Key Points
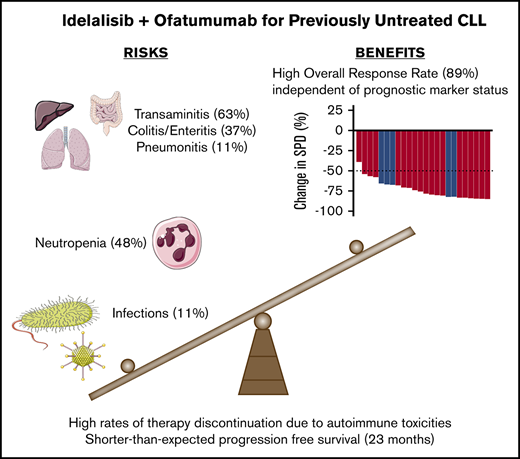
Idelalisib plus ofatumumab have a high ORR but short PFS when used for treatment-naïve CLL.
The short PFS is likely a result of high rates of therapy discontinuation in the setting of transaminitis, colitis, and infections.
Abstract
PI3 kinase (PI3K) activity is critical for survival of neoplastic B cells in patients with chronic lymphocytic leukemia (CLL). Blockade of PI3K signaling with idelalisib is effective for the treatment of relapsed CLL in combination with the anti-CD20 antibody ofatumumab. In this single-arm, open-label, nonrandomized phase 2 study, we investigated the efficacy and safety of idelalisib with ofatumumab in 27 patients with treatment-naïve CLL in need of therapy. Patients were planned to receive idelalisib for 2 monthly cycles, then idelalisib and ofatumumab for 6 cycles, followed by idelalisib indefinitely. The study was closed early and all patients ceased therapy when an increased rate of death as a result of infection was observed on other first-line idelalisib trials. Median time on therapy was 8.1 months, and median duration of follow-up was 39.7 months. We previously reported high rates of hepatotoxicity in a smaller cohort of patients in this trial; toxicities necessitated therapy discontinuation in 15 patients after a median of 7.7 months. The most frequent grade ≥3 adverse events were transaminitis (52% of patients), neutropenia (33%), and colitis/diarrhea (15%). The best overall response rate (ORR) was 88.9%, including 1 complete response. Median progression-free survival (PFS) was 23 months (95% confidence interval [CI], 18-36 months); 11 patients have not yet required second-line therapy. Idelalisib and ofatumumab demonstrated an unacceptable safety profile in the first-line setting, which resulted in a short PFS despite a high ORR. Future development of PI3K inhibitors for use in treatment-naïve CLL will require novel approaches to mitigate toxicities. This trial was registered at www.clinicaltrials.gov as #NCT02135133.
Introduction
Small molecule targeted therapies have revolutionized the management of both previously untreated and relapsed chronic lymphocytic leukemia (CLL). However, these therapies, including ibrutinib, idelalisib, and venetoclax, have limitations when used as single agents. For example, partial remissions (PRs) are the best outcome for the majority of patients who receive monotherapy with kinase inhibitors, even after a prolonged time on treatment.1-4 Such drugs are thus routinely administered indefinitely because they cannot achieve a disease-free state. One potential approach to overcoming such limitations is combination therapy. Historically, the combination of chemotherapy with anti-CD20 monoclonal antibodies achieved durable responses and improved overall survival (OS) with time-limited therapy, thereby setting new standards for the first-line treatment of CLL.5,6 The addition of anti-CD20 monoclonal antibodies to targeted agents could therefore also have enhanced efficacy with nonoverlapping toxicities.
The small molecule inhibitor idelalisib and the anti-CD20 monoclonal antibody ofatumumab are 2 attractive drugs to pair for the treatment of CLL. Idelalisib (also known as GS-1101 or CAL-101) is a PI3Kδ isoform-selective inhibitor. The PI3K pathway is constitutively active in CLL, and preclinical data demonstrate that blockade of PI3K signaling by idelalisib is toxic to CLL cells.7 Inhibition of PI3K also leads to a redistribution of neoplastic lymphocytes from the lymph nodes into the peripheral blood where, in theory, the lymphocytes may be more susceptible to cell death induced by a circulating anti-CD20 antibody.8 Ofatumumab is a fully human anti-CD20 antibody that binds to a different CD20 epitope than rituximab does and induces more potent complement-dependent cytotoxicity than rituximab.9,10 The combination of idelalisib with anti-CD20 antibodies has been primarily explored in the relapsed/refractory setting. Idelalisib in combination with rituximab improves progression-free survival (PFS) and OS compared with rituximab monotherapy,11 and this drug combination is now approved by the US Food and Drug Administration for the treatment of relapsed CLL. In addition, in the relapsed setting, idelalisib and ofatumumab doubled PFS (16.3 vs 8.0 months) and quadrupled the overall response rate (ORR) (75.3% vs 18.4%) when compared with ofatumumab alone.12 These results supported the exploration of the combination of idelalisib and ofatumumab for first-line treatment of CLL, as reported here.
