Abstract
The impact of pharmacologic prophylaxis for venous thromboembolism in patients undergoing neurosurgical intervention remains uncertain. We reviewed the efficacy and safety of pharmacologic compared with nonpharmacologic thromboprophylaxis in neurosurgical patients. Three databases were searched through April 2018, including those for randomized controlled trials (RCTs) and for nonrandomized controlled studies (NRSs). Independent reviewers assessed the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Seven RCTs and 3 NRSs proved eligible. No studies reported on symptomatic proximal and distal deep vein thrombosis (DVT). Two RCTs reported on screening-detected proximal and distal DVTs. We used the findings of these 2 RCTs as the closest surrogate outcomes to inform the proximal and distal DVT outcomes. These 2 RCTs suggest that pharmacologic thromboprophylaxis may decrease the risk of developing asymptomatic proximal DVT (relative risk [RR], 0.50; 95% confidence interval [CI], 0.30-0.84; low certainty). Findings were uncertain for mortality (RR, 1.27; 95% CI, 0.57-2.86; low certainty), symptomatic pulmonary embolism (PE) (RR, 0.84; 95% CI, 0.03-27.42; very low certainty), asymptomatic distal DVT (RR, 0.54; 95% CI, 0.27-1.08; very low certainty), and reoperation (RR, 0.43; 95% CI, 0.06-2.84; very low certainty) outcomes. NRSs also reported uncertain findings for whether pharmacologic prophylaxis affects mortality (RR, 0.72; 95% CI, 0.46-1.13; low certainty) and PE (RR, 0.18; 95% CI, 0.01-3.76). For risk of bleeding, findings were uncertain in both RCTs (RR, 1.57; 95% CI, 0.70-3.50; low certainty) and NRSs (RR, 1.45; 95% CI, 0.30-7.12; very low certainty). In patients undergoing neurosurgical procedures, low certainty of evidence suggests that pharmacologic thromboprophylaxis confers benefit for preventing asymptomatic (screening-detected) proximal DVT with very low certainty regarding its impact on patient-important outcomes.
Introduction
Venous thromboembolism (VTE) is a common life-threatening complication in patients undergoing neurosurgical procedures. The presence of VTE significantly complicates the delivery of care because of bleeding risks associated with treatment. The risk of VTE in this population varies considerably depending on patient comorbidities, the neurosurgical procedure performed, method of VTE diagnosis (screening-detected vs symptomatic VTE), and use of different thromboprophylaxis regimens. Although there are no formal VTE risk stratification models for neurosurgical patients, several VTE risk factors have been identified, including active cancer, advanced age, longer duration of surgery, delayed ambulation, inherited thrombophilia, hospital length of stay, and paresis.1 On a biochemical level, neurosurgical patients have unique risk factors that contribute to VTE because of the release of prothrombotic proteins such as fibrinopeptide A, fibrinogen, factor VIII, thromboplastin from brain tumors, and endothelial injury from traumatic spinal and brain injuries as well as manipulation of central nervous system tissue during surgery.2
The incidence of either screening-detected or asymptomatic deep venous thrombosis (DVT) without prophylaxis in the neurosurgical population has been reported to be as high as 34% in some studies,3 with a mean of 16% across earlier studies.4,5 The rate of postoperative pulmonary embolism (PE) in neurosurgical patients is estimated to be 0% to 4%, with case fatality ranging from 9% to 50%.6
Options for thromboprophylaxis include pharmacologic (eg, low-dose unfractionated heparin [UFH], low molecular weight heparin [LMWH]) or mechanical measures (eg, intermittent pneumatic compression devices, graduated compression stockings [CSs], or inferior vena cava filters). Randomized controlled trials (RCTs) have evaluated multiple approaches to VTE thromboprophylaxis in neurosurgical patients. Although mechanical prophylaxis does not increase the risk of bleeding, its effectiveness in reducing VTE is limited.4 In contrast, pharmacologic thromboprophylaxis may be more likely to reduce VTE, but it increases the risk of bleeding, which is of particular concern with intracranial and intraspinal bleeding in neurosurgical procedures.5
The ideal initial timing and duration of thromboprophylaxis is a topic of debate among neurosurgeons. Previous systematic reviews that evaluated neurosurgical VTE prophylaxis have led to conflicting conclusions.4,5,7-10 There is still considerable uncertainty regarding the impact of pharmacologic thromboprophylaxis in neurosurgical patients on both benefit and harms outcomes.
Methods
This systematic review informed the recommendations of the American Society of Hematology (ASH) clinical practice guidelines on VTE, specifically for pharmacologic thromboprophylaxis in neurosurgical patients.11 We analyzed comparative studies that examined the effects of either UFH or LMWH or low doses of warfarin vs nonpharmacologic thromboprophylaxis on patient-important outcomes in adults who underwent neurosurgical procedures.
Inclusion and exclusion criteria
We included RCTs and nonrandomized controlled studies (NRSs), specifically cohort and case-control studies. We excluded conference abstract reports. Eligible studies included adult patients undergoing neurosurgical procedures. We excluded studies in which all patients required a neurosurgical intervention after acute trauma. We included only studies that reported either UFH or LMWH or low doses of warfarin compared with nonpharmacologic thromboprophylaxis such as placebo, no prophylaxis, or mechanical interventions for VTE prophylaxis (ie, CSs or intermittent pneumatic compression devices).
The outcomes were determined by the ASH guideline panel members after a detailed guideline development process.11 The following outcomes were deemed critical to the decision of whether or not to use pharmacologic anticoagulation for VTE prophylaxis: mortality, symptomatic PE, symptomatic proximal DVT, symptomatic distal DVT, major bleeding, and reoperation. A summary of definitions for PE, DVT, and major bleeding outcomes is provided in the supplemental Data.
Search methods
We searched MEDLINE, EMBASE, and The Cochrane Central Register of Controlled Trials (CENTRAL) from inception to April 2018 without restrictions on the publication’s language. Search strategies for RCTs and NRSs are provided in the supplemental Data. We checked the reference lists of reviewed articles and contacted clinical experts for additional references.
Study selection and data extraction
Ten independent evaluators, working in pairs (L.E.C-L., S.R., F.P., M.B., K.E.O., M.V., A.M.B., S.B., H.B., and A.A.) screened the titles and abstracts obtained through the electronic searches. If a study was deemed potentially relevant, we obtained the full text, and the same 10 evaluators, again working in pairs, made final decisions on eligibility. If reviewers could not resolve disagreement through discussion, a third reviewer adjudicated the decision (J.J.Y.-N.). We contacted the authors if additional information or clarification was needed.
