Key Points
Deferring ultrasound imaging for ≤24 hours with empiric rivaroxaban in patients with suspected DVT is a safe strategy.
The strategy may simplify the diagnostic approach to DVT while improving resource use.
Abstract
Guidelines suggest using empiric low-molecular-weight heparin if the diagnostic workup of deep vein thrombosis (DVT) is expected to be delayed. The role of direct oral anticoagulants for deferred compression ultrasound imaging (CUS) in patients with suspected DVT remains unexplored. The main objective of the study was to assess the safety of deferring CUS with therapeutic doses of rivaroxaban. We prospectively included consecutive outpatients referred to the Emergency Department at Østfold Hospital, Norway, with suspected first or recurrent lower-extremity DVT between February 2015 and November 2018. Patients were discharged with rivaroxaban 15 mg twice daily while awaiting CUS within 24 hours if D-dimer level was ≥0.5 mg/L fibrinogen-equivalent units. The primary outcome was the rate of major bleeding incidents from study inclusion until DVT was confirmed and anticoagulation therapy continued, or otherwise up to 48 hours following administration of the last tablet of rivaroxaban. The secondary outcome was the rate of progressive DVT symptoms or symptoms or signs of pulmonary embolism between hospital discharge until venous thromboembolism was diagnosed. Six hundred twenty-four of 1653 patients referred with suspected DVT were included (37.7%; 95% confidence interval [CI], 35.4-40.1). DVT was diagnosed in 119 patients (19.1%; 95% CI, 16.1-22.3). There were no major bleeding incidents, yielding an observed major bleeding rate of 0% (1-sided 95% CI <0.4). No patients experienced major complications in the interval that CUS was deferred (0%; 95% CI, 0.0-0.6). Deferring CUS for up to 24 hours in patients with suspected DVT with therapeutic doses of rivaroxaban is a safe strategy. This trial was registered at www.clinicaltrials.gov as #NCT02486445.
Introduction
The workup of deep vein thrombosis (DVT) starts with pretest probability assessment and D-dimer testing to determine which patients should be referred for diagnostic compression ultrasonography (CUS) to establish a final diagnosis.1 Guidelines suggest empiric treatment with low-molecular-weight heparin (LMWH) if the workup is prolonged, and the patient has no major risk factors for bleeding.2 Prompt administration of LMWH is recommended in patients with a high pretest probability of DVT. For patients with moderate or low pretest probability, LMWH is suggested if the workup is expected to exceed 4 and 24 hours, respectively.2 Several studies have demonstrated the safety of deferring CUS until on-hours with therapeutic doses of LMWH or unfractionated heparin,3-9 which may alleviate the resource burden of around-the-clock referrals for CUS at hospitals.
Although direct oral anticoagulants are increasingly used in the treatment of DVT, their safety has not been prospectively assessed for suspected DVT in a diagnostic approach deferring CUS. This is important to establish before it may be routinely prescribed in daily practice, as the majority of patients who receive empiric anticoagulation do not have DVT.
In this study, we evaluated the safety and feasibility of deferring CUS for up to 24 hours with therapeutic doses of rivaroxaban in patients with suspected DVT.
Methods
Study population and design
The Rivaroxaban for Scheduled Work-up of Deep Vein Thrombosis Study (the Ri-Schedule study, www.clinicaltrials.gov identifier NCT02486445) was a prospective outcome trial including consecutive outpatients referred from primary care centers to the Emergency Department at Østfold Hospital, Norway, between February 2015 and November 2018. The hospital is the primary referral center for ∼300 000 inhabitants.
Inclusion criteria were ≥18 years of age, referral for first or recurrent suspected lower-extremity DVT, ability and willingness to provide written consent, and no enrollment in the study within the past 3 months. Exclusion criteria were conditions associated with a higher risk of adverse outcomes with rivaroxaban and/or with being discharged awaiting CUS (Table 1). These included expected workup completion within 2 hours, contraindications to rivaroxaban, hemoglobin <11 g/dL, thrombocyte count <100 × 109/L, glomerular filtration rate (GFR) <45 mL/min per 1.73 m2, cancer or chemotherapy in the past 6 months, suspected concurrent pulmonary embolism (PE), comorbidities necessitating admission, suspected leg ischemia or eligibility for thrombolysis, logistical challenges with at-home observation, patient objection to discharge, or physician deeming discharge to be unsafe.