After enrolling the first few patients on this trial, we noticed high rates of an autoimmune hepatitis in patients during idelalisib monotherapy and reported this toxicity in an earlier publication.13 With amendments to the protocol to increase monitoring and require early initiation of steroids to treat the transaminitis, we were able to continue enrollment. However, in early 2016, unpublished analyses by Gilead Pharmaceuticals of other first-line trials of idelalisib-containing combination regimens identified increased rates of serious adverse events and fatalities, generally because of infections. Therefore, in March 2016, all ongoing clinical trials examining idelalisib for the first-line treatment of B-cell malignancies were stopped, including the trial reported here. Despite the fact that development of idelalisib as first-line therapy for CLL has been halted, our experience with this trial is informative. First, other PI3Kδ inhibitors with different toxicity profiles are being developed, and this experience with idelalisib can inform expectations regarding efficacy of these agents in combination with anti-CD20 monoclonal antibodies. Second, the forced cessation of idelalisib provides insight into the consequences of time-limited kinase inhibitor therapy in the absence of a complete response (CR) or minimal residual disease (MRD) negativity. In this light, we present safety and efficacy data from our phase 2 study of idelalisib and ofatumumab as therapy for previously untreated CLL.
Methods
Study design and participants
For this single-arm, open-label, nonrandomized phase 2 trial, we enrolled patients at 3 sites within the Dana-Farber Harvard Cancer Center (DF/HCC; Dana-Farber Cancer Institute [DFCI], Massachusetts General Hospital, and Beth Israel Deaconess Medical Center). Expansion to sites of the CLL Research Consortium (CRC) was planned but never occurred because of the observed toxicity. Eligible patients were required to have a diagnosis of CLL/small lymphocytic lymphoma in need of therapy according to International Workshop on Chronic Lymphocytic Leukemia (iwCLL) 2008 criteria14 but must not have received any prior therapy for CLL. In addition, all patients were required to be age 18 years or older and to have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower. Patients with any degree of cytopenia, as well as any karyotype or molecular abnormalities, were eligible. Normal renal function (creatinine <2 × the upper limit of normal [ULN]) and normal liver function (total bilirubin <1.5 × ULN, alanine aminotransferase and aspartate aminotransferase <2.5 × ULN, subsequently amended to require an alanine aminotransferase strictly less than the ULN) were required. Exclusion criteria included other active malignancies, active infection with HIV, hepatitis B or hepatitis C, active chronic infections requiring treatment, or concurrent administration of the equivalent of >20 mg prednisone per day for any indication. The DF/HCC institutional review board approved the protocol (see supplemental Data), and written informed consent was obtained in accordance with the Declaration of Helsinki from all patients before enrollment.
The study opened to enrollment on 16 June 2014, but was temporarily closed to accrual on 13 August 2014, after the simultaneous development of severe transaminitis in 2 of the initial patients who received study treatment. After amendment of the protocol to include more frequent monitoring of liver function tests and early initiation of steroids for any transaminase abnormalities, the study was reopened in October 2014. On 14 March 2016, the study was permanently closed to new accrual at the request of Gilead Pharmaceuticals because of reports of increased adverse events, including death, in other first-line studies of idelalisib. The day of database lock for the analysis presented here was 19 March 2018.
Procedures
Baseline assessment included history, physical examination, laboratory assessments, bone marrow biopsy, and imaging by computed tomography (CT). Blood samples obtained from enrolled patients were processed and stored at DFCI and also at the CRC Tissue Core at the Moores Cancer Center at the University of California San Diego. ZAP-70 status and immunoglobulin heavy chain variable (IGHV) mutation status were assessed by the CRC Tissue Core per established criteria.15,16 Patients that possessed a leukemic clone with an IGHV sequence that differed by >2% from the germline sequence were classified in the mutated IGHV category. Patients that possessed ZAP-70 expression in ≥20% of leukemic cells were considered to be ZAP-70 positive. TP53 mutation testing was performed by direct sequencing of exons 5-9, and NOTCH1 mutation was tested by pyrosequencing for the most common c.7541_7542delCT mutation.
A schematic of the study design is provided in supplemental Figure 1. All patients received two 28-day cycles of idelalisib lead-in monotherapy, followed by 6 cycles of combination therapy with ofatumumab, followed by idelalisib indefinitely in all participants without excessive toxicity or progressive disease. Idelalisib was administered orally at a dose of 150 mg twice per day. Ofatumumab dosing began with an IV 300-mg dose on cycle 3 day 1, followed by a dose of 1000 mg once per week for 7 weeks, and then a 1000-mg dose every 4 weeks for 16 weeks (6 months total).
The study protocol (supplemental Data) describes dose modifications for toxicities. Briefly, when the patient had an adverse event deemed by the investigator to be related to idelalisib or ofatumumab, the treatment was discontinued until the adverse event resolved to grade 1 or lower. Idelalisib was then resumed at a dose of 100 mg twice per day with the option to increase back to the starting dose after ≥4 weeks. After an amendment to the protocol in response to observed toxicity, patients who developed grade 1 transaminitis continued to receive idelalisib but were required to start prednisone at 40 mg once per day. If at any point a grade 2 or grade 3 transaminitis developed, patients were required to hold idelalisib and receive prednisone at 1 mg/kg. After normalization of the transaminitis, idelalisib could be restarted at 100 mg twice per day with 1 week of overlapping prednisone at full dose before slowly tapering off the steroid. Infection prophylaxis against Pneumocystis jirovecii and varicella zoster reactivation was initially optional, but after 2 cases of P jirovecii pneumonia in this trial, prophylaxis against P jirovecii infection and herpesvirus reactivation were required.
