM and OS were found (data not shown).
Predictors of PFS at both 1 and 2 years were high disease risk (high or very high DRI) and cognitive impairment. Both remained independently associated with PFS at 2 years (for DRI, HR, 1.62; 95% CI, 1.17 to 2.24; P = .004; for cognitive impairment, HR, 1.79; 95% CI, 1.14 to 2.81; P = .01). No geriatric metrics were associated with risk of relapse, and age was not associated with any posttransplant outcome.
Outcomes in patients 60 years or older
In a preplanned subgroup analysis of 224 patients aged ≥60 years, cognitive impairment remained associated with unadjusted 2-year NRM (SHR, 2.73; 95% CI, 1.32 to 5.63; P = .007). There was a borderline significant association between OS and poor patient-reported physical function, defined as MOS-PH score below the cohort median (HR, 1.58; 95% CI, 0.99 to 2.53; P = .05). IADL impairment did not confer higher rates of 2-year NRM (SHR, 0.84; 95% CI, 0.44 to 1.63; P = .61) nor worse 2-year OS (HR, 1.37; 95% CI, 0.93 to 2.04; P = .11) in this subgroup.
Discussion
In this first multi-institutional study of GA prior to alloHCT, we demonstrate for the first time an association between pre-alloHCT cognitive impairment, as measured by BOMC ≥7, and inferior OS due to increased NRM. In contrast to prior reports, IADL was not significantly associated with outcomes.
Mild cognitive impairment is highly prevalent in the older patient population, with best estimates ranging from 14% to 18% in the general population over age 70,26 and 17% to 35% in hematologic malignancy patients over age 75.27 In our alloHCT population aged 50 to 77 years, the prevalence of cognitive impairment by BOMC ≥7 was 17% (40 of 235 subjects), comparable to prior reports despite inclusion of somewhat younger subjects. In our study, there was no difference in rate of cognitive impairment by age, confirming that even “younger” alloHCT patients in the 50- to 59-year age group are vulnerable to this geriatric syndrome.
Limited information is known about the impact of baseline cognitive impairment in the setting of treatment of hematologic malignancies, although cancer-related cognitive impairment has been more extensively described in solid tumors.28 As well, because many different screening and diagnostic tests exist to measure cognitive impairment, with different sensitivity and specificity, comparison of prevalence across studies is challenging. In a recent large single-institutional study of 341 patients age 75 years and older with hematologic malignancies, impaired working memory by 5-word delayed recall was associated with inferior OS, even when adjusting for age, comorbidities, and disease aggressiveness.27 Other studies have shown an inferior impact of cognition on OS in specific patient populations, such as chronic lymphocytic leukemia and AML.29,30 In the setting of alloHCT, evidence suggests that cognitive impairment is prevalent,13–15,23,31 but association with posttransplant outcomes has not been consistently examined. These data identify cognitive impairment as a novel risk factor, independent of function and comorbidity, for NRM and OS in the curative intent setting of alloHCT. Moreover, cognitive impairment was readily assessable using the BOMC, a 6-question screening test commonly considered to be relatively insensitive.
The underlying mechanisms by which cognitive impairment predisposes to higher alloHCT NRM cannot be elucidated from this study, but reasonable potential explanations exist. Patients with mild cognitive impairment may appear unaffected in routine clinical interactions, but have subclinical deficits that may worsen after transplant due to multiple stressors such as chemotherapy, sedative/hypnotics, calcineurin inhibitors, infection, or even the setting of inpatient hospitalization. Cognitive impairment alters decision making even in an idealized research setting.32 Furthermore, cognitive impairment is an established risk factor for postoperative delirium33 and thus may contribute to delirium or falls; these complications have been shown to be associated with inferior NRM and OS after alloHCT.34
We hypothesized that functional impairment as measured by GA would independently predict worse outcome. Surprisingly, the study did not replicate prior findings of the adverse influence of IADL impairment observed in most prior single-center studies,12,14,15 although not all.13 The seemingly conflicting results with prior studies may be reconciled. First, we must acknowledge that although not statistically significant, IADL impairment did show a marginal association with 1-year NRM (HR, 1.41; P = .08 in multivariate analysis), justifying further investigation in larger studies. It is also conceivable that changes in transplant practice have attenuated the negative impact of functional impairment due to increased physician awareness. Functional status, as measured by IADL or other tools, is increasingly being used by transplant physicians as part of standard-of-care assessment for transplant candidacy based on a recent survey.16 The lower rates of functional impairment in the older patients in our cohort indicate some degree of patient selection based on function. Some patients with impaired function may now be excluded or have received targeted intervention for this, such as physical therapy referral, structured exercise, or increased care-giving support, potentially blunting any association with inferior outcomes; some of the subjects in this series (around one-third) were part of such an optimization program.35 Finally, the multi-institutional nature of the study introduced heterogeneity in administration of functional tests as far as both methods of measurement and timing relative to alloHCT.