Exclusion criteria for deferred imaging and empiric rivaroxaban
| Factors with a higher risk of adverse effects of rivaroxaban . |
|---|
| Concomitant anticoagulation* |
| Suspected active or recent bleeding |
| Major risk factors for bleeding† |
| Active cancer or chemotherapy within the past 6 mo |
| Pregnancy or lactation |
| Hemoglobin <11 g/dL or thrombocytes <100 × 109/L |
| GFR <45 mL/min per 1.73 m2 |
| Liver disease with coagulopathy or other bleeding risk |
| Concomitant medications possibly interacting with rivaroxaban |
| Factors with a higher risk of adverse effects of rivaroxaban . |
|---|
| Concomitant anticoagulation* |
| Suspected active or recent bleeding |
| Major risk factors for bleeding† |
| Active cancer or chemotherapy within the past 6 mo |
| Pregnancy or lactation |
| Hemoglobin <11 g/dL or thrombocytes <100 × 109/L |
| GFR <45 mL/min per 1.73 m2 |
| Liver disease with coagulopathy or other bleeding risk |
| Concomitant medications possibly interacting with rivaroxaban |
| Conditions or situations in which scheduled workup is deemed inappropriate . |
|---|
| Suspicion of concurrent PE |
| Comorbidities necessitating admission |
| Suspected ischemia or eligibility for thrombolysis |
| Physician considers discharge unsafe |
| Patient objects to discharge |
| Logistical challenges |
| Workup can be completed within 2 h |
| Conditions or situations in which scheduled workup is deemed inappropriate . |
|---|
| Suspicion of concurrent PE |
| Comorbidities necessitating admission |
| Suspected ischemia or eligibility for thrombolysis |
| Physician considers discharge unsafe |
| Patient objects to discharge |
| Logistical challenges |
| Workup can be completed within 2 h |
Regular prescription or empiric anticoagulation for suspected DVT.
Current or recent gastrointestinal ulceration; presence of malignant neoplasms at high risk of bleeding; recent brain or spinal injury; recent brain, spinal, or ophthalmic surgery; recent intracranial hemorrhage; known or suspected esophageal varices; arteriovenous malformations; vascular aneurysms; major intraspinal, or intracerebral vascular abnormalities.
Interventions
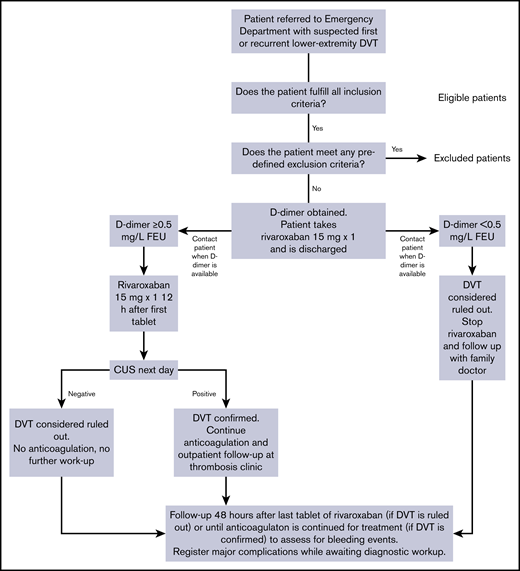
The study design is outlined in Figure 1. Dedicated study nurses and doctors screened patients for enrollment. If the patient was ≥18 years old, had not been included in the study within the past 3 months, and provided written consent, study personnel obtained pregnancy tests for women of childbearing age, as well as hemoglobin and GFR levels with point-of-care devices. If the patient did not meet any of the predefined exclusion criteria for discharge with rivaroxaban and deferred CUS as outlined in Table 1, the patient was enrolled in the study. Excluded patients remained in the Emergency Department until the relevant workup had completed.
Included patients underwent a clinical examination including assessment of the 3-tier Wells score before admission blood tests, including D-dimer, were obtained,10 as per routine management. Wells score was assessed for later analyses and did not guide further management. Patients were next administered 1 tablet of rivaroxaban 15 mg and discharged with another tablet of 15 mg to take at home. The patients were advised to contact the Emergency Department if they experienced symptom progression, symptoms of PE, or bleeding complications. Study personnel contacted patients by phone when D-dimer results were available. D-dimer was analyzed by the immunoturbidometric method of STA-Liatest D-Di Plus (Stago Diagnostics, Asnieres, France) on the STA-R Evolution Analyzer. If D-dimer levels were <0.5 mg/L fibrinogen-equivalent units (FEUs), then DVT was considered to be ruled out. Patients were instructed not to take the second tablet of rivaroxaban and consult their family doctor for evaluation of other diagnoses.
If D-dimer levels were ≥0.5 mg/L FEUs, patients were instructed to take the second tablet of rivaroxaban 12 hours after the first. They were given an appointment for whole-leg CUS the following morning and within 24 hours of inclusion. The final diagnosis was based on this CUS examination. As such, we considered DVT ruled out in patients who had either negative D-dimer or where CUS did not reveal DVT. The safety of ruling out venous thromboembolism (VTE) on the basis of a negative D-dimer without clinical pretest probability assessment is an investigational practice with a low risk of a missed diagnosis suggested by some studies,11,12 including a prior study of our department.13 Validation of these findings was outside the scope of the current study.