Restaging assessments with CT scans and a bone marrow biopsy were obtained after the completion of cycle 2 and before the initiation of cycle 10. CT scans were also performed at the end of cycles 4 and 8. Adverse events were assessed at baseline, throughout the treatment, and during the 28-day period after treatment discontinuation. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, except for hematologic toxicities which were evaluated according to iwCLL 2008 guidelines.14 Patients were removed from protocol treatment for progressive disease, unacceptable toxicity, or patient preference. Patients receiving idelalisib at the time of study cessation in March 2016 were required to stop idelalisib immediately, although they could elect to continue to receive ofatumumab monotherapy if they were within cycles 3 through 8 of therapy (n = 2).
Outcomes
The primary end point of this study was the ORR of patients with previously untreated CLL after 10 months of therapy with idelalisib and ofatumumab. Secondary end points included PFS, OS, the CR rate after 10 cycles of combination therapy, and safety analyses. To categorize responses and determine progression, we used a modification of the iwCLL 2008 criteria, which allows for a PR with lymphocytosis.14,17 Overall response was defined as CR (with or without MRD), CR with incomplete marrow recovery, PR, or PR with lymphocytosis. We defined PFS as the time from date of initiation of investigational therapy to date of the first documentation of disease progression or death irrespective of cause, whichever occurred first. We defined OS as the time from date of initiation of investigational therapy to date of death as a result any cause. Living patients were censored at date of last contact.
Statistical analysis
We considered a 75% ORR to be of interest; further testing was not to be pursued if the ORR was 55% or lower. The planned sample size was thus 50 efficacy-evaluable patients, which would be sufficient to detect a 75% response rate with 90% power and 4% type I error. The study was planned to have a Simon 2-stage design with a preliminary response assessment after 31 patients were evaluated for the primary endpoint. However, because the study closed after accruing 27 eligible patients, the response analysis described here is the first formal analysis of this study. ORR, PFS, OS, and safety analyses were performed on the intent-to-treat population. Median PFS and OS were calculated by using the Kaplan-Meier method. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC) and R v2.13.2 (the CRAN project).
Results
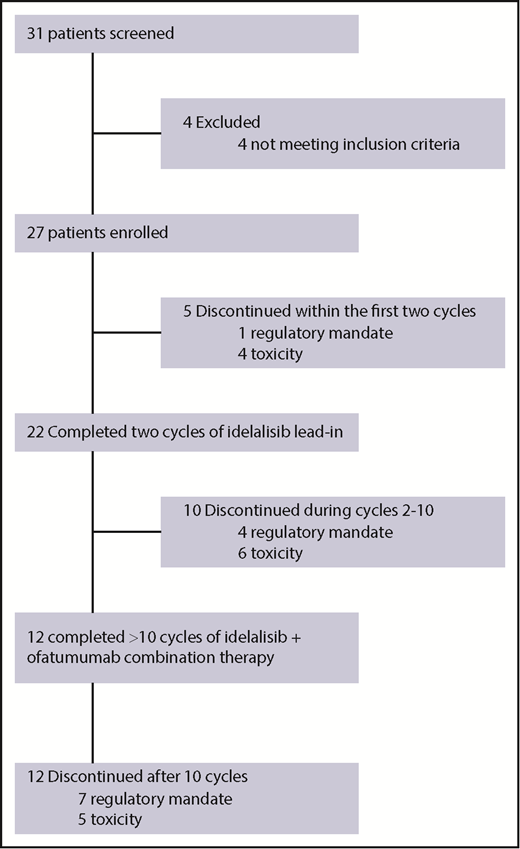
We screened 31 patients between 16 June 2014, and 14 March 2016, and 27 patients ultimately received at least 1 dose of study therapy (Figure 1). All patients had previously untreated CLL or small lymphocytic lymphoma, but the disease of 1 enrolled patient was later reclassified as lymphoplasmacytic lymphoma (this patient was included in all efficacy and safety analyses). Baseline characteristics for the 27 enrolled patients are included in Table 1. With regard to high-risk prognostic features, 5 patients (18.5%) had TP53-aberrant disease (1 patient had 17p deletion only, 2 patients had TP53 mutation only, and 2 patients had both 17p deletion and TP53 mutation). Three patients (11.1%) had 11q deletion, and 13 (48.1%) had unmutated IGHV.