Eight independent evaluators (L.E.C.-L., S.R., F.P., M.B., M.V., A.M.B., H.B., and A.A.), working in pairs, read all reports of eligible studies in detail and summarized the pertinent details in a standard data extraction sheet (type of study, methodology, characteristics of participants, results, outcome measurements, and an evaluation of the risk of bias or study limitations). Reviewers discussed any disagreements and consulted a third reviewer (J.J.Y.-N.) if necessary.
Assessment of risk of bias in included studies
Two review authors (J.J.Y.-N. and M.B.) independently assessed risk of bias for each RCT and NRS using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Intervention12 , and version 1.0 of the Cochrane assessment tool: Risk of Bias in Non-Randomized Studies-of Intervention (ROBINS-I).13
Data analysis
We calculated the relative risk (RR) and 95% confidence intervals (95% CIs) for dichotomous outcomes. For NRSs, we calculated the measure of the relative effect based on information reported by authors. We selected baseline risks (control group risk) to calculate absolute effects measures from a systematic review that estimated the incidence of thromboembolic disease in postoperative patients who had spinal surgery.8 We estimated absolute effect measures to facilitate the decision-making process for the effect of pharmacologic thromboprophylaxis in individual neurosurgical patients.14 We contacted authors if details about study design or descriptive statistics for outcomes were not presented in the original articles. We did not impute data.
We assessed inconsistency between studies by visual inspection of forest plots, in particular extent of overlap of CIs, the Q statistic (with P ≤ .05 as a suggestion of important statistical heterogeneity), and the I2 value. We planned to explore reasons for inconsistency by prespecified differences in type of intervention. We planned, if 10 or more studies were available for a particular outcome, to create a funnel plot to assess publication bias by visual inspection.
We assessed the treatment effect through mean difference for continuous outcomes and RR for dichotomous outcomes for individual studies, and we used random-effects models to pool study data. We presented all measures with 95% CIs. We carried out all statistical analyses using Review Manager 5.3 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). We considered 1 main group for subgroup analysis based on the type of neurosurgical intervention (craniotomy vs spinal surgery vs craniotomy and spinal surgery). We planned analyses to determine the effect of including or excluding the studies with high risk of bias on estimates of treatment effect.
We used the GRADE methodology to rate the certainty of the body of evidence for each outcome as high, moderate, low, or very low.15 The assessment included judgments addressing risk of bias, imprecision, inconsistency, indirectness, and publication bias. We also rated the certainty of the body of evidence using a GRADE approach for observational studies.16 We created evidence profile and summary of findings tables for each population using GRADE’s electronic tool GRADEpro GDT (www.gradepro.org). To assess the usefulness of including NRSs, we applied the GRADE guidance using ROBINS-I as a part of GRADE’s certainty rating process.17 A senior methodologist (H.J.S.) checked all GRADE tables and ratings of the certainty of the body of evidence.
Results
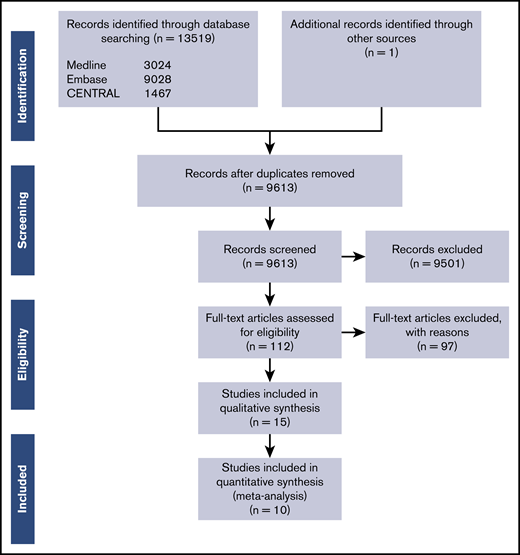
The MEDLINE, EMBASE, and CENTRAL database search yielded 10 538 unique records after duplicates were removed. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram, and the PRISMA checklist is included in the supplemental Data.18 We reviewed the full text of 112 publications in detail.
Overall, 7 RCTs19-25 and 3 NRSs (retrospective cohort studies)26-28 met our inclusion criteria. Five RCTs reported the effect of pharmacologic thromboprophylaxis vs nonpharmacologic intervention on the development of mortality19-21,23,24 ; 3 RCTs reported the development of symptomatic PEs19,22,25 ; 7 RCTs reported on the risk of major bleeding19-25 ; and 2 RCTs reported on reoperation.20,23 No RCTs reported on symptomatic proximal and distal DVT. Therefore, we used data from 2 RCTs that reported on screening-detected proximal19,24 and distal19 DVTs as surrogate outcomes for symptomatic proximal and distal DVTs for the purposes of this analysis. The 3 NRSs reported information about mortality,26,28 PE,26,28 and major bleeding.26-28
One RCT3 reported the incidence of asymptomatic DVT, and another RCT29 reported the incidence of asymptomatic and symptomatic DVT. These 2 RCTs reported the outcomes as a composite outcome, regardless of whether the DVT was proximal or distal. One RCT30 reported findings of asymptomatic proximal and distal DVT, but the authors did not describe how many DVTs, proximal or distal, were identified in the intervention or placebo arm. Because these 3 RCTs did not report findings for the outcomes to be included in the guideline, and because we could not use the information for the surrogate outcomes, we did not incorporate this body of evidence in our meta-analysis.
One NRS31 reported no qualitative difference in the rate of hemorrhagic complications and PE between neurosurgical patients who received pharmacologic thromboprophylaxis with UFH compared with control patients who did not receive pharmacologic thromboprophylaxis. Because this information was not reported quantitatively, we could not combine these data in our meta-analysis.
Another NRS32 reported PE in symptomatic and asymptomatic patients with subarachnoid hemorrhage and ventriculostomies and the number of DVTs for both proximal and distal DVT together. Outcomes were measured through helical computed tomography angiogram by protocol and venous ultrasound, respectively. In the group receiving pharmacologic thromboprophylaxis, 6 of 138 patients developed PE compared with none of 53 patients in the nonpharmacologic thromboprophylaxis group. Cases of DVT were less frequent in patients who received pharmacologic thromboprophylaxis (28 of 188) compared with those who did not (4 of 53). These data were not combined in the meta-analysis because the authors explored DVT in neurosurgical patients regardless of whether it was proximal or distal, and PE was investigated by protocol (screening-detected). No additional information from the included studies was required; therefore, we did not contact the authors. Table 1 presents the characteristics of the 10 eligible studies according to the study design. The studies that were excluded and the reasons for their exclusion are detailed in the supplemental Data.