Patients were contacted by phone 48 hours after taking the last tablet of rivaroxaban to assess for bleeding events. The 48-hour range was chosen based on the time needed to eliminate rivaroxaban.14 For patients who had been diagnosed with VTE and therefore had continued anticoagulation treatment, we assessed for bleeding events in the interval preceding DVT being confirmed and anticoagulation continued for treatment purposes. Additionally, we registered whether the patients who had been diagnosed with VTE had experienced progressive symptoms or symptoms or signs of PE before the diagnosis was confirmed.
Objectives and end points
The main objective of the study was to determine the safety of rivaroxaban in the prediagnostic phase of DVT workup, ie, the interval from when the patient was included until the diagnosis could be confirmed or ruled out.
The secondary objectives were to determine the overall safety and feasibility of the deferred workup strategy.
The primary outcome was the proportion of patients in whom DVT had been ruled out who suffered a major bleeding incident within 48 hours after ingesting the last tablet of rivaroxaban or otherwise until DVT had been confirmed and anticoagulation continued for treatment purposes. Bleeding events were classified according to the criteria of the Control of Anticoagulation Subcommittee of the International Society on Thrombosis and Haemostasis,15,16 whereby major bleeding is defined as fatal or symptomatic bleeding in a critical area or organ and/or bleeding causing a fall in hemoglobin level of ≥20 g/dL or leading to transfusion of ≥2 U whole blood or red cells.
The secondary safety outcomes were the incidence of clinically relevant nonmajor and minor bleeding events16 and major complications while awaiting CUS. Major complications were defined as the worsening of DVT symptoms or the development of symptoms or signs of PE (number of patients with major complications/number of patients diagnosed with VTE). This was based on any of the following criteria occurring: hemodynamic instability, worsening of vital signs (increased respiratory or resting pulse rate after 15 minutes of rest, decrease in resting systolic blood pressure, or decrease in SaO2 by >20% compared with baseline), increased leg circumference by >10%, and/or progressive symptoms, such as worsening pain or dyspnea until VTE was confirmed.
Moreover, we assessed the rate of VTE events within 3 months of follow-up in patients in whom DVT was ruled out at baseline either by negative D-dimer or negative CUS.
The secondary feasibility outcome was the proportion of patients who did not meet any of our predefined exclusion criteria for deferred CUS with rivaroxaban (Table 1) and were included in the study out of all otherwise eligible patients (patient age ≥18 years, able and willing to provide written consent, and not included within the past 3 months).
Statistical analyses
We estimated the expected bleeding rate based on the number of patients in studies on LMWH (n = 729 patients), in whom no major bleeding events were observed.3-5,7 This yielded an observed major bleeding rate of 0% and 95% confidence interval (CI) of 0.0% to 0.6%. Based on this, we assumed a frequency of observed major bleeding with rivaroxaban at ≤0.2% with a 1-sided 95% confidence limit of <0.8%. With these assumptions, a significance level of 5% and a power of 80% (β = 20%), we set the sample size at 620 patients.
The study outcomes are expressed as proportion in descriptive summary percentage and 95% CIs, calculated by Clopper-Pearson exact method.17 Baseline characteristics are expressed in median with interquartile range (IQR) for continuous variables and numbers and percentages for categorical variables. The software package used was IBM SPSS Statistics, Version 25.
Safety and adjudication
One fatal bleeding event or 2 nonfatal, major bleeding events were set as criteria for stopping the study. An independent adjudication committee would determine causes of bleeding or death, and an independent safety committee was responsible for terminating the study if deemed necessary.
Ethics approval
The study was approved by the Regional Committee for Medical and Health Research Ethics (REK), reference number 2014/377. The researchers adhered to the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and the International Conference on Harmonisation–Good Clinical Practice Guideline.
Results
Baseline characteristics
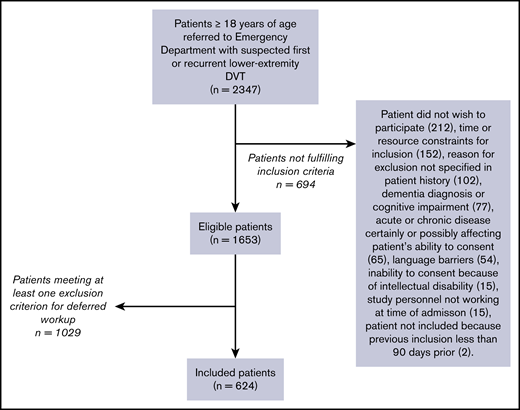
Figure 2 provides an overview of the patient flow. Screening of consecutive outpatients mainly took place when dedicated study personnel recruited from the pool of the Emergency Department staff was working, 8 to 13 hours of the day during weekdays.
Two thousand three hundred forty-seven patients who presented to the Emergency Department with suspected DVT were screened for participation. Of these, 1653 patients (70.4%; 95% CI, 78.5-72.3) were ≥18 years of age, provided written consent, and were not included in the past 3 months.