Patient demographics and baseline clinical characteristics
| Characteristic . | n . | % . |
|---|---|---|
| Total number of patients | 27 | |
| Median age (range), y | 69 (57-84) | |
| Sex | ||
| Male | 21 | 77.8 |
| Female | 6 | 22.2 |
| ECOG performance status | ||
| 0 | 23 | 85.2 |
| 1 | 4 | 14.8 |
| Diagnosis | ||
| CLL | 23* | 85.2 |
| SLL | 4 | 14.8 |
| Rai stage at therapy initiation | ||
| 1 | 3 | 11.1 |
| 2 | 5 | 18.5 |
| 3 | 4 | 14.8 |
| 4 | 15 | 55.6 |
| Cytogenetics (Döhner hierarchy) | ||
| 17p deletion | 3 | 11.1 |
| 11q deletion | 3 | 11.1 |
| Trisomy 12 | 7 | 25.9 |
| 13q deletion | 8 | 29.6 |
| Normal | 6 | 22.2 |
| IGHV status | ||
| Mutated >2% | 14 | 51.8 |
| Unmutated | 13 | 48.1 |
| ZAP-70 status | ||
| Negative | 9 | 33.3 |
| Positive | 14 | 51.9 |
| Not recorded | 4 | 14.8 |
| High-risk features | ||
| 17p deletion only | 1 | 3.7 |
| TP53 mutation only | 2 | 7.4 |
| Both TP53 mutation and 17p deletion | 2 | 7.4 |
| NOTCH1 mutation | 3 | 11.1 |
| Bulky adenopathy† | 8 | 29.6 |
| Median β2 microglobulin (range), mg/L | 5.2 (2.4-14.6) |
| Characteristic . | n . | % . |
|---|---|---|
| Total number of patients | 27 | |
| Median age (range), y | 69 (57-84) | |
| Sex | ||
| Male | 21 | 77.8 |
| Female | 6 | 22.2 |
| ECOG performance status | ||
| 0 | 23 | 85.2 |
| 1 | 4 | 14.8 |
| Diagnosis | ||
| CLL | 23* | 85.2 |
| SLL | 4 | 14.8 |
| Rai stage at therapy initiation | ||
| 1 | 3 | 11.1 |
| 2 | 5 | 18.5 |
| 3 | 4 | 14.8 |
| 4 | 15 | 55.6 |
| Cytogenetics (Döhner hierarchy) | ||
| 17p deletion | 3 | 11.1 |
| 11q deletion | 3 | 11.1 |
| Trisomy 12 | 7 | 25.9 |
| 13q deletion | 8 | 29.6 |
| Normal | 6 | 22.2 |
| IGHV status | ||
| Mutated >2% | 14 | 51.8 |
| Unmutated | 13 | 48.1 |
| ZAP-70 status | ||
| Negative | 9 | 33.3 |
| Positive | 14 | 51.9 |
| Not recorded | 4 | 14.8 |
| High-risk features | ||
| 17p deletion only | 1 | 3.7 |
| TP53 mutation only | 2 | 7.4 |
| Both TP53 mutation and 17p deletion | 2 | 7.4 |
| NOTCH1 mutation | 3 | 11.1 |
| Bulky adenopathy† | 8 | 29.6 |
| Median β2 microglobulin (range), mg/L | 5.2 (2.4-14.6) |
SLL, small lymphocytic lymphoma.
One patient’s diagnosis was later reclassified to lymphoplasmacytic lymphoma.
Bulky indicates the presence of ≥1 node with a diameter ≥5 cm.
All 27 patients in the intent-to-treat analysis received idelalisib (Figure 1). Toxicities necessitated treatment discontinuation in 15 patients (4 during idelalisib lead-in, 6 during combination therapy, and 5 while receiving idelalisib monotherapy during cycles 8 and above). Toxicities that were thought to be treatment-related and resulted in treatment discontinuation included transaminitis (n = 5), enteritis/colitis (n = 5), pneumonitis (n = 2), rash (n = 2), and autoimmune hemolytic anemia (n = 1). Therapy was discontinued in the remaining 12 patients at the request of Gilead Pharmaceuticals, which halted all trials of idelalisib in the first-line setting in March 2016. Thirteen patients completed the combination portion of the study (8 cycles of idelalisib plus ofatumumab). The median time on therapy including all patients was 8.1 months (range, 0.3-20.6 months). For patients who discontinued treatment because of toxicity, the median time on therapy was 7.7 months (range, 0.7-20.2 months), whereas patients who discontinued treatment per mandate had a longer median time on therapy of 12.8 months (range, 0.3-20.7 months). The median duration of follow-up was 39.7 months (range, 13.1-44.8 months).
Because only 12 of the 27 patients completed 10 cycles of therapy (the treatment duration required for primary end point assessment), we instead report ORR as determined by best overall response. In the intent-to-treat population, the ORR was 88.9% (24 of 27 patients), including 21 PRs, 2 PRs with lymphocytosis, and 1 CR. The patient who had a CR was MRD positive. Three patients had stable disease; all of these patients received <1 cycle of idelalisib before therapy was discontinued because of toxicity (n = 2) or mandate (n = 1). All patients (n = 5) with TP53-aberrant disease and all patients with IGHV unmutated disease (n = 13) had a response to therapy. No prognostic factors (del17p, IGHV mutational status, Rai stage, or performance status) were significant predictors of response, likely reflecting the high ORR in this study.
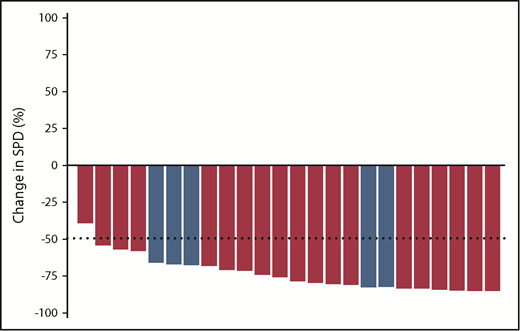
Figure 2 shows the best percent change in nodal size (sum of the product of the perpendicular diameters of the 6 largest lymph nodes) based on measurements obtained through serial CT scans. All 24 patients with lymph node enlargement at baseline and with at least 1 postbaseline efficacy assessment before second-line therapy had a reduction in nodal burden.
