Key Points
This multicenter phase 2 study explores BV as consolidation after ABVD in the first-line setting for treating non-bulky limited-stage HL.
BV consolidation led to high PET negativity and favorable survival outcomes in risk-stratified patients with limited-stage HL.
Abstract
Approximately 90% of limited-stage Hodgkin lymphoma (HL) patients are projected to be cured with standard therapy, but many do not live their expected life span because of late treatment–related complications. New treatment paradigms are needed to reduce the use of radiation therapy (RT) as well as conventional chemotherapy drugs while improving upon current standard-of-care survival outcomes. In this phase 2 multicenter study, patients with non-bulky limited-stage HL received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by brentuximab vedotin (BV) consolidation. Forty-one patients were enrolled, and patient characteristics included median age of 29 years (range, 19 to 67 years), 58% were female, 45% had unfavorable disease, and 98% had stage II disease. Based on positron emission tomography (PET)–based risk stratification, patients received 2 to 6 cycles of ABVD followed by 6 cycles of BV. After ABVD followed by BV, 95% of evaluable patients (37 out of 39; 95% confidence interval [CI], 83%-99%) achieved PET-negative status. In the intent-to-treat patient population, the estimated 3-year progression-free survival (PFS) rate was 92%, and the overall survival (OS) rate was 97%, with a median follow-up of 47 months. All 37 patients who achieved negative PET status after BV consolidation effectively avoided RT and remain in remission with estimated 3-year PFS and OS rates of 100%. In conclusion, BV demonstrates encouraging clinical activity when it follows ABVD therapy in limited-stage HL. Early incorporation of BV may reduce the use of RT as well as conventional chemotherapy drugs while achieving favorable survival outcomes in risk-stratified patients with non-bulky limited-stage HL. This trial was registered at www.clinicaltrials.gov as #NCT01578967.
Introduction
Hodgkin lymphoma (HL) is one of the most common cancer types in young adults with the incidence peaking in people in their 20s.1 Despite the identification of clinical prognostic factors and the optimal use of primary and secondary treatments, HL remains fatal for more than 10% of patients. Furthermore, the morbidity of therapy is substantial and long-lasting, even in patients who are cured.2 Radiation therapy (RT), although it is considered one of the most active treatment modalities in HL, has been largely associated with increased risk of late treatment–related complications, such as secondary malignancies. Given the unclear overall survival (OS) advantage associated with RT, the use of this modality remains a controversial topic in the treatment of HL.3,4 In addition, both RT and anthracycline exposure have been associated with significantly increased risk of cardiovascular complications in a dose-dependent manner.5 Thus, treatment of HL requires a careful balance between providing effective therapy to cure the disease while minimizing the risk of excessive long-term treatment-related complications, especially in the adolescent and young adult patient population.6
Brentuximab vedotin (BV) is an antibody-drug conjugate targeting CD30 that is approved by the US Food and Drug Administration (FDA) for the treatment of patients with relapsed classical HL or for consolidation in patients with classical HL who are at high risk of relapse after autologous hematopoietic stem cell transplantation (ASCT).7,8 The FDA later expanded the approval of BV for stage III or IV classical HL in combination with doxorubicin, vinblastine, and dacarbazine (A+AVD) to replace bleomycin in the first-line setting.9 We hypothesized that BV might be effective in eliminating any potential residual disease after chemotherapy, which would reduce the use of RT as consolidative treatment in first-line therapy of limited-stage HL. This phase 2 study was conducted to evaluate BV as consolidation therapy to minimize the use of RT while maintaining current standard-of-care survival outcomes in patients with limited-stage HL.
Methods
Patients
Patients between 18 and 60 years of age with previously untreated histologically confirmed stage I or II classical HL were eligible for the study. Patients were required to have measurable disease shown by the presence of lesions that could be accurately measured in 2 dimensions by computed tomography (CT), but patients with bulky lymphadenopathy, defined as lymphadenopathy >7.5 cm in the largest diameter, were excluded. Detailed eligibility and protocol information can be found in the supplemental Data.