One thousand twenty-nine of the 1653 patients (62.3%; 95% CI, 59.9-64.6) met ≥1 exclusion criterion for deferred workup with rivaroxaban (Table 2). Of these 1029 patients, 185 patients (18.0%; 95% CI, 15.7-20.5) were diagnosed with DVT.
Exclusion criteria for deferred workup in eligible patients (N = 1653)
| . | n (%) with criterion . | n (%) with only criterion . |
|---|---|---|
| Anticoagulation* | 447 (27.0) | 329 (19.9) |
| Empiric anticoagulation treatment in primary care before referral | 328 (19.8) | 245 (14.8) |
| Regular prescription of anticoagulation treatment | 129 (7.8) | 76 (4.6) |
| Both empiric and regular use of anticoagulation treatment | 10 (0.6) | 8 (0.5) |
| Patient objects to discharge | 192 (11.6) | 117 (7.1) |
| Physician deems discharge unsafe | 189 (11.4) | 89 (5.4) |
| Suspected active or recent bleeding | 70 (4.2) | 11 (0.7) |
| GFR <45 mL/min per 1.73 m2 | 66 (4.0) | 17 (1.0) |
| Active cancer or chemotherapy within the past 6 mo | 65 (3.9) | 23 (1.4) |
| Major risk factors for bleeding | 59 (3.6) | 4 (0.2) |
| Logistical challenges for at-home observation | 45 (2.7) | 16 (1.0) |
| Workup expected to complete within 2 h | 44 (2.7) | 28 (1.7) |
| Medications possibly interacting with rivaroxaban | 44 (2.7) | 10 (0.6) |
| Hemoglobin <11 g/dL and/or thrombocytes <100 × 109/L | 39 (2.4) | 6 (0.4) |
| Pregnancy or lactation | 23 (1.4) | 14 (0.8) |
| Suspected concurrent PE | 22 (1.3) | 2 (0.1) |
| Comorbidities necessitating admission | 20 (1.2) | 2 (0.1) |
| Suspected ischemia or eligibility for thrombolysis | 4 (0.2) | 0 (0) |
| Liver disease† | 2 (0.1) | 0 (0) |
| . | n (%) with criterion . | n (%) with only criterion . |
|---|---|---|
| Anticoagulation* | 447 (27.0) | 329 (19.9) |
| Empiric anticoagulation treatment in primary care before referral | 328 (19.8) | 245 (14.8) |
| Regular prescription of anticoagulation treatment | 129 (7.8) | 76 (4.6) |
| Both empiric and regular use of anticoagulation treatment | 10 (0.6) | 8 (0.5) |
| Patient objects to discharge | 192 (11.6) | 117 (7.1) |
| Physician deems discharge unsafe | 189 (11.4) | 89 (5.4) |
| Suspected active or recent bleeding | 70 (4.2) | 11 (0.7) |
| GFR <45 mL/min per 1.73 m2 | 66 (4.0) | 17 (1.0) |
| Active cancer or chemotherapy within the past 6 mo | 65 (3.9) | 23 (1.4) |
| Major risk factors for bleeding | 59 (3.6) | 4 (0.2) |
| Logistical challenges for at-home observation | 45 (2.7) | 16 (1.0) |
| Workup expected to complete within 2 h | 44 (2.7) | 28 (1.7) |
| Medications possibly interacting with rivaroxaban | 44 (2.7) | 10 (0.6) |
| Hemoglobin <11 g/dL and/or thrombocytes <100 × 109/L | 39 (2.4) | 6 (0.4) |
| Pregnancy or lactation | 23 (1.4) | 14 (0.8) |
| Suspected concurrent PE | 22 (1.3) | 2 (0.1) |
| Comorbidities necessitating admission | 20 (1.2) | 2 (0.1) |
| Suspected ischemia or eligibility for thrombolysis | 4 (0.2) | 0 (0) |
| Liver disease† | 2 (0.1) | 0 (0) |
Regular prescription or empiric anticoagulation for suspected DVT.
Associated with coagulopathy or other bleeding risk.
Renal function was assessed with the point-of-care device in 388 patients. In the remaining patients, laboratory renal function results were either available at inclusion or the physician attending preferred to wait for these. All 388 patients either had previously known renal function impairment or GFR >45 mL/min per 1.73 m2.