Best nodal response to treatment with idelalisib and ofatumumab in evaluable patients. Best nodal response for 24 patients evaluable for lymph node response (3 patients were not evaluable: 1 because of lack of baseline adenopathy and 2 because of short duration of treatment without follow-up scans before receiving the next line of therapy). Blue bars represent patients with TP53 aberrant disease. SPD, sum of the products of the perpendicular diameters of measured lymph nodes.
Best nodal response to treatment with idelalisib and ofatumumab in evaluable patients. Best nodal response for 24 patients evaluable for lymph node response (3 patients were not evaluable: 1 because of lack of baseline adenopathy and 2 because of short duration of treatment without follow-up scans before receiving the next line of therapy). Blue bars represent patients with TP53 aberrant disease. SPD, sum of the products of the perpendicular diameters of measured lymph nodes.
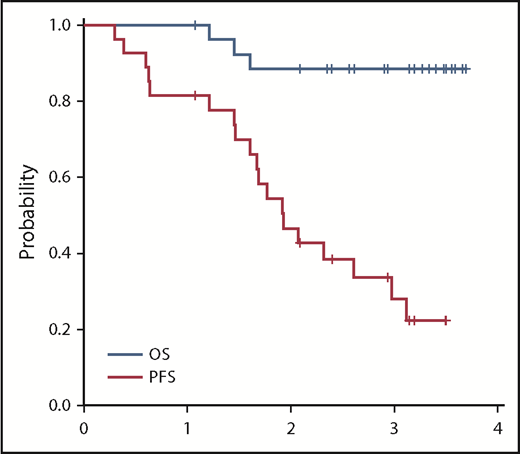
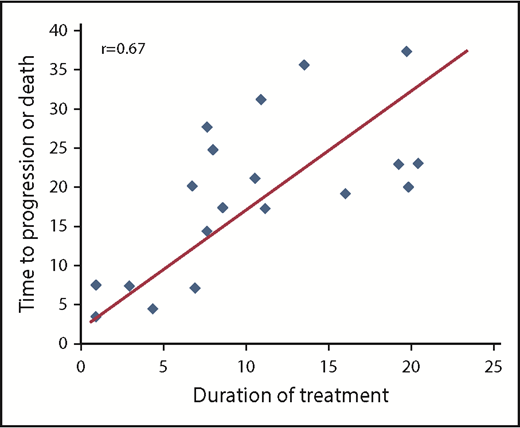
Median PFS time was 23 months (95% confidence interval [CI], 18-36 months; Figure 3). The PFS rate at 3 years was 28% (95% CI, 12%-47%). At the time of data cutoff, 9 patients had not yet had disease progression despite discontinuation of therapy, with median follow-up in these patients of 28.9 months. In univariable Cox regression analysis, the presence of TP53-aberrant disease (17p deletion, TP53 mutation, or both) was associated with a shorter PFS with a hazard ratio of 4.37 (95% CI, 1.11-17.15; P = .035). Patients with a TP53 aberration had a median PFS of 17.6 months (range, 4.6-23.2 months) vs a PFS of 24.3 months (range, 0.9-42.3 months) in those without a TP53 aberration. In addition, a duration of therapy <12 months vs ≥12 months was associated with a shorter PFS (hazard ratio, 2.86; 95% CI, 1.12-8.08; P = .038). Patients who were treated for <1 year had a median PFS of 17.6 months (range, 0.9-38.3 months) compared with a median PFS of 35.7 months (range, 19.5-42.3 months) for patients who were treated for >1 year. Consistent with this, among the 19 patients who experienced disease progression or death, duration of treatment correlated with time to progressive disease or death (r = 0.67; P = .002; Figure 4). Although in general, the duration of treatment correlated with time to progression, 5 patients who received a short duration of treatment (<8 months) had a prolonged PFS of >20 months (supplemental Table 1), with 3 of the responses still ongoing at the time of data cutoff. All 5 of these patients were IGHV mutated without 17p deletion or TP53 mutation. Given the high rates of treatment discontinuation, we also examined PFS from time of study drug discontinuation. The median time from treatment discontinuation to disease progression was 9.0 months (range, 0.2-34.0 months).
Correlation between duration of treatment and time to progression or death. Only data points from the 19 patients who had disease progression or death are included.
Correlation between duration of treatment and time to progression or death. Only data points from the 19 patients who had disease progression or death are included.
Thirteen patients have received second-line therapy after disease progression (supplemental Table 2). The median time to next treatment was 6.8 months. Although second-line treatments were varied, in general they were efficacious. For example, 7 of 8 patients who received subsequent BTK inhibitors had a PR. Of 3 patients who received subsequent chemoimmunotherapy, 2 had PRs and 1 discontinued therapy because of rash. In total, rashes to second-line therapy were noted in 4 patients, 3 of whom required discontinuation of therapy.
Median OS has not yet been reached (Figure 3). Three-year OS was 88% (95% CI, 68%-96%). No patients died during treatment; 3 patients died in the follow-up period. Two deaths (1 as a result of marasmus/malnutrition and 1 as a result of renal failure) occurred in patients who had suffered preceding colitis/enteritis that was possibly treatment related. A third death resulted from an ischemic stroke and was felt to be unrelated to investigational therapy.