Study design and treatment
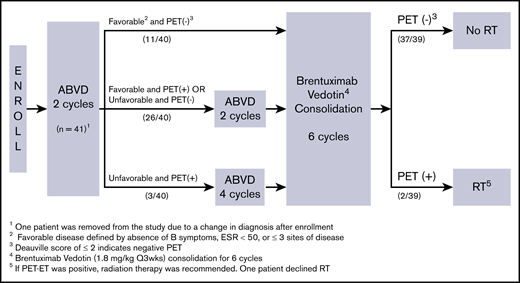
This was a single-arm phase 2 open-label study of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by BV consolidation (Lineberger Comprehensive Cancer Center 1115). Patients were enrolled and treated at 6 US study sites through the University of North Carolina Cancer Network. The protocol was approved by the institutional review board for each study site and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent (approved by the institutional review board) before any study-specific procedures began. The primary objective of the study was to estimate the complete response (CR) rate based on positron emission tomography (PET) scans, defined by Deauville score ≤2, after ABVD followed by BV consolidation. Secondary objectives were to assess progression-free survival (PFS) and OS rates. The study schema is depicted in Figure 1. Briefly, all patients were started with 2 cycles of ABVD (doxorubicin 25 mg/m2, bleomycin 10 U/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2), and an interim PET scan was obtained after 2 cycles of ABVD (PET-2). Patients with favorable disease, defined by the absence of B symptoms, erythrocyte sedimentation rate <50, and 2 or fewer sites of disease, who achieved negative PET status after 2 cycles of ABVD proceeded directly to BV consolidation with no further ABVD chemotherapy and received BV at 1.8 mg/kg every 3 weeks for 6 cycles. Patients with favorable disease who had positive PET-2 or those with unfavorable disease who had negative PET-2 received an additional 2 cycles of ABVD followed by BV consolidation. Patients with unfavorable disease who had positive PET-2 received an additional 4 cycles of ABVD followed by BV consolidation. Patients who were unable to achieve at least a partial response after ABVD and those who progressed at any point during ABVD therapy were removed from protocol treatment. Patients with negative PET status at the end of BV (PET-BV) were assigned to no further therapy. RT was recommended only if PET-BV was positive.
Protocol outline with the study schema. ABVD followed by BV. Q3wks, once every 3 weeks.
Protocol outline with the study schema. ABVD followed by BV. Q3wks, once every 3 weeks.
Study assessment
Safety was monitored from the time of informed consent up to 30 days after the last dose of BV and included capturing adverse events (AEs), abnormal laboratory values, and vital signs. Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. The efficacy was determined on the basis of response assessments made according to the Revised Response Criteria for Malignant Lymphoma, and investigators based treatment decisions on these assessments.10 For the PET response criteria, the Deauville 5-point scale was used by individual institutions.11 A clinical response of progressive disease, stable disease, partial response, or CR was determined at each assessment for all patients. PET-2 status was obtained within 10 days at the end of cycle 2 of ABVD. PET-BV status was obtained 8 weeks after BV consolidation. PET status was considered negative if the patient had a Deauville score of 2 or lower. Patients received follow-up PET/CT scans or contrast CT scans at a minimum of every 6 months for the first 2 years and annually for years 3 to 5.
Statistical analysis
Sample size was determined on the basis of historical data from a randomized trial by the United Kingdom National Cancer Research Institute (UK RAPID), in which ∼75% of patients had a CR by PET scan (Deauville score of ≤2) before RT.3 The target rate for CR was >85% before RT could be omitted in this study, which was a 10% improvement compared to the UK RAPID study, while maintaining PFS similar to that in the combined modality treatment (CMT) arm of the same study. A total sample size of 40 would be required to estimate the proportion within ±16.2%, and the half-width of an exact 95% confidence interval (CI) was expected to be even smaller because the percentage was anticipated to be closer to 100%. As an example, 85% (34 of 40) would yield a 95% CI of 70.2% to 94.3% with a half-width of 12.1% whereas 95% (38 of 40) would yield a 95% CI of 83.1% to 99.4% with a half-width of 8.2%. Sequential boundaries were used as an early futility stopping rule if too many patients on the study required RT. A Pocock type stopping boundary was used to halt accrual with an RT rate of >0.15 considered unacceptable. For example, if 3 of the first 23 enrolled patients required RT, the study would be terminated for futility. Exact 95% binomial CIs were calculated. The Kaplan-Meier method was used to estimate OS and PFS. OS time was calculated from date of diagnosis, and PFS time was calculated from start of ABVD therapy.