Six hundred twenty-four patients (37.7%; 95% CI, 35.4-40.1) were included. Their baseline characteristics are summarized in Table 3. Median age was 65 years (IQR, 54-73), and 342 patients (54.8%; 95% CI 50.8-58.8) were female. One hundred nineteen patients (19.1%; 95% CI, 16.1-22.4) were diagnosed with DVT at baseline. Of these, 89 (74.8%; 95% CI, 66.0-82.3) were proximal and 30 (25.2%; 95% CI, 17.7-34.0) were isolated distal thromboses. D-dimer was positive in 475 patients (76.1%), negative in 143 patients (22.9%), and missing in 6 patients (1.0%). In patients with positive D-dimer, 137 (28.8%), 261 (54.9%), and, 77 (16.2%) patients were classified as high, moderate, and low probability, respectively. In patients with negative D-dimer, 20 (14.0%), 83 (58.0%), and 40 (28.0%) patients were classified as high, moderate, and low probability, respectively.
Demographics and characteristics
| . | Included patients (N = 624) . |
|---|---|
| Age, median (IQR), y | 65 (54-73) |
| Female sex, n (%) | 342 (55) |
| Symptoms duration, median (IQR), d | 7 (4-14) |
| Positive D-dimer,* n (%) | 475 (76) |
| Low probability for DVT,† n (%) | 117 (19) |
| Moderate probability for DVT,† n (%) | 348 (56) |
| High probability for DVT,† n (%) | 159 (25) |
| DVT at baseline, n (%) | 119 (19) |
| Risk factors for VTE, n (%) | |
| Previous VTE | 91 (15) |
| VTE in first-degree relatives | 118 (19) |
| Current smoking | 129 (21) |
| Recent travel >4 h | 194 (31) |
| Recent inactivity | 84 (14) |
| Surgery within past 12 wk | 37 (6) |
| Known thrombophilia | 17 (3) |
| Hormonal contraceptives | 23 (4) |
| Hormone-replacement therapy | 46 (7) |
| . | Included patients (N = 624) . |
|---|---|
| Age, median (IQR), y | 65 (54-73) |
| Female sex, n (%) | 342 (55) |
| Symptoms duration, median (IQR), d | 7 (4-14) |
| Positive D-dimer,* n (%) | 475 (76) |
| Low probability for DVT,† n (%) | 117 (19) |
| Moderate probability for DVT,† n (%) | 348 (56) |
| High probability for DVT,† n (%) | 159 (25) |
| DVT at baseline, n (%) | 119 (19) |
| Risk factors for VTE, n (%) | |
| Previous VTE | 91 (15) |
| VTE in first-degree relatives | 118 (19) |
| Current smoking | 129 (21) |
| Recent travel >4 h | 194 (31) |
| Recent inactivity | 84 (14) |
| Surgery within past 12 wk | 37 (6) |
| Known thrombophilia | 17 (3) |
| Hormonal contraceptives | 23 (4) |
| Hormone-replacement therapy | 46 (7) |
D-dimer missing in n = 6.
According to the 3-tier Wells score.
Enrollment ended when reaching the predefined sample size.
Study outcomes
The study outcomes are summarized in Table 4. All patients were followed up according to study protocol. There were no major bleeding events in patients in whom DVT was ruled out or in patients with confirmed DVT (0/624 [0.0%]; 1-sided 95% CI < 0.4). Moreover, no patients (0/624 [0.0%]; 95% CI, 0.0-0.6) suffered worsening of presenting symptoms or developed symptoms or signs of PE in the interval between inclusion until VTE was diagnosed. There were 505 patients in whom DVT was ruled out at baseline either by negative D-dimer or negative CUS. No patients with initial negative CUS were diagnosed with VTE within 3 months of follow-up for a 3-month VTE rate of 0.0% (95% CI, 0.0-1.0). Two patients who did not undergo CUS at baseline because of negative D-dimer were diagnosed with DVT for a 3-month VTE rate of 1.4% (95% CI, 0.2-5.0). One patient was in the low-probability subgroup, and the other patient was in the high-probability subgroup.
Primary and secondary outcomes
| . | n (%) . | 95% CI . |
|---|---|---|
| Safety, bleeding events | ||
| Major | 0 (0) | <0.4 |
| Clinically relevant nonmajor | 3 (0.5) | 0.1-1.4 |
| Minor | 60 (9.6) | 7.4-12.2 |
| Major complications* | 0 (0) | 0.0-0.6 |
| Feasibility | ||
| Patients included in the study | 624 (37.7) | 35.4-40.1 |
| . | n (%) . | 95% CI . |
|---|---|---|
| Safety, bleeding events | ||
| Major | 0 (0) | <0.4 |
| Clinically relevant nonmajor | 3 (0.5) | 0.1-1.4 |
| Minor | 60 (9.6) | 7.4-12.2 |
| Major complications* | 0 (0) | 0.0-0.6 |
| Feasibility | ||
| Patients included in the study | 624 (37.7) | 35.4-40.1 |
Worsening of symptoms, development of symptoms, or signs of PE between inclusion and diagnosis of VTE.