Table 2 shows the adverse events reported in this study. We previously reported a high rate of transaminitis, likely autoimmune-mediated, in an earlier cohort of patients on this trial.13 Transaminitis remained frequent in this larger population, occurring in 63% of patients (grade ≥3 in 52% of patients). Onset of transaminitis showed a bimodal distribution, with 13 patients experiencing transaminitis early (at a median of 24 days on idelalisib, before introduction of ofatumumab) and the remaining 4 patients experiencing transaminitis much later (median time to onset, 133 days). Other adverse events of interest, likely related to idelalisib use, included colitis/diarrhea (37%; grade ≥3, 15%; median time to onset, 8.4 months) and pneumonitis (11%; grade ≥3, 7%; median time to onset, 117 days). Neutropenia was the second most common adverse event (48%; grade ≥3, 33%), but there were no cases of febrile neutropenia. Opportunistic infections included 2 cases of P jirovecii pneumonia (both occurred during cycle 4 in patients receiving the combination of idelalisib plus ofatumumab, and both resolved with therapy); 1 of these patients had a concomitant brain abscess resulting from an unknown organism (thought to be P jirovecii) that also resolved on trimethoprim-sulfamethoxazole. After these initial cases, pharmacologic prophylaxis for both P jirovecii and herpesviruses was mandated, and no subsequent P jirovecii infections occurred. Additional opportunistic infections included 1 instance each of Aspergillus pneumonia (cycle 6, during combination therapy), cytomegalovirus colitis (cycle 17, on idelalisib monotherapy), and herpes simplex virus esophagitis (cycle 4, during combination therapy). The rate of ofatumumab-related infusion reactions (any grade, 22%; grade ≥3, 4%) was lower than previously reported for the combination of chlorambucil and ofatumumab in previously untreated CLL (any grade, 67%; grade ≥3, 10%).18 We measured immunoglobulin levels at cycles 2, 4, and 10. Excluding patients with a monoclonal gammopathy or those receiving IV immunoglobulin (IVIg), immunoglobulin G levels decreased over time. Compared with baseline, these decreases at cycle 2 (median, −77 mg/dL; range, −550 to +151 mg/dL; P = .28) and cycle 4 (median, −81 mg/dL; range, −427 to +153 mg/dL; P = .06) were not significant, but by cycle 10, there was a significant decrease in IgG levels (median, −140 mg/dL; range −629 to +5 mg/dL; P = .03) (supplemental Figure 2).
Adverse events experienced by at least 2 patients on study, ordered by decreasing frequency of occurrence of any grade toxicity
| Event . | Any grade . | Grade 3 or 4 . |
|---|---|---|
| ALT increase | 17 (63) | 14 (52) |
| AST increase | 17 (63) | 11 (41) |
| Decreased neutrophil count | 13 (48) | 9 (33) |
| Fever | 11 (41) | 0 (0) |
| Colitis/diarrhea | 10 (37) | 4 (15) |
| Fatigue | 8 (30) | 0 (0) |
| Rash (maculopapular) | 8 (30) | 2 (7) |
| Nausea | 7 (26) | 0 (0) |
| Infusion-related reaction | 6 (22) | 1 (4) |
| Myalgia | 5 (19) | 1 (4) |
| Abdominal pain | 4 (15) | 0 (0) |
| Chills | 4 (15) | 0 (0) |
| Anemia | 3 (11) | 1 (4) |
| Constipation | 3 (11) | 0 (0) |
| Cough | 3 (11) | 0 (0) |
| Dysgeusia | 3 (11) | 0 (0) |
| Limb edema | 3 (11) | 0 (0) |
| Other infections and infestations | 3 (11) | 2 (7) |
| Pneumonitis | 3 (11) | 2 (7) |
| Vomiting | 3 (11) | 0 (0) |
| Increased alkaline phosphatase | 2 (7) | 1 (4) |
| Anorexia | 2 (7) | 0 (0) |
| Increased bilirubin | 2 (7) | 0 (0) |
| Bronchial infection | 2 (7) | 0 (0) |
| Dyspnea | 2 (7) | 0 (0) |
| Gastroesophageal reflux disease | 2 (7) | 0 (0) |
| Hyponatremia | 2 (7) | 2 (7) |
| Lung infection | 2 (7) | 2 (7) |
| Malaise | 2 (7) | 0 (0) |
| Acneiform rash | 2 (7) | 0 (0) |
| Weight loss | 2 (7) | 1 (4) |
| Decreased white blood cell count | 2 (7) | 0 (0) |
| Event . | Any grade . | Grade 3 or 4 . |
|---|---|---|
| ALT increase | 17 (63) | 14 (52) |
| AST increase | 17 (63) | 11 (41) |
| Decreased neutrophil count | 13 (48) | 9 (33) |
| Fever | 11 (41) | 0 (0) |
| Colitis/diarrhea | 10 (37) | 4 (15) |
| Fatigue | 8 (30) | 0 (0) |
| Rash (maculopapular) | 8 (30) | 2 (7) |
| Nausea | 7 (26) | 0 (0) |
| Infusion-related reaction | 6 (22) | 1 (4) |
| Myalgia | 5 (19) | 1 (4) |
| Abdominal pain | 4 (15) | 0 (0) |
| Chills | 4 (15) | 0 (0) |
| Anemia | 3 (11) | 1 (4) |
| Constipation | 3 (11) | 0 (0) |
| Cough | 3 (11) | 0 (0) |
| Dysgeusia | 3 (11) | 0 (0) |
| Limb edema | 3 (11) | 0 (0) |
| Other infections and infestations | 3 (11) | 2 (7) |
| Pneumonitis | 3 (11) | 2 (7) |
| Vomiting | 3 (11) | 0 (0) |
| Increased alkaline phosphatase | 2 (7) | 1 (4) |
| Anorexia | 2 (7) | 0 (0) |
| Increased bilirubin | 2 (7) | 0 (0) |
| Bronchial infection | 2 (7) | 0 (0) |
| Dyspnea | 2 (7) | 0 (0) |
| Gastroesophageal reflux disease | 2 (7) | 0 (0) |
| Hyponatremia | 2 (7) | 2 (7) |
| Lung infection | 2 (7) | 2 (7) |
| Malaise | 2 (7) | 0 (0) |
| Acneiform rash | 2 (7) | 0 (0) |
| Weight loss | 2 (7) | 1 (4) |
| Decreased white blood cell count | 2 (7) | 0 (0) |
Data are n (%).