Results
Patients
Patient characteristics are detailed in Table 1. Forty-one patients were enrolled at 6 US sites between April 2012 and December 2015. One patient was considered unevaluable because of a change in diagnosis from HL to gray zone lymphoma after ABVD treatment was started. The median age was 29 years (range, 19-67 years) with 70% of patients younger than age 40 years at the time of study enrollment. A higher proportion of patients were female (58%) than male. Most patients (98%) had stage II HL, with 45% having features of unfavorable disease. The German Hodgkin Lymphoma Group (GHSG) criteria were used to categorize favorable vs unfavorable groups, although some modifications were made because bulky disease was excluded from the study. Eight patients were categorized as unfavorable on the basis of having ≥3 sites of disease, whereas 5 patients were categorized on the basis of an elevated erythrocyte sedimentation rate. Risk stratification was not specified in 5 patients, although they were reported to have at least 1 of the 3 risk factors. All patients had non-bulky disease except 1 female patient who was granted a single-subject exception for a conglomerate mediastinal nodal mass measuring 10 cm.
Characteristics of patients with limited-stage HL treated with ABVD followed by BV consolidation (N = 40)
| Characteristic . | n . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 29 (19-67) | |
| <40 | 28 | 70 |
| Sex | ||
| Female | 23 | 58 |
| Male | 17 | 42 |
| Race | ||
| White | 36 | 90 |
| African American | 3 | 8 |
| Unknown | 1 | 2 |
| Stage | ||
| IA | 1 | 2 |
| IB | 0 | 0 |
| IIA | 29 | 73 |
| IIB | 10 | 25 |
| Prognosis | ||
| Favorable | 22 | 55 |
| Unfavorable | 18 | 45 |
| Characteristic . | n . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 29 (19-67) | |
| <40 | 28 | 70 |
| Sex | ||
| Female | 23 | 58 |
| Male | 17 | 42 |
| Race | ||
| White | 36 | 90 |
| African American | 3 | 8 |
| Unknown | 1 | 2 |
| Stage | ||
| IA | 1 | 2 |
| IB | 0 | 0 |
| IIA | 29 | 73 |
| IIB | 10 | 25 |
| Prognosis | ||
| Favorable | 22 | 55 |
| Unfavorable | 18 | 45 |
Eleven patients (28%) received no more than 2 cycles of ABVD based on their favorable prognostic features at baseline and the negative PET-2 status per protocol, and 26 patients (65%) received 4 cycles of ABVD. Three patients received more than 4 cycles of ABVD before BV consolidation. Of 40 patients included in the study, 39 patients completed treatment per protocol and were considered evaluable for response, and 1 patient, who discontinued the study treatment with BV before response assessment was obtained, was included in the intent-to-treat survival and toxicity analysis only.
Efficacy and survival
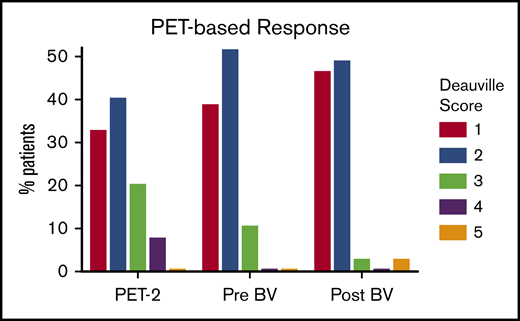
PET-based responses are depicted in Figure 2. Of 39 patients evaluable for response who completed 2 to 6 cycles of ABVD followed by BV consolidation, 37 patients (95% CI, 83%-99%) achieved a CR with negative PET status (Deauville score ≤2). As expected, more patients converted to PET-negative status as they received additional therapy. Of 11 patients who had positive PET-2, 8 patients had a Deauville score of 3, and 3 patients had a Deauville score of 4. None of the 8 patients with a Deauville score of 3 at PET-2 scanning relapsed, but 1 of the 3 patients with a Deauville score of 4 eventually relapsed. Four patients were PET positive at the end of ABVD treatment, and all of them achieved negative PET status after BV consolidation and were still in remission at the time of data cutoff. At the end of BV consolidation, 1 patient was PET-BV positive with a Deauville score of 3. This patient subsequently had a biopsy that confirmed disease progression and received RT followed by ASCT. The other patient with positive PET-BV status had primary refractory disease with a Deauville score of 5 and declined RT to proceed directly with ASCT.