Notably, 1 patient suffered a major bleeding event, which was adjudicated not to meet the primary end point for 2 reasons. First, the patient in question was included despite experiencing melena, hence fulfilling the exclusion criterion for scheduled workup of active bleeding (Table 1). Second, the event occurred beyond the predefined study period, 70 hours after taking 1 tablet of rivaroxaban. The event therefore was adjudicated as protocol violation. Taking it into account would have yielded a major bleeding rate of 0.2% (1-sided 95% CI <0.7).
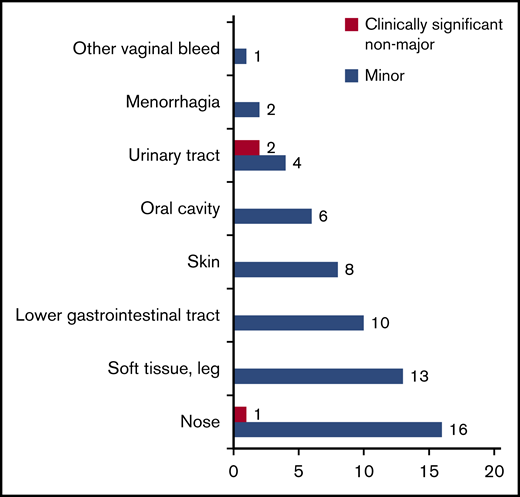
In total, 63 minor and clinically relevant nonmajor bleedings occurred in 61 patients (10.1%; 95% CI, 7.8-12.7). Figure 3 provides an overview of number and origin of bleeding events. In patients where DVT was confirmed, there were 9 bleeding events in 9 patients (7.6%; 95% CI, 3.5-13.9). Of these, all were minor but for one clinically relevant nonmajor incident of hematuria. In patients where DVT was ruled out, there were 54 bleeding events in 52 patients (10.5%; 95% CI, 8.0-13.0). Of these, 52 were minor and 2 were adjudicated as clinically relevant nonmajor: 1 incident of epistaxis and 1 incident of hematuria.
All 60 minor bleeding events were mild, and at follow-up, 37 had recovered spontaneously and 23 were recovering. Of the bleedings still recovering, 1 patient had experienced increased though currently diminishing menstrual bleeding, while the remainder were either recovering subcutaneous hematomas (n = 9) or hematomas detected on CUS (n = 13) while the patients were worked up for DVT.
As for the secondary feasibility outcome, 1029 patients (62.3%) met at least 1 exclusion criterion for scheduled workup. Numbers and percentages meeting the different criteria are detailed in Table 2. Three hundred twenty-eight patients (19.8%; 95% CI, 17.9-21.9) had received empiric LMWH in primary care before referral, and it was the only exclusion criterion for 245 patients (14.8%; 95% CI, 13.1-16.6). Of the 328 patients who had received LMWH in primary care before referral, weight and dosage were available in patients’ records for 286 patients. Of these, 159 patients (55.6%; 95% CI, 49.6-61.4) had received at least the minimum therapeutic dosage for VTE.
Eighty-nine patients (5.4%) only met the criterion of “physician deems discharge unsafe," and the 73 patients with documented reasons did so because more likely or concurrent diagnoses needed management (n = 51), the Emergency Department physician attending deemed DVT unlikely after evaluation (n = 14), or they had pronounced DVT symptoms needing instant consideration (n = 8).
Discussion
Principal findings
We found that deferring CUS for up to 24 hours with therapeutic rivaroxaban is a safe and feasible strategy for patients with suspected DVT. While others have suggested that direct oral anticoagulants may be safe in the diagnostic workup of VTE,18 our study is to our knowledge the first prospective trial to assess this question.
There have been no clinical trials addressing the benefits and disadvantages of administering vs withholding anticoagulants in prolonged workup of DVT, and guidelines have a grade 2C level of recommendation.2 The main benefit of empiric anticoagulation is faster initiation of treatment in patients who are ultimately diagnosed with DVT, preventing proximal extension of the clot, PE, and perhaps postthrombotic syndrome. A disadvantage is system and patient cost, albeit subject to varying legislation between countries.19-21 However, the main potential disadvantage is risking bleeding complications in patients without DVT. The favorable safety profile demonstrated in this and similarly designed studies applying LMWH or unfractionated heparin for deferred workup, all yielding no major bleedings,3-9 support the use of empiric anticoagulation in prolonged workup of DVT. Our conclusion is in line with suggestions from guidelines and what is, in our experience, already a relatively commonplace practice despite the grade 2C evidence. A recent article describing anticoagulation therapy patterns in the ∼10 000 patients included in the large prospective observational GARFIELD-VTE registry demonstrated that 13.4% of patients had started anticoagulant treatment before the diagnosis being confirmed, of whom 17.0% started on a direct oral anticoagulant without parenteral bridging despite guidelines recommending LMWH in this setting.22
A fair amount of patients (10.1%) experienced a bleeding event (Figure 3), of which 95.2% were minor. Minor or “nuisance” bleeding events lack a rigorous definition, may be underreported in large data trials,16 and may be more open to interpretation and the physician’s inclination to report. We believe several aspects of our study contributed to the observed bleeding rate. First, we documented all bleeding events to avoid reporting bias. Several of these were trivial, such as light recurrent or light epistaxis when blowing the nose (8/15), habitual or light gum bleed when brushing teeth (3/6), or easier bruising (n = 9). Second, we reported bleeding events despite probable causative factors. For instance, judging by presenting history and symptoms, it is likely that many of the lower-extremity hematomas detected by CUS were present before the patient received rivaroxaban. However, as CUS was performed after the patients had taken rivaroxaban, we cannot conclude whether hematomas preceded rivaroxaban administration. Third, the study design involving thorough patient information and follow-up may have affected the patient’s propensity for reporting bleeding events.