Characteristics of studies included in the review
| Study . | N . | Patient description . | Type of neurosurgery . | Pharmacologic thromboprophylaxis . | Co-interventions . | Route, dose, and time of pharmacologic thromboprophylaxis . | Time to follow-up . | DVT diagnostic technique . | Outcomes assessed . |
|---|---|---|---|---|---|---|---|---|---|
| RCT | |||||||||
| Agnelli et al20 | 307* | Age 18 y or older | Elective cranial or spinal surgery | LMWH (enoxaparin) | Thigh-length TED CS in all patients | Subcutaneous injections, 40 mg per day for 8 ± 1 d | 60 d | Ultrasonography and venography in case of positive ultrasonography | M, PE, DVT†, MB |
| Cerrato et al3 | 100* | Age 40 y or older | Elective neurosurgical procedures | UFH | None reported | Subcutaneous injections, 5000 IU every 8 h for at least 7 d | Not reported | 125I-labeled fibrinogen | DVT‡ |
| Constantini et al21 | 103* | Age 40 y or older | Craniotomy for brain tumor removal | UFH | None reported | Subcutaneous injections, 5000 IU every 12 h for 7 d or until full ambulation | Not reported | Not reported§ | M, DVT||, MB, RO |
| Dickinson et al22 ¶ | 66* | Age 18 y or older with a diagnosis of an intracranial neoplasm | Craniotomy or stereotactic biopsy | LMWH (enoxaparin) | Thigh-high TED CS in all patients only before random assignment | Subcutaneous injections, 30 mg every 12 h until hospital discharge from neurosurgery service | Not reported | Duplex ultrasonography | M, DVT#, MB |
| Gruber et al23 | 50* | Adults undergoing lumbar disc operations | Herniated lumbar disc operations | Heparin-DHE | None reported | Subcutaneous injections, 2500 IU; 0.5 mg every 12 h for at least 7 d or until hospital discharge | Not reported | Phlebogram, plethysmography, Doppler ultrasound, or an 125I fibrinogen test | PE, DVT||, MB |
| Halim et al30 | 74* | Adult with ASCI | Anterior and/or posterior surgical approach | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day for 8 wk | 2 weeks | Color Doppler venous ultrasonography | DVT** |
| Hamidi and Riazi24 | 89* | Age 18 to 75 y | Elective instrumental spinal surgery | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day within 12 h before surgery | 2 wk and 8 mo after surgery | Compression Doppler ultrasonography | M, DVT**, MB, RO |
| Nurmohamed et al25 | 485* | Age 18 y or older | Craniotomy or spinal column surgery for a tumor or injury | LMWH (nadroparin calcium) | TED CS in all patients | Subcutaneous injections, 7500 IU anti-factor Xa per day for 10 d or until hospital discharge | 56 d after surgery | B-mode compression ultrasonography and venogram in case of positive ultrasonography | M, proximal DVT††, MB |
| Rokito et al26 ¶ | 110* | Age 18 y or older | Major reconstructive spinal surgery | Warfarin (coumadin) | Thigh-high TED CS in all patients | 10 mg before surgery; doses were adjusted accordingly to prothrombin time | 1 y | Duplex ultrasonography and venography in case of positive ultrasonography | PE, DVT**, MB |
| Sonaglia et al31 | 157* | Age 18 y or older | Neurosurgery for brain or spinal tumor | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day for not less than 7 d | Not reported | Venography | Proximal and distal DVT# |
| NRS | |||||||||
| Bauman et al27 | 254 | Had movement disorders (Parkinson disease, essential tremor, dystonia) | Deep brain stimulation surgery | UFH | CS in all patients and pneumatic, compression boots postoperatively | Subcutaneous injections, 50 mg before surgery and 50 mg every 12 h after surgery. Duration of pharmacologic thromboprophylaxis not reported | Not reported | Doppler ultrasonography | M, DVT||, PE, MB |
| Dermody et al28 | 174 | Neurosurgical patients, who underwent screening with once-per-week VDUS of the bilateral lower extremities | Endovascular coiling or clipping, craniotomy, stereotactic, biopsy, spine surgery, trans sphenoidal surgery | UFH or LMWH (enoxaparin) | Mechanical prophylaxis | UFH: 5000 IU, 2 or 3 times per day; enoxaparin, not reported | 6 mo | Venous duplex ultrasound | MB, DVT‡‡ |
| Hacker et al29 | 522 | Neurosurgical postoperative patients admitted to SICU | Cervical spinal cord, decompression, cervical laminectomy, craniotomy/craniectomy, decompressive laminectomy, nasal sinuses surgery | UFH | Lower extremity compression boots | Subcutaneous injections, 5000 IU every 8 h until hospital discharge | Until death or until discharged from the hospital | Ultrasound evaluation | M, DVTa, PE, MB |
| Khaldi et al32 | 2638 | Underwent a neurosurgical procedure | Multilevel lumbar surgeries, major spine surgery, head surgery | UFH | Mechanical DVT prophylaxis | Subcutaneous injections, 5000 IU every 12 h | Not reported | Duplex ultrasonography | PE, DVTa, MBb |
| Zachariah et al33 | 241 | Had subarachnoid hemorrhage and external ventricular drain | None reported | UFH, LMWH, or warfarin | Sequential compression devices | Not reported | Not reported | Venous ultrasound | DVT‡‡ |
| Study . | N . | Patient description . | Type of neurosurgery . | Pharmacologic thromboprophylaxis . | Co-interventions . | Route, dose, and time of pharmacologic thromboprophylaxis . | Time to follow-up . | DVT diagnostic technique . | Outcomes assessed . |
|---|---|---|---|---|---|---|---|---|---|
| RCT | |||||||||
| Agnelli et al20 | 307* | Age 18 y or older | Elective cranial or spinal surgery | LMWH (enoxaparin) | Thigh-length TED CS in all patients | Subcutaneous injections, 40 mg per day for 8 ± 1 d | 60 d | Ultrasonography and venography in case of positive ultrasonography | M, PE, DVT†, MB |
| Cerrato et al3 | 100* | Age 40 y or older | Elective neurosurgical procedures | UFH | None reported | Subcutaneous injections, 5000 IU every 8 h for at least 7 d | Not reported | 125I-labeled fibrinogen | DVT‡ |