PET-based response by Deauville scores. PET-2, after 2 cycles of ABVD; pre-BV, after 2 to 6 cycles of ABVD; post-BV (after BV consolidation).
PET-based response by Deauville scores. PET-2, after 2 cycles of ABVD; pre-BV, after 2 to 6 cycles of ABVD; post-BV (after BV consolidation).
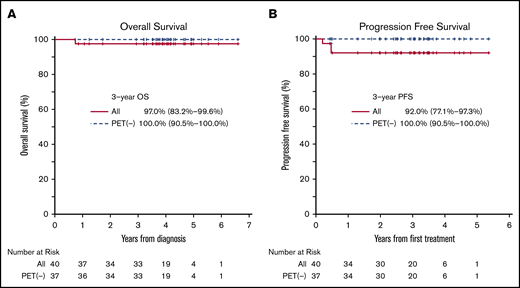
The median OS and PFS were not reached for all patients with a median follow-up of 47 months (Figure 3). The estimated 3-year OS rate was 97% (95% CI, 83%-100%), and the PFS rate was 92% (95% CI, 77% to 97%) for the intent-to-treat patient population. All 37 patients who achieved a CR with a negative PET-BV status were still in remission at the time of data cutoff with estimated 3-year PFS of 100% (95% CI, 91%-100%). Notably, all 11 patients who received no more than 2 cycles of ABVD before receiving BV consolidation remained in remission. The remission after BV consolidation was durable with the median duration of remission being 3.1 years from the time the last dose of BV was administered. Two patients who had positive PET-BV status successfully underwent ASCT, and both patients remain alive with no evidence of relapse. In a subgroup analysis, no difference in survival was observed between favorable and unfavorable risk groups; 1 of 22 patients in the favorable-risk group relapsed compared with 1 of 18 patients in the unfavorable-risk group.
Kaplan-Meier estimates of outcomes by PET-based response in patients with limited-stage HL treated with ABVD followed by BV consolidation. (A) Overall survival. (B) Progression-free survival.
Kaplan-Meier estimates of outcomes by PET-based response in patients with limited-stage HL treated with ABVD followed by BV consolidation. (A) Overall survival. (B) Progression-free survival.
Safety
BV consolidation was generally well tolerated, and 36 patients received the full 6 doses of BV, with 4 patients requiring dose reduction. Patients who completed fewer than 6 doses of BV discontinued treatment either because of an AE or patient decision (non-AE). The major grade ≥3 AEs reported during BV consolidation are summarized in Table 2. BV-related grade ≥3 toxicities included neutropenia (7.5%), peripheral neuropathy (2.5%), and rash (2.5%). There was 1 grade 5 toxicity resulting from sepsis, hepatotoxicity, and pancreatitis, a very rare but known complication of BV, after 4 cycles of ABVD and 1 dose of BV. All reported grade ≥4 toxicities were associated with this single event. Peripheral sensory neuropathy was the most common nonhematologic AE, although most (87%) were grade 1, and 2 patients had grade 2. There was no pulmonary toxicity associated with BV consolidation.