Important limitations exist in this observational study. First, the ideal set of tools and thresholds remains undefined. Cognitive testing presents a major dilemma, as cognitive screening tests such as the BOMC are not diagnostic.36 However, the gold-standard comprehensive neuropsychological testing is laborious and time consuming. Furthermore, cognitive screening tests such as the BOMC limit the ability to study subdomains of cognition (eg, memory, executive function, attention, and concentration). Similarly, the tools and thresholds for functional status may not be optimized for the studied population.37,38 The BMT CTN 1704 trial, a large national study prospectively utilizing a standard set of health status tools pre-alloHCT among patients 60 years and older, will be a critical study to potentially confirm these results (NCT03992352). This study uses a slightly more detailed cognitive test, the Montreal Orientation Memory Concentration test, rather than the BOMC.
The observational nature of the present study precluded evaluation for toxicities (eg, delirium) or better adjudicating non–disease-related causes of death. The heterogeneity in patients and regimens inherent in the multicenter nature of this study represents a strength as far as generalizability, although also introduces variability that may limit detecting prognostic utility of GA metrics. Finally, this sample included only a limited number of patients over age 70, and one-third were under age 60; nonetheless, geriatric vulnerabilities such as IADL impairment and cognitive impairment were prevalent in each age group, and the latter was associated with inferior outcomes.
Moving forward, this data suggest that the routine assessment of cognitive impairment in older patients preparing to undergo alloHC may aid in risk-stratification and may even encourage alloHCT in older adults lacking cognitive impairment. Pending a confirmatory study, we recommend that, rather than being used as an exclusion for alloHCT, the finding of cognitive impairment by a screening test should prompt additional workup, interventions to improve or preserve cognitive function (eg, behavioral, pharmacologic, or exercise-based interventions),39–41 and increased caregiver support.35 Studies to reduce the risk of NRM targeted to this vulnerable population are a high priority so as to maximize the availability and safety of alloHCT for those in need.
E-mail requests for original data may be directed to the corresponding author, Rebecca L. Olin, at rebecca.olin@ucsf.edu.