| Constantini et al21 | 103* | Age 40 y or older | Craniotomy for brain tumor removal | UFH | None reported | Subcutaneous injections, 5000 IU every 12 h for 7 d or until full ambulation | Not reported | Not reported§ | M, DVT||, MB, RO |
| Dickinson et al22 ¶ | 66* | Age 18 y or older with a diagnosis of an intracranial neoplasm | Craniotomy or stereotactic biopsy | LMWH (enoxaparin) | Thigh-high TED CS in all patients only before random assignment | Subcutaneous injections, 30 mg every 12 h until hospital discharge from neurosurgery service | Not reported | Duplex ultrasonography | M, DVT#, MB |
| Gruber et al23 | 50* | Adults undergoing lumbar disc operations | Herniated lumbar disc operations | Heparin-DHE | None reported | Subcutaneous injections, 2500 IU; 0.5 mg every 12 h for at least 7 d or until hospital discharge | Not reported | Phlebogram, plethysmography, Doppler ultrasound, or an 125I fibrinogen test | PE, DVT||, MB |
| Halim et al30 | 74* | Adult with ASCI | Anterior and/or posterior surgical approach | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day for 8 wk | 2 weeks | Color Doppler venous ultrasonography | DVT** |
| Hamidi and Riazi24 | 89* | Age 18 to 75 y | Elective instrumental spinal surgery | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day within 12 h before surgery | 2 wk and 8 mo after surgery | Compression Doppler ultrasonography | M, DVT**, MB, RO |
| Nurmohamed et al25 | 485* | Age 18 y or older | Craniotomy or spinal column surgery for a tumor or injury | LMWH (nadroparin calcium) | TED CS in all patients | Subcutaneous injections, 7500 IU anti-factor Xa per day for 10 d or until hospital discharge | 56 d after surgery | B-mode compression ultrasonography and venogram in case of positive ultrasonography | M, proximal DVT††, MB |
| Rokito et al26 ¶ | 110* | Age 18 y or older | Major reconstructive spinal surgery | Warfarin (coumadin) | Thigh-high TED CS in all patients | 10 mg before surgery; doses were adjusted accordingly to prothrombin time | 1 y | Duplex ultrasonography and venography in case of positive ultrasonography | PE, DVT**, MB |
| Sonaglia et al31 | 157* | Age 18 y or older | Neurosurgery for brain or spinal tumor | LMWH (enoxaparin) | CS in all patients | Subcutaneous injections, 40 mg per day for not less than 7 d | Not reported | Venography | Proximal and distal DVT# |
| NRS | |||||||||
| Bauman et al27 | 254 | Had movement disorders (Parkinson disease, essential tremor, dystonia) | Deep brain stimulation surgery | UFH | CS in all patients and pneumatic, compression boots postoperatively | Subcutaneous injections, 50 mg before surgery and 50 mg every 12 h after surgery. Duration of pharmacologic thromboprophylaxis not reported | Not reported | Doppler ultrasonography | M, DVT||, PE, MB |
| Dermody et al28 | 174 | Neurosurgical patients, who underwent screening with once-per-week VDUS of the bilateral lower extremities | Endovascular coiling or clipping, craniotomy, stereotactic, biopsy, spine surgery, trans sphenoidal surgery | UFH or LMWH (enoxaparin) | Mechanical prophylaxis | UFH: 5000 IU, 2 or 3 times per day; enoxaparin, not reported | 6 mo | Venous duplex ultrasound | MB, DVT‡‡ |
| Hacker et al29 | 522 | Neurosurgical postoperative patients admitted to SICU | Cervical spinal cord, decompression, cervical laminectomy, craniotomy/craniectomy, decompressive laminectomy, nasal sinuses surgery | UFH | Lower extremity compression boots | Subcutaneous injections, 5000 IU every 8 h until hospital discharge | Until death or until discharged from the hospital | Ultrasound evaluation | M, DVTa, PE, MB |
| Khaldi et al32 | 2638 | Underwent a neurosurgical procedure | Multilevel lumbar surgeries, major spine surgery, head surgery | UFH | Mechanical DVT prophylaxis | Subcutaneous injections, 5000 IU every 12 h | Not reported | Duplex ultrasonography | PE, DVTa, MBb |
| Zachariah et al33 | 241 | Had subarachnoid hemorrhage and external ventricular drain | None reported | UFH, LMWH, or warfarin | Sequential compression devices | Not reported | Not reported | Venous ultrasound | DVT‡‡ |
ASCI, acute spinal cord injury; DHE, dihydroergotamine; M, mortality; MB, major bleeding; RO, reoperation; SICU, surgical intensive care unit; TED, thrombosis embolic deterrent; VDUS, venous duplex ultrasound.
Number of patients randomly assigned.
Symptomatic proximal DVT, screening-detected proximal DVT, screening-detected distal DVT, symptomatic proximal and distal DVT (any symptomatic DVT).
Asymptomatic DVT, but authors did not make distinction in proximal or distal DVT.
Authors reported DVT only by clinical evidence.
Symptomatic proximal and distal DVT (any symptomatic DVT).
Three-arm RCT.
Screening-detected proximal and distal DVT (any asymptomatic DVT).
Symptomatic proximal and distal DVT (any symptomatic DVT), screening-detected proximal and distal DVT (any asymptomatic DVT).
Screening-detected proximal DVT, symptomatic proximal and distal DVT (any symptomatic DVT), screening-detected proximal and distal DVT (any asymptomatic DVT).
No distinction between proximal, distal, symptomatic, or asymptomatic DVT.
DVT events were not reported.
Findings were reported qualitatively.
Risk of bias in studies included in the analyses
Overall risk of bias was deemed low to very low in individual RCTs (see supplemental Data). For RCTs, the most significant concerns were related to incomplete outcome data and lack of blinding of participants and personnel. Regarding the NRSs, we evaluated risk of bias in 4 outcomes that were reported in 5 studies. All 4 outcomes presented very serious risk of bias. We found inappropriate methods to control for all important confounding domains across all outcomes in all NRSs.
Effect of interventions
Table 2 summarizes the findings for all research questions. The supplemental Data contains the full evidence profile with more detailed explanations along with forest plots of RCTs and NRSs.