Summarized, we cannot conclude whether the observed proportion of 10.1% is particularly high or low, but we believe there was an overall low threshold for reporting bleeding events and overall low clinical relevance of the majority of the bleeding events.
This notwithstanding, our patient with melena adjudicated as protocol violation underlines the importance of precluding patients at high risk of bleeding from empiric anticoagulation treatment. Although we do not know the natural progression in this case, rivaroxaban likely exacerbated or accelerated the course of the patient’s signs and symptoms.
Regarding feasibility of the strategy, 37.7% of patients did not meet any of our predefined exclusion criteria for deferred workup with rivaroxaban (Table 2), and we believe more patients would be included in future implementation. The 245 patients with empiric LMWH in primary care as their only exclusion criterion would likely add to the eligible proportion if scheduled workup had been standard management and could have increased the number to 869 patients (52.6%; 95% CI, 50.1-55.0).
Eighty-nine patients were excluded because the treating physicians deemed discharge unsafe. In most cases, this was because other workup was necessary to rule out other conditions, or the suspicion of DVT was withdrawn upon closer look. In a clinical setting, the criteria for deferred workup would only apply to patients with a primary DVT suspicion in the first place. Therefore, we consider that this criterion will not exclude as many patients in future implementation.
A few other aspects of our predefined criteria merit mentioning. For future implementation, we would recommend establishing hemoglobin levels and pregnancy status for eligible women through point-of-care devices. Estimating point-of-care GFR did not yield previously unknown GFR <45 mL/min per 1.73 m2. As such, we believe there is no need to determine GFR levels, as long as the patient’s history is not suggestive of compromised renal function. As for platelets, we did not routinely await laboratory results before administering rivaroxaban and instead asked all patients about signs suggestive of thrombocytopenia.
We have previously found that stand-alone D-dimer may safely rule out DVT with a 3-month VTE rate of 0.3% (95% CI, 0.1-1.9) in 298 patients with negative D-dimer.13
In the current study, there was a low 3-month VTE rate with a higher upper limit of the 95% CI than yielded in our previous study. Regardless, the aim of this study was to explore whether the diagnostic workup of DVT could safely be deferred for up to 24 hours with empiric rivaroxaban without adversely affecting patients in this time frame, not to determine whether it is safe to withhold CUS in select patients altogether. No patients experienced major complications from deferring CUS, whereas stand-alone D-dimer in ruling out DVT need validation before their safety in routine use may be considered as supported by the findings of this study.
Several benefits of deferring CUS until hospital on-hours have been described in previous studies conducted at university and community hospitals. Arnaoutakis et al estimated an annual cost savings of ∼$12 000 with the termination of off-hour imaging without affecting patient outcomes, possibly a higher figure if other reimbursements had been taken into account as well.23 Potential cost savings was also suggested by Bauld et al.9 Langan et al found increased retention of sonographers after off-hour CUS had decreased by 89%, which could possibly be attributed to satisfaction with diminished off-hour workload.7 Improved sonographer satisfaction was also a benefit noted in a study conducted by Chaer et al, as well as more laboratory time for inpatient studies.4
Our study has several novel implications elaborating on the findings of previous trials.
First, it introduces an alternative empiric anticoagulant for patients with suspected DVT, which has the benefits of oral administration, potentially lower cost, as well as standard, not weight-required dosing. The latter may be of particular benefit, as only 56% of patients in our study received the minimum therapeutic dosage of LMWH according to weight. Second, our criteria will aid primary and emergency care physicians in deciding which patients can wait for referral until hospital on-hours. Deferred workup strategies may reduce wait time for patients and improve resource use during hospital peak or off-hours, possibly channeling 40% to 50% of patients to on-hour workup.