Adverse events associated with BV after ABVD
| Toxicity . | Grade . | Total . | ||
|---|---|---|---|---|
| 3 . | 4 . | 5 . | ||
| Neutropenia | 3 | 3 | ||
| Infections | 1 | 1 | 2 | |
| Alanine aminotransferase increased | 1 | 1 | ||
| Anemia | 1 | 1 | ||
| Ascites | 1 | 1 | ||
| Increased aspartate aminotransferase | 1 | 1 | ||
| Increased bilirubin | 1 | 1 | ||
| Increased creatinine | 1 | 1 | ||
| Hepatic failure | 1 | 1 | ||
| Hyperglycemia | 1 | 1 | ||
| Hypophosphatemia | 1 | 1 | ||
| Pancreatitis | 1 | 1 | ||
| Peripheral sensory neuropathy | 1 | 1 | ||
| Decreased platelet count | 1 | 1 | ||
| Pleural effusion | 1 | 1 | ||
| Maculo-papular rash | 1 | 1 | ||
| Other renal and urinary disorders (specify) | 1 | 1 | ||
| Syncope | 1 | 1 | ||
| Toxicity . | Grade . | Total . | ||
|---|---|---|---|---|
| 3 . | 4 . | 5 . | ||
| Neutropenia | 3 | 3 | ||
| Infections | 1 | 1 | 2 | |
| Alanine aminotransferase increased | 1 | 1 | ||
| Anemia | 1 | 1 | ||
| Ascites | 1 | 1 | ||
| Increased aspartate aminotransferase | 1 | 1 | ||
| Increased bilirubin | 1 | 1 | ||
| Increased creatinine | 1 | 1 | ||
| Hepatic failure | 1 | 1 | ||
| Hyperglycemia | 1 | 1 | ||
| Hypophosphatemia | 1 | 1 | ||
| Pancreatitis | 1 | 1 | ||
| Peripheral sensory neuropathy | 1 | 1 | ||
| Decreased platelet count | 1 | 1 | ||
| Pleural effusion | 1 | 1 | ||
| Maculo-papular rash | 1 | 1 | ||
| Other renal and urinary disorders (specify) | 1 | 1 | ||
| Syncope | 1 | 1 | ||
Discussion
The main goal of this exploratory phase 2 study was to evaluate BV as consolidation to omit RT in >85% of patients, a 10% improvement over historical data from the UK RAPID trial, while maintaining a PFS rate comparable to CMT in limited-stage HL. The study design was based on the hypothesis that BV consolidation would be effective in eliminating any potential residual disease after ABVD and lead to improved long-term survival rates without the need for RT in risk-stratified patients. In this study, 95% of patients achieved CR with a negative PET status, all of whom remained in remission with an estimated 3-year PFS of 100% at a median follow-up of 47 months. Furthermore, approximately one-third of patients in the study received no more than 2 cycles of ABVD on the basis of the risk-adapted design, all of whom remained in remission at the time of data cutoff. The results of our study are in line with those of the GHSG HD10 study, which showed excellent clinical outcomes associated with 2 cycles of ABVD when followed by RT at 20 Gy in patients with favorable-risk limited-stage HL.12 Our study substituted BV for RT and demonstrated that early incorporation of targeted therapy and risk-adapted approach may reduce not only the use of RT but also conventional chemotherapy drugs while maintaining excellent disease control in limited-stage HL.
Standard therapy for limited-stage HL currently involves the use of chemotherapy, such as ABVD, with or without RT.13,14 Although approximately 90% of limited-stage HL patients are projected to be cured with the standard approach, many do not live their expected life span because of late treatment–related complications.15,16 In several large retrospective studies, survivors of HL who received RT experienced a significantly higher incidence of secondary malignancies than those who were treated with chemotherapy alone.15,17-19 It is expected that more modern RT techniques such as involved-site or node RT will reduce toxicity,20 but the long-term effects of these newer RT techniques are still largely unknown.21 In addition, both RT and anthracycline exposure have been associated with increased risk of cardiovascular complications, including congestive heart failure and coronary artery disease, in a dose-dependent manner even at lower doses.5,22,23 Furthermore, salvage therapy, which often involves high-dose chemotherapy followed by stem cell transplantation, may also lead to other long-term complications, such as secondary acute myeloid leukemia and myelodysplastic syndrome.2,24 For these reasons, cumulative incidence of cause-specific mortality from long-term treatment-related complications far exceeds that of HL-specific mortality in HL survivors, with the all-cause mortality rate approaching 30% at 15 years of follow-up even in the modern era.6,25 Furthermore, the relative risks of treatment-related morbidity and mortality continue to rise even 2 to 3 decades after initial HL diagnosis.26 Early incorporation of targeted therapy could potentially allow a less toxic but equally effective treatment of patients with limited-stage HL.