Summary of findings
| Outcome . | No. of participants12 (follow-up) . | Certainty of the evidence (GRADE) . | RR . | 95% CI . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with nonpharmacologic prophylaxis . | Risk difference with pharmacologic prophylaxis . | |||||
| Mortality, RCTs | 1029 (5 RCTs) | ⨁⨁◯◯ Low* | 1.27 | 0.57 to 2.86 | 35 per 1000 | 9 more per 1000 (15 fewer to 65 more) |
| Mortality, NRSs | 674 (2 observational studies) | ⨁◯◯◯ Very low†‡ | 0.72 | 0.46 to 1.13 | 115 per 1000 | 32 fewer per 1000 (62 fewer to 15 more) |
| PE, as described by the moderate marker state; RCTs, symptomatic PE | 434 (3 RCTs) | ⨁◯◯◯ Very low§|| | 0.84 | 0.03 to 27.42 | Study population | |
| 14 per 1000 | 2 fewer per 1000 (13 fewer to 359 more) | |||||
| Low | ||||||
| 2 per 1000¶ | 0 fewer per 1000 (2 fewer to 53 more) | |||||
| PE, as described by the moderate marker state; NRS, symptomatic PE | 776 (2 observational studies) | ⨁◯◯◯ Very low†||# | 0.18 | 0.01 to 3.76 | Study population | |
| 5 per 1000 | 4 fewer per 1000 (5 fewer to 13 more) | |||||
| Low | ||||||
| 2 per 1000¶ | 2 fewer per 1000 (2 fewer to 6 more) | |||||
| Symptomatic DVT as inferred from screening-detected proximal DVT, as described by the moderate marker state; RCTs, screening-detected proximal DVT | 744 (2 RCTs) | ⨁⨁◯◯ Low**†† | 0.50‡‡ | 0.30 to 0.84 | Study population | |
| 113 per 1000 | 56 fewer per 1000 (79 fewer to 18 fewer)‡‡ | |||||
| Low | ||||||
| 3 per 1000a | 2 fewer per 1000 (2 fewer to 1 fewer) | |||||
| Symptomatic DVT as inferred from screening-detected distal DVT, as described by the severe marker state, screening-detected distal DVT | 259 (1 RCT) | ⨁◯◯◯ Very low††b | 0.54‡‡ | 0.27 to 1.08 | Study population | |
| 194 per 1000 | 68 fewer per 1000 (141 fewer to 16 more)‡‡ | |||||
| Low | ||||||
| 1 per 1000a | 0 fewer per 1000 (0 fewer to 0 fewer) | |||||
| Major bleeding, RCTs | 1156 (7 RCTs) | ⨁⨁◯◯ Lowc | 1.57 | 0.70 to 3.50 | 17 per 1000 | 10 more per 1000 (5 fewer to 43 more) |
| Major bleeding, NRSs | 930 (3 observational studies) | ⨁◯◯◯ Very lowd | 1.45 | 0.30 to 7.12 | 7 per 1000 | 3 more per 1000 (5 fewer to 40 more) |
| Reoperation, RCTs | 192 (2 RCTs) | ⨁◯◯◯ Very lowe | 0.43 | 0.06 to 2.84 | 31 per 1000 | 18 fewer per 1000 (29 fewer to 57 more) |
| Outcome . | No. of participants12 (follow-up) . | Certainty of the evidence (GRADE) . | RR . | 95% CI . | Anticipated absolute effects . | |
|---|---|---|---|---|---|---|
| Risk with nonpharmacologic prophylaxis . | Risk difference with pharmacologic prophylaxis . | |||||
| Mortality, RCTs | 1029 (5 RCTs) | ⨁⨁◯◯ Low* | 1.27 | 0.57 to 2.86 | 35 per 1000 | 9 more per 1000 (15 fewer to 65 more) |
| Mortality, NRSs | 674 (2 observational studies) | ⨁◯◯◯ Very low†‡ | 0.72 | 0.46 to 1.13 | 115 per 1000 | 32 fewer per 1000 (62 fewer to 15 more) |
| PE, as described by the moderate marker state; RCTs, symptomatic PE | 434 (3 RCTs) | ⨁◯◯◯ Very low§|| | 0.84 | 0.03 to 27.42 | Study population | |
| 14 per 1000 | 2 fewer per 1000 (13 fewer to 359 more) | |||||
| Low | ||||||
| 2 per 1000¶ | 0 fewer per 1000 (2 fewer to 53 more) | |||||
| PE, as described by the moderate marker state; NRS, symptomatic PE | 776 (2 observational studies) | ⨁◯◯◯ Very low†||# | 0.18 | 0.01 to 3.76 | Study population | |
| 5 per 1000 | 4 fewer per 1000 (5 fewer to 13 more) | |||||
| Low | ||||||
| 2 per 1000¶ | 2 fewer per 1000 (2 fewer to 6 more) | |||||
| Symptomatic DVT as inferred from screening-detected proximal DVT, as described by the moderate marker state; RCTs, screening-detected proximal DVT | 744 (2 RCTs) | ⨁⨁◯◯ Low**†† | 0.50‡‡ | 0.30 to 0.84 | Study population | |
| 113 per 1000 | 56 fewer per 1000 (79 fewer to 18 fewer)‡‡ | |||||
| Low | ||||||
| 3 per 1000a | 2 fewer per 1000 (2 fewer to 1 fewer) | |||||
| Symptomatic DVT as inferred from screening-detected distal DVT, as described by the severe marker state, screening-detected distal DVT | 259 (1 RCT) | ⨁◯◯◯ Very low††b | 0.54‡‡ | 0.27 to 1.08 | Study population | |
| 194 per 1000 | 68 fewer per 1000 (141 fewer to 16 more)‡‡ | |||||
| Low | ||||||
| 1 per 1000a | 0 fewer per 1000 (0 fewer to 0 fewer) | |||||
| Major bleeding, RCTs | 1156 (7 RCTs) | ⨁⨁◯◯ Lowc | 1.57 | 0.70 to 3.50 | 17 per 1000 | 10 more per 1000 (5 fewer to 43 more) |
| Major bleeding, NRSs | 930 (3 observational studies) | ⨁◯◯◯ Very lowd | 1.45 | 0.30 to 7.12 | 7 per 1000 | 3 more per 1000 (5 fewer to 40 more) |
| Reoperation, RCTs | 192 (2 RCTs) | ⨁◯◯◯ Very lowe | 0.43 | 0.06 to 2.84 | 31 per 1000 | 18 fewer per 1000 (29 fewer to 57 more) |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence: high certainty, we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty, we are moderately confident in the effect estimate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); low certainty, our confidence in the effect estimate is limited (the true effect may be substantially different from the estimate of the effect); very low certainty, we have very little confidence in the effect estimate (the true effect is likely to be substantially different from the estimate of effect).
Studies that carried large weight for the overall effect estimate are rated as unclear risk of bias because of lack of information about the sequence generation process in 3 of 5 studies and lack of concealment in 2 of 5 studies. Very serious imprecision. Wide CI with only 45 events in total.
Serious risk of bias. Studies did not analyze findings adjusting for confounding factors.
Serious inconsistency. Unexplained inconsistency, with point estimates widely different and CIs not overlapping (P value χ2 = 0.12; I2 = 43%). Serious imprecision. 95% CI is consistent with the possibility for important benefit and large harm exceeding a minimal important difference, including only 66 events in total.
Studies that carried large weight for the overall effect estimate were rated as unclear risk of bias because of lack of blinding of outcome assessment in 2 of 4 studies. Serious inconsistency. Moderate heterogeneity between studies. I2 = 64% (P = .10).
Very serious imprecision. Wide CI with only 5 events in total.
A systematic review of 25 NRSs published by Glotzbecker8 on elective spinal surgeries (cervical spine, lumbar laminectomy, lumbar spinal fusion, spinal trauma, spinal tumors) reported an incidence of symptomatic PEs of 0.2% (34 of 15 204).
Serious inconsistency. Moderate inconsistency, with point estimates widely different and CIs not overlapping (P value χ2 = 0.18; I2 = 41%).