Acknowledgments
Individual investigators were supported as follows: Hellman Fellows award, University of California, San Francisco (R.L.O.); Leukemia and Lymphoma Society grant 6136-14 (M.A.); Marrow on the Move (M.A,); National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) grant 1 U54 GM115458 (V.R.B. and T.K.); NIH, National Cancer Institute (NCI) grants P30 CA036727 (V.R.B.) and 5T32CA009566-32; University of Chicago (B.D.); NIH, National Institute on Aging grant T32AG000212; and the University of California, San Francisco (L.-W.H.). The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported primarily by NIH, NCI Public Health Service grant U24CA076518; NIH, National Heart, Lung, and Blood Institute (NHLBI) grants U24HL138660, R21HL140314, and U01HL128568); the NIH, National Institute of Allergy and Infectious Diseases (NIAID); US Health Resources and Services Administration grants HHSH250201700006C, SC1MC31881-01-00, and HHSH250201700007C; and US Office of Naval Research grants N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705. Additional federal support was provided by NHI, NCI grants P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141; NIH, NHLBI grants R01HL126589, R01HL129472, R01HL130388, R01HL131731; NIH, NIAID grants R01AI128775, U01AI069197, and U01AI126612; and the Biomedical Advanced Research and Development Authority (BARDA). Support was also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, Japan Hematopoietic Cell Transplantation Data Center, St Baldrick’s Foundation, the National Marrow Donor Program, the Medical College of Wisconsin, and the following commercial entities: AbbVie, Actinium Pharmaceuticals, Inc, Adaptive Biotechnologies, Adienne SA, Allovir, Inc, Amgen, Inc, Anthem, Inc, Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, Inc, bluebird bio, Inc, Bristol Myers Squibb Co, Celgene Corp, Chimerix, Inc, CSL Behring, CytoSen Therapeutics, Inc, Daiichi Sankyo Co, Ltd, Gamida-Cell, Ltd, Genzyme, GlaxoSmithKline, HistoGenetics, Inc, Incyte Corp, Janssen Biotech, Inc, Janssen Pharmaceuticals, Inc, Janssen/Johnson & Johnson, Jazz Pharmaceuticals, Inc, Kiadis Pharma, Kite Pharma, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Mallinckrodt LLC, Medac GmbH, Merck & Co, Inc, Merck Sharp & Dohme Corp, Mesoblast, Millennium, the Takeda Oncology Co, Miltenyi Biotec, Inc, Novartis Oncology, Novartis Pharmaceuticals Corp, Omeros Corp, Oncoimmune, Inc, Orca Biosystems, Inc, Pfizer, Inc, Phamacyclics, LLC, Regeneron Pharmaceuticals, Inc, REGiMMUNE Corp, Sanofi Genzyme, Seattle Genetics, Sobi, Inc, Takeda Oncology, Takeda Pharma, Terumo BCT, Viracor Eurofins, and Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: R.L.O., C.F., M.C.P., and A.A. designed the study; R.L.O., C.F., M.C.P., M.A., V.R.B., B.D., S.A.G., L.-W.H., T.K., R.J.L., L.P., U.R.P., D.J.W., and A.A. collected and assembled data; R.L.O., C.F., M.C.P., M.A., V.R.B., B.D., S.A.G., L.-W.H., T.K., S.M.L., R.J.L., L.P., U.R.P., D.J.W., and A.A. analyzed and interpreted the data; R.L.O. and A.A. wrote the manuscript; and all authors participated in manuscript development and gave final approval.
Conflict-of-interest disclosure: R.L.O. received research funding from Daiichi Sankyo, Astellas, Pfizer, AstraZeneca, Clovis, Ignyta, MedImmune, Mirati Therapeutics, Novartis, and Spectrum; received consultancy fees and research funding from Genentech; received consultancy fees and honoraria from Jazz Pharmaceuticals; and received consultancy fees from Revolution Medicine and Amgen. M.C.P. received research funding from Kite Pharmaceuticals, Novartis, and BMS; and consultancy fees from Medigene, Amgen, and Pfizer. V.R.B. received research funding from Jazz Pharmaceuticals and Tolero Pharmaceuticals; received consultancy fees from Agios, AbbVie, Omeros, Partner Therapeutics, Takeda, National Marrow Donor Program, CSL Behring, and Pfizer; and received consultancy fees and research funding from Incyte. S.A.G. received research funding from Miltenyi and Omeros; received consultancy fees and research funding from Celgene, Amgen, Takeda, Actinium, and Johnson & Johnson; and received consultancy fees from Jazz Pharmaceuticals, Kite, Spectrum Pharmaceuticals, Janssen, and Novartis. U.R.P. received research funding from Bayer and Incyte; and received consultancy fees from Jazz. A.A. received research funding from Miltenyi. The remaining authors declare no competing financial interests.
Correspondence: Rebecca L. Olin, Helen Diller Family Comprehensive Cancer Center, University of California, 400 Parnassus Ave, Box 0324, San Francisco CA 94143; e-mail: rebecca.olin@ucsf.edu.
References
Author notes
The full-text version of this article contains a data supplement.