Two patients, both with IGHV unmutated disease, discontinued idelalisib and had a subsequent constellation of symptoms consistent with an acute tumor flare. One patient in an ongoing PR discontinued idelalisib per the regulatory mandate after 19 cycles of therapy. Six days after stopping the drug, he reported a pruritic rash, swollen painful lymph nodes, and a temperature of 38.5°C (101.3°F). He received prednisone 60 mg once per day, and after 1 week, he reported resolution of his symptoms. He started second-line therapy 15 days later. A second patient experiencing a PR discontinued idelalisib per regulatory mandate after completing 7 cycles of therapy. Six days later, he reported left upper quadrant pain, increased nodal size, subjective fever, and fatigue. CT scans confirmed an increase in spleen and nodal size. He was started on prednisone 60 mg once per day with resolution of his symptoms 5 days later. Seventeen days later, he was started on second-line therapy.
Discussion
In this phase 2 study of 27 patients with previously untreated CLL in need of therapy, the combination of idelalisib plus ofatumumab had encouraging efficacy but a challenging safety profile that limits its potential as a first-line option. Viewed in the context of other first-line regimens containing idelalisib, the 88.9% ORR of the idelalisib-ofatumumab combination is similar to the 97% ORR published for the combination of idelalisib-rituximab for the first-line treatment of CLL in patients age 65 years or older.19 Additional ORRs presented in abstract form include 81% for idelalisib monotherapy in patients older than age 65 years20 and 79.4% for idelalisib and rituximab in patients of any age with 17p-deleted disease.21 The latter study also reported a 41% rate of grade ≥3 transaminitis and a 37% rate of grade ≥3 neutropenia, comparable to the rates in our study. Data from that trial, in conjunction with our finding that the majority of transaminitis cases occurred before the introduction of ofatumumab, suggest that it is idelalisib, and not the unique anti-CD20 antibody used in combination, that drives the development of transaminitis.
Despite similar ORRs, the median PFS of 23 months seen with idelalisib plus ofatumumab is much shorter than the median PFS previously published for first-line idelalisib plus rituximab in older patients, which was not reached after a median time on therapy of 22.4 months.19 Although this could be attributed to the different anti-CD20 antibodies or the difference in patient population enrolled (eg, Rai high-risk disease in 70% of patients here vs 42% of patients in the study by O’Brien et al,19 all ages eligible here vs only older patients in the O’Brien study), it is most likely the result of the shorter time on treatment in this trial (8.1 months). Supporting this hypothesis is our finding that a longer time to progression correlated with a longer time on treatment. Although these times are normally expected to correlate in trials in which patients remain on therapy for as long as they are responding, these times could be independent in a trial with forced cessation of therapy. We did not see a difference in PFS between patients who stopped treatment for toxicity reasons and those who stopped treatment for regulatory reasons, although this analysis is limited by confounding variables (eg, higher rates of IGHV-mutated disease in patients who experience early severe transaminitis and stop treatment because of toxicity13 ) and the relatively small number of patients enrolled on this trial.
Because all patients discontinued therapy for either toxicity or regulatory reasons, this study provides a unique perspective on the benefits and drawbacks of time-limited therapy with kinase inhibitors in patients not achieving CR or undetectable MRD. As expected, discontinuation of therapy while a patient was in PR often resulted in disease progression. However, patients who received at least 1 year of therapy had substantially longer PFS compared with those receiving therapy of shorter duration. Furthermore, disease prognostic factors predicted PFS after fixed-duration therapy. Those with TP53 aberrations had a shorter PFS, whereas in contrast, 5 patients with favorable prognostic factors, including mutated IGHV status and absence of TP53 aberrations, had a PFS of >20 months after receiving therapy for <8 months. Thompson et al22 also found that the durability of response after cessation of idelalisib treatment because of toxicity depended on the underlying disease biology. Time-limited therapy does carry the risk of a rebound effect, with 2 patients here experiencing rapid disease progression after abrupt discontinuation of idelalisib. This has also been seen with ibrutinib,23 suggesting that this risk may be shared among kinase inhibitors. In sum, these hypothesis-generating results suggest that time-limited therapy may be an option for patients with favorable disease biology, but that clinicians must be vigilant for any rebound effect at the time of therapy discontinuation. These findings also imply that when data on the outcomes of treatment cessation are reported, careful attention should be paid to the underlying prognostic factors in patients who discontinue therapy.