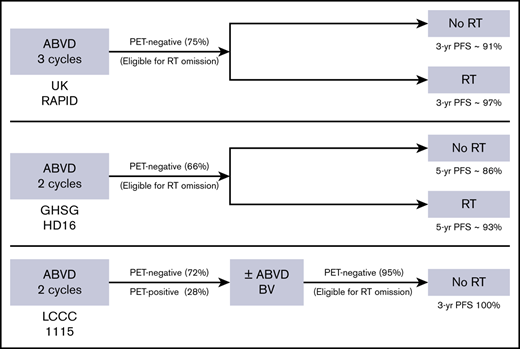
In this study, estimated 3-year PFS and OS rates for the entire group were 92% and 97%, respectively, which compare favorably with historical data. Recently, there have been concerted efforts to incorporate risk-adapted approaches to identify HL patients in whom RT could potentially be omitted without compromising disease control. In the UK RAPID study, patients with limited-stage HL who achieved PET-negative status (Deauville score of ≤2) after 3 cycles of ABVD were randomly assigned to no further treatment vs consolidative RT.3 In the intent-to-treat population with a negative PET scan after 3 cycles of ABVD, the 3-year PFS rate was 90.8% for the chemotherapy-only arm vs 94.6% for CMT. The study did not meet the noninferiority criteria for the omission of RT, and more importantly, 25% of the study population was deemed inappropriate for the omission of RT based on their positive PET status at the time of randomization.
A phase 2 US intergroup study led by the Alliance (formerly Cancer and Leukemia Group B) took a similar risk-adapted approach but used less stringent criteria for negative interim PET status with a Deauville score of ≤3 as the cutoff.27 After 2 cycles of ABVD, 91% of patients had a Deauville score of ≤3 and went on to receive 2 additional cycles of ABVD. The estimated 3-year PFS was 91% in PET-negative patients after 4 cycles of ABVD. An intergroup trial (HD10) by European Organisation for Research and Treatment of Cancer/Lymphoma Study Association/Fondazione Italiana Linfomi randomly assigned patients to either CMT or risk-adapted therapy.28 For patients with a negative PET-2 status (Deauville score of ≤2), 5-year PFS was in favor of ABVD plus RT (99.0% for favorable risk and 92.1% for unfavorable risk) compared with ABVD only (87.1% for favorable risk and 89.6% for unfavorable risk). ABVD failed to demonstrate noninferiority compared with CMT, and risk of relapse was significantly higher when RT was omitted, especially in the favorable risk group. For patients with positive PET-2 in the same study, 5-year PFS was 77.4% after additional ABVD plus RT and 90.6% after dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) plus RT. The estimated 3-year PFS was 82% for patients with positive PET-2 in the current study. Considering significant short-term and long-term toxicities associated with BEACOPP plus RT, BV consolidation may be an attractive option in this patient population.
An international study (HD16) led by the GHSG randomly assigned patients with limited-stage favorable-risk HL to either CMT with 20 Gy of RT or ABVD alone after a negative PET-2 status.29 Of 1007 evaluable patients, 667 (66%) achieved negative PET-2 status (Deauville score of ≤2). Among the patients who achieved negative PET-2 status, the 5-year PFS rate was 93.4% with CMT vs 86.1% with ABVD. There was no statistically significant difference in OS between the 2 groups. The authors concluded that RT cannot be omitted from standard CMT without clinically relevant loss of tumor control in patients with limited-stage favorable-risk HL, even if they achieve negative PET-2 status. In this study, omission of RT does not seem to compromise disease control when BV is substituted for RT in patients who are PET-BV negative, suggesting that PET-BV may confer an even stronger predictive value than PET-2 when BV is used as consolidation to eliminate any potential residual disease after chemotherapy.