Studies that carried large weight for the overall effect estimate were rated as high risk of bias because of lack of information about the incomplete outcome data.
Serious indirectness. Patients were identified through screening ultrasound. None of the patients developed symptomatic VTE before venography.
If any DVT detected by screening was considered a surrogate, then 6 RCTs and 2 NRSs measured it; there was a total of 137 events (53 in the prophylaxis group and 84 in the no prophylaxis group) among 927 patients for the RCTs, and 72 events (32 in the prophylaxis group and 40 in the no prophylaxis group) among 415 patients for the NRSs. For the RCTs, the RR would be 0.65 (95% CI, 0.47-0.89), and the risk difference would be 64 fewer per 1000 (from 21 fewer to 96 fewer) using the control group event rate of 17.7%, or 1 fewer per 1000 (from 1 fewer to 2 fewer) based on the baseline risk of 0.32%. For the NRSs, the RR would be 0.48 (95% CI, 0.29-0.81), and the risk difference would be 96 fewer per 1000 (from 35 fewer to 131 fewer) using the control group event rate of 18.4%, or 2 fewer per 1000 (from 1 fewer to 2 fewer) based on the baseline risk of 0.32%.
Rates of proximal and distal symptomatic DVT in patients receiving no prophylaxis and undergoing elective spinal surgeries (cervical spine, lumbar laminectomy, lumbar spinal fusion, spinal trauma, spinal tumors) were reported in Glotzbecker8 as 1.6% (46 of 2956) for DVTs and 0.2% (34 of 15 204) for PEs. We applied the assumption that approximately 20% of symptomatic DVTs are proximal, 80% are distal, and 5% of the distal DVTs are severe.
One study that carried large weight for the overall effect estimate was rated as high risk of bias because of lack of incomplete outcome data. Very serious imprecision. The 95% CI is consistent with the possibility for important benefit and large harm exceeding a minimal important difference, including only 40 events in total.
Studies that carried large weight for the overall effect estimate were rated as unclear risk of bias because of lack of random sequence generation and lack of concealment in 4 of 7 studies. Serious imprecision. The 95% CI is consistent with the possibility for important benefit and large harm exceeding a minimal important difference, including only 24 events in total.
Serious risk of bias. Studies assessed comorbidities associated with high risk of DVT such as obesity, heart failure, cancer, history of DVT, pregnancy, tobacco use, and history of hypercoagulable disorder. However, authors did not adjust for confounding factors. Very serious imprecision. The 95% CI is consistent with the possibility for important benefit and large harm exceeding a minimal important difference, including only 6 events in total.
Studies that carried large weight for the overall effect estimate rated as unclear risk of bias because of lack of random sequence generation and allocation concealment in 1 of 2 studies and lack of blinding of outcome assessment in 1 of 2 studies. Very serious imprecision. Wide CI with only 4 events in total.
Mortality.
Five RCTs10-21,23,24 reported the effect of pharmacologic thromboprophylaxis on mortality. No effect of pharmacologic prophylaxis was found on mortality (RR, 1.27; 95% CI, 0.57-2.86). We also found 2 NRSs26,28 that reported mortality in patients with movement disorders who underwent deep brain stimulation surgery and postoperative patients admitted to the surgical intensive care unit. In 1 NRS,26 investigators observed no deaths in patients who received UFH or in patients who did not receive pharmacologic thromboprophylaxis. In the other NRS,28 pharmacologic prophylaxis with UFH did not reduce mortality in neurosurgical patients (RR, 0.72; 95% CI, 0.46-1.13). The overall certainty of the body of evidence ranged from low to very low because of the risk of bias, inconsistency, and imprecision.
Symptomatic PE.
Three RCTs19,22,25 reported symptomatic PEs. The evidence was essentially uninformative with respect to relative effects on PEs with extremely wide CIs (RR, 0.84; 95% CI, 0.03-27.42). Two NRS reported symptomatic PEs. One NRS26 reported the development of symptomatic PEs in 2 of 121 neurosurgical patients who did not receive UFH, and none of the patients who received UFH developed the outcome being evaluated. This finding was also uninformative with respect to relative effects (RR, 0.18; 95% CI, 0.01-3.76). In the other NRS,28 no cases of PE were observed in 522 neurosurgical patients. In terms of absolute effect, the difference between groups was 4 fewer events of PE per 1000 patients with CIs from 5 in 1000 fewer to 13 in 1000 more. The overall certainty of the body of evidence was very low because of the risk of bias, inconsistency, and imprecision.
Symptomatic proximal DVT.
No study reported on symptomatic proximal DVT. Two RCTs19,24 reported on screening-detected asymptomatic proximal DVT, which was deemed to be the closest surrogate outcome for consideration. Pharmacologic thromboprophylaxis was associated with a reduction of proximal DVT (RR, 0.50; 95% CI, 0.30-0.84). No information about symptomatic proximal DVT was reported in NRSs. The certainty of the body of evidence was low because of the risk of bias and indirectness, because the outcome important to patients was symptomatic proximal DVT. In addition, we explored data for the following 2 outcomes.
Symptomatic distal DVT.
No study reported on symptomatic distal DVT. One RCT19 reported on screening-detected distal DVT when pharmacologic thromboprophylaxis was administered in patients who underwent elective cranial or spinal surgery. We used data for this outcome as a surrogate for symptomatic distal DVT. No convincing effect of pharmacologic thromboprophylaxis was found for distal DVT (RR, 0.60; 95% CI, 0.33-1.08). No NRSs addressed this outcome. The overall certainty of the evidence was very low because of the risk of bias, indirectness (because the outcome of interest was symptomatic distal DVT), and imprecision.
Any screening-detected DVT.
Data from the 6 RCTs21,23-25,29,30 that reported screening-detected proximal or distal DVT together were pooled separately. Pharmacologic thromboprophylaxis in neurosurgical patients reduced the risk of developing proximal or distal DVT (RR, 0.67; 95% CI, 0.49-0.91). Two NRSs27,32 reported a benefit of pharmacologic thromboprophylaxis in reducing any screening-detected proximal or distal DVT events (RR, 0.48; 95% CI, 0.29-0.77). The overall certainty of the body of evidence was very low because of the risk of bias and imprecision.
Any symptomatic DVT.
Seven RCTs19,20,22-25,29 reported findings pooling proximal and distal symptomatic DVT events. There was a risk reduction in developing symptomatic DVT with pharmacologic thromboprophylaxis (RR, 0.55; 95% CI, 0.36-0.85) in neurosurgical patients. Three NRSs reported on symptomatic DVT. In 1 NRS,26 pharmacologic prophylaxis did not reduce the incidence of symptomatic DVT (RR, 0.30; 95% CI, 0.01-7.38) with UFH. The other 2 NRSs28,31 did not provide information on symptomatic DVT events. The overall certainty of the body of evidence was very low because of the risk of bias and imprecision.