Limitations here include the small numbers of patients enrolled (partially attributable to early closure of the trial), the single-arm design precluding direct comparison with other first-line therapies, and the heterogeneous variation in treatment duration from patient to patient because of toxicities. Despite these limitations, this trial demonstrates that inhibition of PI3K signaling is a therapeutic approach with significant benefit in treatment-naïve CLL: 12 patients were tolerating therapy well (6 of them for >1 year) at the time they were required to stop, and no patients had disease progression while receiving the study drugs. The risk-benefit profile of PI3K inhibition in the first-line setting may be more favorable with other PI3K inhibitors in clinical development. Umbralisib and copanlisib have lower reported rates of transaminitis, colitis, and pneumonitis than idelalisib,24,25 but the mechanisms underlying these differences in rates remain unclear (the autoimmune toxicities in particular may be an on-target effect of PI3K inhibition13 ). Our findings demonstrate that although abrogation of PI3K signaling achieves responses in most untreated patients with CLL, these responses lack durability in many patients, likely because premature discontinuation as a result of toxicity (seen in 57% of patients here) leads to a shorter-than-expected PFS. Future investigative efforts must identify ways of effectively blocking this pathway while simultaneously mitigating toxicities.
The full-text version of this file contains a data supplement.
Acknowledgments
The authors thank all the patients and their families who participated in this trial and donated extra blood and bone marrow for analysis.
This work was supported by Gilead and Novartis; both companies provided their study drug at no cost. Additional funding was provided by the National Institutes of Health, National Cancer Institute (NCI) grant R01-CA213442 (J.R.B.), in part by NCI grant PO1-CA81534 to the CLL Research Consortium (L.Z.R. and T.J.K.), by the Melton Family Fund for CLL Research, and by the Susan and Gary Rosenbach Fund for Lymphoma Research.
This was an investigator-initiated study designed by senior author J.R.B., who held the investigational new drug application. Both Gilead Pharmaceuticals and GlaxoSmithKline (initially) and Novartis (subsequently) provided funding as well as their study drug at no cost. Neither Gilead nor Novartis had any role in the study design, data collection, data analysis, data interpretation, or writing of this report, which were entirely under the supervision of J.R.B., who had full access to all the data and the final responsibility for the decision to submit the article for publication.
Authorship
Contribution: J.R.B. designed the trial; B.L.L. collected and analyzed data; H.T.K. performed statistical analyses; M.S.D., J.S.A., A.S.F., C.A.J., P.A.A., R.M.J., J.E.A., D.C.F., and J.R.B. enrolled and treated patients and collected data; J.F., S.M.F., and J.R.H. collected patient samples and individual patient data; T.J.K. and L.Z.R. collected samples and performed analysis; J.R.B. and B.L.L. produced the first version of the manuscript, which was circulated for comments to the other authors; and all authors analyzed and interpreted the data, critically revised draft versions, and approved the final manuscript.
Conflict-of-interest disclosure: J.R.B. has consulted for Janssen, Gilead Pharmaceuticals, Celgene, Sun, Novartis, AbbVie, Pfizer, Acerta, AstraZeneca, Beigene, Sunesis, Loxo, Astellas, RedX, Pharmacyclics, Roche/Genentech, Verastem, and TG Therapeutics; has received grants from Gilead Pharmaceuticals, Verastem, Sun, and Loxo; and has served on data safety monitoring boards for Morphosys and Invectys. M.S.D. received institutional research grants from Genentech, Pharmacyclics, MEI Pharma, Acerta Pharma, Verastem, Bristol-Myers Squibb, Surface Oncology, and TG Therapeutics and is a consultant/advisory board member for AbbVie, Roche/Genentech, Pharmacyclics, Janssen, Gilead Pharmaceuticals, AstraZeneca, MEI Pharma, Verastem, and TG Therapeutics. J.S.A. served as a consultant or advisory board member for Kite Pharma, Roche/Genentech, Gilead Pharmaceuticals, Celgene, Seattle Genetics, AbbVie, and Incyte. C.A.J. served as a consultant or advisory board member for Kite Pharma and Pharmacyclics. P.A.A. served as a consultant or advisory board member for Bristol-Myers Squibb, Merck, Infinity Pharmaceuticals, Pfizer, Affimed, and Adaptive Biotechnologies and received institutional research funding from Merck, Bristol-Myers Squibb, Affimed, Adaptive Biotechnologies, Roche, Tensha Therapeutics, Otsuka Pharmaceutical, and Sigma-Tau Pharmaceuticals (now Leadiant Biosciences). J.E.A. received consulting fees from Gilead and Regeneron. T.J.K. has received grants from Oncternal, Roche/Genentech, and Pharmacyclics and served as a consultant and/or advisory board member for Gilead Pharmaceuticals, Celgene, Roche/Genentech, and Pharmacyclics. D.C.F. served as a consultant and/or advisory board member for Seattle Genetics, Celgene, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer R. Brown, Dana-Farber Cancer Institute, Mayer Building, Room 226, 450 Brookline Ave, Boston, MA 02215; e-mail: jennifer_brown@dfci.harvard.edu.