This study represents one of the first efforts to incorporate both targeted and risk-adapted approaches for first-line therapy in limited-stage HL with the longest median follow-up reported to date. Kumar et al30 evaluated 4 cycles of A+AVD with or without RT in limited-stage unfavorable risk HL, and Abramson et al31 used a lead-in cycle of BV followed by 4 cycles of A+ AVD. Similar to our findings, both studies showed promising clinical outcomes with PET-negative CR rates ranging from 91% to 93% after 4 cycles of A+AVD, which equates to 8 doses of BV. A sequential approach with BV as consolidation was used in our study on the basis of the emerging safety data from a phase 1 study of concurrent A(B)VD and BV at the time of study design, which demonstrated unacceptable pulmonary toxicity when ABVD and BV were given concurrently.32 In addition, sequential administration may reduce the risk of other potential toxicities, such as febrile neutropenia, compared with the concurrent treatment. No patients treated with up to 6 doses of BV sequentially after ABVD experienced pulmonary toxicity in this study. Peripheral neuropathy was the most common nonhematologic AE, but the large majority of AEs were grade 1 or 2. This study was not designed to follow the course of neuropathy, although recent data from the ECHELON-1 trial showed that 78% of patients who received A+AVD (12 doses of BV) had either complete resolution or improvement of peripheral neuropathy at 3 years of follow-up.33 Notably, 1 patient developed grade 5 treatment-related AEs complicated by systemic fungal infection, hepatotoxicity, and pancreatitis after receiving 4 cycles of ABVD and 1 dose of BV. It was difficult to ascertain the primary cause because all events occurred within a short time frame. Hepatotoxicity and pancreatitis are known but rare conditions associated with BV. The risk of all grades of hepatotoxicity reported in clinical trials is 1.4%, and the risk of all grades of hepatotoxicity from post-marketing data is 0.61%, with the risk of all grades of acute pancreatitis even lower at ∼0.2%.34,35 Fatal hepatotoxic events have been documented in less than 0.01% of more than 15 000 patients treated with BV. It is notable that this study maintained favorable survival outcomes despite being negatively impacted by this rare event.
Table 3 represents a comprehensive summary of various trials which used risk-adapted approach in limited-stage HL. Clearly, this study is limited by its small sample size as a single-arm study, and it is important to note that it was originally designed as an exploratory phase 2 study and was not intended to be practice changing. In addition, the patient populations may be slightly different among these studies, which make the direct comparison even more difficult. Quality-of-life measures and cost-benefit analysis have also become important aspects of the modern clinical trial design, but this study was not intended to address complex secondary measures such as these. Future studies will require a careful design to not only compare the direct costs involved in patient care during active treatment but also examine decades of financial as well as psychosocial consequences in managing the long-term mortality and morbidity associated with HL treatment.
Studies using PET-directed approaches in limited-stage HL
| Study . | PET-negative rate (% considered for RT omission) . | Treatment of PET-negative patients . | Median follow-up, mo . | 3- to 5-y PFS for PET-negative patients . | |
|---|---|---|---|---|---|
| n . | % . | ||||
| United Kingdom RAPID3 | 75%* | ABVD × 3 cycles ABVD × 3 cycles + RT | 62 | 211 209 | 90.8 94.6 |
| EORTC/LYSA/FIL H1026 | 81%† | ABVD × 4-6 cycles ABVD × 3-4 cycles + RT | 54 | 540 519 | 88.3 93.4 |
| ALLIANCE/CALGB 5060427 | 91%‡ | ABVD × 4 cycles | 46 | 135 | 91 |
| GHSG HD1629 | 66%* | ABVD × 2 cycles ABVD × 2 cycles + RT | 46 | 300 328 | 86.1 93.4 |
| Current study | 95%* | ABVD × 2-6 cycles + BV | 47 | 37 | 100 |
| Study . | PET-negative rate (% considered for RT omission) . | Treatment of PET-negative patients . | Median follow-up, mo . | 3- to 5-y PFS for PET-negative patients . | |
|---|---|---|---|---|---|
| n . | % . | ||||
| United Kingdom RAPID3 | 75%* | ABVD × 3 cycles ABVD × 3 cycles + RT | 62 | 211 209 | 90.8 94.6 |
| EORTC/LYSA/FIL H1026 | 81%† | ABVD × 4-6 cycles ABVD × 3-4 cycles + RT | 54 | 540 519 | 88.3 93.4 |
| ALLIANCE/CALGB 5060427 | 91%‡ | ABVD × 4 cycles | 46 | 135 | 91 |
| GHSG HD1629 | 66%* | ABVD × 2 cycles ABVD × 2 cycles + RT | 46 | 300 328 | 86.1 93.4 |
| Current study | 95%* | ABVD × 2-6 cycles + BV | 47 | 37 | 100 |
CALGB, Cancer and Leukemia Group B; EORTC/LYSA/FIL, European Organisation for Research and Treatment of Cancer/Lymphoma Study Association/Fondazione Italiana Linfomi; GHSG, German Hodgkin Study Group.