Major bleeding.
Ten studies, 7 RCTs19-25 and 3 NRSs,26-28 reported on major bleeding. In the 7 RCTs, pharmacologic thromboprophylaxis in patients who underwent neurosurgical procedures did not show an increase in major bleeding compared with nonpharmacologic thromboprophylaxis (RR, 1.57; 95% CI, 0.70-3.50). In 226,27 of 3 NRSs, no cases of major bleeding were reported in neurosurgical patients who received pharmacologic thromboprophylaxis or in those who did not. One NRS28 reported the same very small number of neurosurgical patients who developed major bleeding for those who received pharmacologic thromboprophylaxis and for those who did not (RR, 1.45; 95% CI, 0.30-7.12). In absolute terms, the difference between groups was 3 more events of major bleeding per 1000 patients with CIs from 5 in 1000 fewer to 40 in 1000 more. The certainty of the body of evidence of the risk of major bleeding ranged from low to very low because of the risk of bias and imprecision.
Reoperation.
Two RCTs20,23 evaluated the risk of reoperation after pharmacologic thromboprophylaxis. Results were uninformative in terms of relative effects, with CIs including reductions in RR of more than 50% and almost threefold increases (RR, 0.43; 95% CI, 0.06-2.84). In absolute terms, the difference between groups was 18 fewer events of reoperation per 1000 patients with CIs from 29 in 1000 fewer to 57 in 1000 more. The certainty of the body of evidence was very low because of the risk of bias and imprecision.
Subgroup and sensitivity analyses
The results of the subgroup analysis for the type of neurosurgical intervention in RCTs are provided in the supplemental Data. We carried out subgroup analysis for the outcomes for which evidence was available: mortality, PE, major bleeding, and reoperation. None of the analyses supported the existence of a subgroup effect. Sparse data reported from the NRSs did not allow us to conduct a subgroup analysis. The number of studies in each particular population for any individual outcome proved insufficient to conduct sensitivity analyses.
Discussion
Findings from 7 RCTs and 3 NRS provide evidence having low to very low certainty for prevention of VTEs with pharmacologic thromboprophylaxis in neurosurgical patients. In these RCTs and NRSs, results suggest that pharmacologic thromboprophylaxis possibly prevents asymptomatic proximal DVT (assessed with screening-detected DVT), with uncertain impact on mortality, symptomatic PE, reoperation, and asymptomatic distal DVT (assessed by screening-detected DVT).
Two RCTs provided indirect low-certainty evidence of a benefit of pharmacologic thromboprophylaxis for prevention of asymptomatic proximal DVTs (assessed by screening-detected DVT) in neurosurgical patients. We did not find evidence to indicate a difference in effect in subgroups of populations based on the type of surgery. We also found no effect of pharmacologic thromboprophylaxis on major bleeding in either RCTs or NRSs. The certainty of the body of evidence was low or very low.
Although pharmacologic prophylaxis in neurosurgical patients has been used more commonly in practice, this systematic review questions whether routine pharmacologic prophylaxis is effective in preventing symptomatic PE or mortality. The results of this review should be taken in the context of the low certainty of evidence that did not detail outcomes based on comorbidities or surgery type. Therefore, the decision to use pharmacologic prophylaxis in this population should consider the clinical characteristics of individual patients that may confer higher risk for VTE. Such patients may include those undergoing craniotomy or spinal surgery for malignancy,33 patients with severe traumatic brain injury,34 or patients with spinal cord injury.35 . Other characteristics associated with increased VTE risk are longer duration of surgery, prolonged hospitalization, or discharge to a subacute nursing facility.36-38 These factors are associated with a prothrombotic state and/or prolonged immobilization resulting in a higher risk of VTE. Therefore, these patients may benefit from a more aggressive approach to prevention of VTE, including pharmacologic thromboprophylaxis. Alternatively, patients who are otherwise at low risk of VTE, such as patients having elective neurosurgical procedures and who do not have neurologic deficits, would be well-served with mechanical thromboprophylaxis alone.
Our systematic review has several strengths. We established explicit eligibility criteria, conducted a comprehensive literature search, and assessed eligibility and extracted data in duplicate. Our list of outcomes is more comprehensive and more specific to VTE thromboprophylaxis than that of previous systematic reviews.4,5,10 We assessed the risk of bias in NRSs using the ROBINS-I tool, which allowed us to identify issues in estimates of the benefits or harms in the NRSs. For the first time, the ROBINS-I assessment was integrated with the GRADE risk of bias criteria that used a framework recently reported in GRADE guidelines.17 We also rated the certainty of the body of evidence using guidance from the GRADE Working Group. Moreover, we included both RCTs and NRSs that compared pharmacologic thromboprophylaxis with a control group to broaden the results and applicability of our findings in both cranial and spinal neurosurgeries.
This review also has several limitations, mostly inherent in the evidence. We found only 7 RCTs, and 3 NRSs that addressed prevention of VTE after neurosurgical patients had received pharmacologic thromboprophylaxis. The explanation for this finding is that we focused on specific VTE outcomes for prevention of VTEs in neurosurgical patients. Specifically, we included data from studies that reported symptomatic PE and screening-detected proximal or distal DVT and did not find studies that reported proximal or distal symptomatic DVT in neurosurgical patients. An additional limitation is the over-representation of patients undergoing elective procedures in the studies that were included. These patients likely have an overall low likelihood of developing VTEs or mortality compared with patients undergoing urgent neurosurgical procedures for tumors, ruptured aneurysms, or traumatic brain or spinal cord injuries. Although we found that pharmacologic thromboprophylaxis may reduce the rates of screening-detected proximal DVT, the clinical significance remains uncertain. Conversely, in part because of limited sample sizes, the evidence provides only low to very low confidence in estimates for our outcomes. Serious considerations about the susceptibility to bias and imprecision of the pool estimates (Table 2) were noted in most of the studies.
Other systematic reviews have explored the association between pharmacologic prophylaxis for VTE in neurosurgical patients. In 2008, Collen et al4 identified 30 studies (18 RCTs and 12 prospective cohort studies) that compared pharmacologic thromboprophylaxis with UFH, LMWH, or low-dose warfarin to one another or to mechanical thromboprophylaxis (intermittent compression devices or CSs), or placebo. Intermittent compression devices and LMWH were associated with reducing DVT events compared with placebo (RR, 0.41; 95% CI, 0.21-0.78) and CSs (RR, 0.60; 95% CI, 0.44-0.81). No other comparisons showed differences in either DVT or PE events. Estimates for the comparisons of LMWH vs UFH, LMWH vs nonpharmacologic management, and UFH vs placebo were also not associated with intracranial hemorrhage (ICH), minor bleeding, major bleeding, or death. However, rates of minor bleeding were lower when patients did not receive heparin peri-operatively (0.04 per 1000 patients; 95% CI, 0.00-3.7 lower).