Strengths and limitations
The strengths of our study include its prospective design, standardized assessment, collection of data, and classification of bleeding events for all patients. No patients were lost to follow-up, and outcomes were adjudicated by an independent adjudication committee. Unlike other studies, we included patients with suspected recurrent DVT, who comprised a considerable proportion of the study cohort of 15%. Moreover, we applied the same diagnostic strategy to all patients irrespective of pretest likelihood of DVT (to our knowledge only the second study to do so).9 As such, our findings suggest that rivaroxaban may safely be administered to low-risk patients and that CUS may safely be deferred for up to 24 hours in high-risk patients. Both are groups where the benefits to risk ratio might be more uncertain and where it is particularly desirable to avoid adverse effects of the respective interventions.
A limitation of our study is that it was conducted in a single center. Hence, external validity of our findings remains to be established. Moreover, we performed a single-arm study rather than a randomized controlled trial, which would have required a larger sample size than feasible for the scope of this study. Lastly, our exclusion criteria for scheduled workup limit generalizability to the whole outpatient population, and our findings cannot be extrapolated to patients with suspected concurrent PE, cancer, lower hemoglobin, or GFR than predefined. Ultimately, there were several reasons for why we erred on the side of caution at the expense of generalizability. First, based on previous studies from our department, we expected that ∼80% of the included patients would end up having DVT ruled out,13 and less conservative criteria would particularly disfavor these patients in case of bleeding. Second, there have to our knowledge not been studies randomizing patients with suspected DVT to receive or not empiric treatment, and the uncertainty of potential benefits warranted a cautious approach. Lastly, as patients were discharged and not observed in hospital, we decided that additional safety considerations were necessary.
Importantly, a strategy involving deferred workup and empiric anticoagulation will likely always be inappropriate for a substantial proportion of any outpatient population depending on the characteristics of the population in question.
However, some of the criteria might be modified for future implementation. A high GFR threshold was chosen as a moderately reduced renal function of creatinine clearance <50 mL/min warrants dose reduction in certain situations. Hemoglobin <11 g/dL was raised during the study from <10 g/dL as an extra safety precaution after the inclusion of a patient with ongoing melena. Lastly, cancer patients were excluded, as the role of direct anticoagulants in these patients was unknown at the time of designing the study.
In conclusion, we found that deferring CUS with therapeutic doses of rivaroxaban in patients with suspected DVT where CUS was not readily available was not associated with major bleeding or other major adverse events. Our strategy resulted in 37.7% of patients being discharged to await further diagnostic considerations at home. The strategy may simplify the diagnostic workup of DVT while improving resource use.
Deidentified individual participant data that underlie the reported results may be requested after publication to investigators, whose proposed use of the data has been approved by an independent review committee identified for this purpose to achieve aims in the approved proposal. Information regarding accessing data and obtaining study protocol can be directed to the corresponding author, Synne G. Fronas (e-mail: s.g.fronas@gmail.com).
Acknowledgments
The authors would like to thank Brynhild Jørgensen, Jens Stene-Johansen, Mohamed Qarbosh, Håkon Rørberg, Mathias Perminow, Adeline Svendsen, and Bitte Therese Broch for enrollment and follow-up of patients; Åse-Berit Mathisen for laboratory assistance; and Heidi Hassel Pettersen and Christina Roaldsnes for study coordination.
The study was supported in part by research funding from Bayer, the South-Eastern Norway Regional Health Authority, and the Østfold Hospital Trust.
Authorship
Contribution: S.G.F. participated in data acquisition and management of the trial, analyzed and interpreted the data, and drafted and revised the manuscript; A.E.A.D. participated in protocol drafting, study management, interpretation, and revision of the manuscript; H.S.W. participated in protocol drafting, data interpretation, and revision of the manuscript; C.T.J. participated in data acquisition and daily management of the study and revision of the manuscript; J.G. participated in study management and drafting and revising the manuscript; N.R. participated in data acquisition and study management and revision of the manuscript; R.H. participated in study design, input regarding end-point selection and statistical analyses, and revision of the manuscript; F.A.K. participated in data analyses, interpretation, and revision of the manuscript; and W.G. was trial manager and responsible for the design, planning, initiation, and conduction of the study and participated in data acquisition and interpretation and revision of the manuscript.
Conflict-of-interest disclosure: S.G.F. and C.T.J. received grants from Bayer, the South-Eastern Norway Regional Health Authority, and the Østfold Hospital Trust to the conduct of the study (no personal fees). A.E.A.D. received grants and personal fees from Pfizer AS and personal fees from Bristol-Myers Squibb and Novartis Norway AS outside the submitted work. F.A.K. received research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, MSD and Actelion, the Dutch Heart foundation, and the Dutch Thrombosis association outside the submitted work. W.G. received grants from Bayer, the South-Eastern Norway Regional Health Authority, and Østfold Hospital Trust for the conduct of this study; received grants from Bayer, Bristol-Myers Squibb, and Novartis outside the submitted work; and participated on advisory boards for Amgen and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Synne G. Fronas, Clinic of Internal Medicine, Østfold Hospital Trust, Kalnesveien 300, 1714 Grålum, Norway; e-mail: s.g.fronas@gmail.com.