Deauville ≤2.
International Harmonization Project (IHP) criteria.
Deauville ≤3.
Another challenge lies in identifying the population of HL patients who may benefit most from novel approaches. In this study, no more than 2 cycles of ABVD were sufficient for achieving excellent disease control without RT in favorable-risk patients. On the basis of data from the HD16 trial, which showed inferior disease control when this patient population was treated with ABVD alone, BV consolidation could be an attractive option to support omitting RT in this setting. Conversely, it remains to be seen whether the excess cost and the excess time (∼10 weeks) of BV consolidation are truly warranted to reduce ABVD chemotherapy while achieving incremental improvements in PFS. Future studies should be designed to identify groups of patients who may not need BV and to determine how much improvement in PFS would justify the use of targeted agents in this patient population. As an example, RT might be more appropriate in older male patients with focal disease involvement outside the chest area, and clinical tools are being developed to better guide this type of treatment decision.36 In addition, this study used maximal tumor dimension (MTD) of >7.5 cm instead of the traditional 10 cm to define bulky disease to ensure that patients would not be undertreated.37 However, it remains unclear which MTD should be used to reasonably predict the potential benefit of RT. A recent follow-up study of the UK RAPID trial showed that patients with MTD of ≥5 cm had a significantly higher risk for relapse when RT was omitted even after their PET status was negative at the end of ABVD.38 It requires further exploration to determine whether RT can be safely omitted for HL with higher MTDs when targeted agents are used. Finally, the role of bleomycin is becoming increasing unclear, especially when BV is added in the first-line setting.9,30,31 A follow-up study (NCT03233347) was subsequently designed to evaluate an abbreviated course of A+AVD followed by nivolumab consolidation in limited-stage HL, and this study is currently ongoing.
In summary, ABVD followed by BV consolidation showed promising clinical activity in previously untreated non-bulky limited-stage HL. Our data demonstrate that early incorporation of targeted agents and the risk-adapted approach may provide a novel treatment strategy to transform the current standard for HL therapy by reducing the use of RT as well as conventional chemotherapy drugs without compromising the disease control.
Presented in part at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 6-9 December 2014, the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL, 3-7 June 2016, and the 14th International Conference on Malignant Lymphoma, Lugano, Switzerland, 14-17 June 2017.
Requests for data can be addressed to Steven I. Park via e-mail at steven.park@atriumhealth.org.
Acknowledgment
This work was supported by Seattle Genetics, Inc.
Authorship
Contribution: S.I.P. and S.M.A. contributed to study design, collected and interpreted the data, and wrote the article; T.C.S., O.O., N.M.R., L.E.B., and N.G. collected and interpreted the data and wrote the article; and A.M.D. and J.F.N. contributed to study design, analyzed and interpreted the data, and wrote the article.
Conflict-of-interest disclosure: S.I.P. received honoraria and research funding/grants from Seattle Genetics, Inc., honoraria/research funding from Bristol-Myers Squibb, G1 Therapeutics, Teva, and Gilead, and is a member of the medical advisory board for Rafael Pharma. S.M.A. received research funding (to his institution for clinical trials) from Seattle Genetics, Bristol-Myers Squibb, Celldex, Regeneron, Trillium, LAM Therapeutics, and Pfizer. N.M.R. received honoraria and research funding from Bristol-Myers Squibb and honoraria from Teva, Gilead, Janssen, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Steven I. Park, Levine Cancer Institute, 1021 Morehead Medical Dr, Suite 3100, Charlotte, NC 28204; e-mail: steven.park@atriumhealth.org.
References
Author notes
The full-text version of this article contains a data supplement.