The systematic review by Collen et al4 included more studies than ours for 3 reasons. First, Collen et al included comparisons of mechanical thromboprophylaxis vs placebo or LMWH vs UFH. We did not consider mechanical thromboprophylaxis interventions vs placebo because patient compliance with mechanical prophylaxis is low and often difficult to assess in retrospective studies. We also did not include the comparison of LMWH vs UFH because both interventions have been shown to have similar effects for VTE thromboprophylaxis as well as for intracerebral hemorrhage risk in patients who underwent craniotomy.10 Second, the prospective cohort studies included by Collen et al did not have a group of patients who did not receive thromboprophylaxis to compare thromboprophylaxis intervention. In contrast, we included only NRSs that reported a comparison or control group for VTE pharmacologic thromboprophylaxis. Third, although we made a clear distinction between proximal and distal screening-detected DVT events, Collen et al did not report on the anatomical location of the blood clot in DVT. Our approach allowed us to increase our accuracy in estimating the actual effect of the interventions in each of the outcomes measured. We also identified additional RCTs that were not reported in the systematic review by Collen et al, and we reported patient-important outcomes for patient decision-making purposes.
In 2011, Hamilton et al10 conducted a systematic review to evaluate the efficacy and safety of low doses of UFH or LMWH compared with placebo for VTE thromboprophylaxis in patients who underwent cranial neurosurgery. The systematic review found 6 RCTs that compared heparin (UFH or LMWH) vs placebo or mechanical methods; these were also included in our systematic review. The systematic review by Hamilton et al reported findings for symptomatic and asymptomatic VTE. They concluded that heparin prophylaxis reduces the risk of symptomatic and asymptomatic VTE (RR, 0.58; 95% CI, 0.45-0.75) but there was no association with an increase of ICH (RR, 1.48; 95% CI, 0.63-3.44) or extracranial major bleeding (RR, 0.91; 95% CI, 0.38-2.17), but there was an increase in minor bleeding (RR, 2.28; 95% CI, 1.02-5.10). Authors of this review used the Jadad scale to assess the certainty of individual RCTs, which is no longer perceived to be an appropriate tool to assess risk of bias. As with the study by Collen et al,4 the outcome reporting of the Hamilton et al systematic review differed from our approach, particularly for the DVT outcomes. Moreover, Hamilton et al did not include spinal neurosurgical procedures in their review. Our work included a broader spectrum of neurosurgical procedures as well as reported findings for specific components of VTE: proximal and distal screening-detected DVT and PE.
In 2018, Khan et al5 carried out a systematic review for VTE pharmacologic thromboprophylaxis in patients undergoing cranial or spinal neurosurgeries. They found 5 RCTs that we also identified in our systematic review. They also found 2 publications that we did not include in our systematic review because they did not meet our inclusion criteria. They reported a benefit of pharmacologic thromboprophylaxis to reduce DVT compared with placebo (odds ratio [OR], 0.51; 95% CI, 0.37-0.71). Safety outcomes did not show significant increase of major ICH (OR, 1.42; 95% CI, 0.61-3.30), major extracranial hemorrhage (OR, 0.98; 95% CI, 0.29-3.36), minor bleeding complications (OR, 1.28; 95% CI, 0.50-3.24), or spinal hemorrhage complications. The authors combined symptomatic and screening-detected DVT and did not divide findings regarding proximal or distal screening-detected DVT as we did.
Our systematic review provides an update on the role of pharmacologic thromboprophylaxis for patients with cranial and spinal neurosurgery based on evidence from both RCTs and NRSs. We found low certainty of evidence that pharmacologic thromboprophylaxis reduces asymptomatic proximal DVT established through evidence from screening-detected proximal DVT in neurosurgical patients. We did not find a convincing effect of pharmacologic thromboprophylaxis on any other VTE or major bleeding outcomes.
Further research must be carried out to increase the certainty of the body of evidence, including greater precision of the findings. Future RCTs should use rigorous methods in terms of randomization, blinding of participants and personnel, and complete and transparent outcome reporting. In particular, RCTs should focus on assessing both asymptomatic and symptomatic proximal and distal DVT. Additional RCTs may help evaluate the effects of using pharmacologic thromboprophylaxis over other VTE patient-important outcomes with and without mechanical thromboprophylaxis.
In conclusion, different pharmacologic and nonpharmacologic strategies have been proposed for VTE thromboprophylaxis in neurosurgical patients. The current evidence suggests that pharmacologic thromboprophylaxis reduces the development of asymptomatic proximal DVT (assessed using a screening-detected proximal DVT surrogate outcome) in neurosurgical patients. However, the finding are hampered because of the risk of bias as well as the use of an indirect measure for patient-important outcomes (ie, asymptomatic thrombosis provides only indirect evidence for symptomatic thrombosis). Future RCTs must be carried out to evaluate the impact of pharmacologic thromboprophylaxis on patient-important VTE outcomes as well as to determine the potential harmful effects of these interventions.
For original data, please contact Juan José Yepes-Nuñez via e-mail at yepesnjj@mcmaster.ca.
The full-text version of this article contains a data supplement.
Acknowledgment
This systematic review was performed as part of the ASH clinical practice guidelines on VTE. The entire guideline development process was funded by ASH.
Authorship
Contribution: J.J.Y.-N., P.D., D.R.A., S.B., W.W., R.N., G.P.M., and H.J.S. conceived and designed the study; J.J.Y.-N., L.E.C.-L., S.R., M.B., K.E.O., F.P., M.V., A.M.B., S.B., H.B., A.A., W.W., and G.P.M. acquired data; J.J.Y.-N., W.W., R.N., G.P.M., and H.J.S. analyzed and interpreted the data; J.J.Y.-N., A.R., M.R., P.D., D.R.A., W.W., and H.J.S. drafted the article; A.R., M.R., P.D., D.R.A., W.W., R.N., G.P.M., and H.J.S. critically revised the article; J.J.Y.-N., A.R., M.R., P.D., D.R.A., L.E.C.-L., S.R., M.B., K.E.O., F.P., M.V., A.M.B., S.B., H.B., A.A., W.W., R.N., G.P.M., and H.J.S. reviewed the submitted version of manuscript; and J.J.Y.-N. approved the final version of the manuscript on behalf of all of the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan José Yepes-Nuñez, Department of Health Research Methods, Evidence, and Impact, McMaster University, Health Sciences Centre, Room 2C14, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: yepesnjj@mcmaster.ca.
References
Author notes
The full-text version of this article contains a data supplement.