Key Points
Response to bortezomib-based therapy is related to the secretory status of M-protein in myeloma.
Myeloma patients with unmeasurable M-protein have a poor clinical outcome.
Abstract
The treatment of multiple myeloma (MM) with proteasome inhibitor (PI) bortezomib has significantly improved the survival of patients with MM. The 26S proteasome inhibitor targets the unfolded protein response (UPR) by inhibiting proteasome degradation of ubiquitinated paraprotein, subsequently leading to the lethal accumulation of paraprotein within the endoplasmic reticulum. According to secretory status of monoclonal immunoglobulin, newly diagnosed MM (NDMM) is divided into measurable and unmeasurable disease, which includes oligosecretory, nonsecretory, and nonproducer myeloma. The present study analyzed the clinical characteristics of 822 patients with NDMM who had either measurable or unmeasurable diseases and received bortezomib- or thalidomide-based therapies. Our results showed that the median progression-free survival (PFS) and overall survival (OS) of patients with MM was significantly longer in patients with measurable disease than those in oligosecretory, nonsecretory, and nonproducer MM (PFS: 27, 18, 19, and 2.0 months, respectively [P < .001]; OS: 51, 30, 22, and 2.0 months, respectively [P < .001]). Within the unmeasurable group, patients with nonproducer myeloma showed the shortest PFS and OS. Importantly, compared with thalidomide treatment, bortezomib significantly improved the PFS and OS of patients with MM with measurable disease (PFS: 25 and 33 months [P = .022], respectively; OS: 41 and 58 months [P < .001], respectively), but not those with unmeasurable disease (PFS: 18 and 16 months [P = .617], respectively; OS: 22 and 27 months [P = .743], respectively). Our results indicate that bortezomib-based therapy performed no better than thalidomide-based treatment in patients with unmeasurable MM. The results need to be confirmed in other patient cohorts, preferably in the context of a prospective trial.
Introduction
Multiple myeloma (MM) is an incurable clonal plasma cell malignancy characterized by the production of monoclonal protein (M-protein) in most patients. However, MM is heterogenous with respect to different M-protein secretory status, and 10% to 15% of patients have no measurable M-protein at diagnosis. According to the International Myeloma Working Group, measurable disease is defined as serum M-protein level of at least 1 g/dL or urine M-protein level of at least 200 mg/24 hours, whereas an M-protein level below this threshold is considered unmeasurable disease.1,2 Unmeasurable disease includes oligosecretory and nonsecretory MM: oligosecretory MM has M-protein in serum or urine, but below the criteria of measurable disease; and nonsecretory MM has no M-protein in serum or urine. Within nonsecretory MM, nonsecretory myeloma is characterized by positive κ or λ light chain detected by immunohistochemistry, whereas nonproducer MM has no detectable κ or λ light chain by immunohistochemistry. To date, it is unclear whether the M-protein secretion status has an effect on the outcome of patients treated in real-world settings in China.
The survival of patients with MM has been dramatically improved after treatment with bortezomib3 ; however, not all patients benefit from the treatment.4 Moreover, bortezomib is associated with adverse events including peripheral neuropathy, gastrointestinal symptoms, and thrombocytopenia,5 which can be reduced, but not eliminated, by subcutaneous administration, as well as modification of dose and schedule (ie, weekly) of bortezomib.6,7 Therefore, it is important to identify which subtype of myeloma is sensitive to bortezomib treatment. Because the antitumor activity of bortezomib, at least in part, depends on the degradation of misfolded or unfolded proteins through the unfolded protein response (UPR),8 the quantity of unfolded immunoglobulin (Ig) may affect the efficacy of bortezomib.9
We postulate that low levels of M-protein in patients with unmeasurable disease may not activate the UPR, and that bortezomib may therefore not induce MM cell apoptosis and clinical responses. We here analyzed the efficacy of bortezomib treatment in Chinese patients with MM with measurable disease, as well as oligosecretory, nonsecretory, and nonproducer MM. Overall, our results from real-world clinical practice showed that patients with measurable disease had improved PFS and OS with bortezomib-based therapy compared with thalidomide-based treatment, whereas patients with unmeasurable disease fared worse regardless of whether they received bortezomib- or thalidomide-based strategies.
Design and methods
Study design
We carried out a retrospective study in 822 patients with newly diagnosed MM (NDMM) by International Myeloma Working Group criteria,10 who received either bortezomib-based or thalidomide-based induction therapies in China between 2002 and 2015. The study was performed in accordance with the Declaration of Helsinki, and was approved by the local ethics committee of State Key Laboratory of Experimental Hematology, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College. All patients provided written informed consent. Clinical baseline, molecular cytogenetic subtype, immunophenotype, and follow-up information of these patients was collected and analyzed. To assess outcome associated with M-protein status, patients were classified as having measurable disease vs unmeasurable disease, and within unmeasurable disease, as having oligosecretory, nonsecretory, or nonproducer MM.
All 822 patients with symptomatic MM received either thalidomide-based (group A) or bortezomib-based (group B) therapy. Thalidomide-based therapy was carried out as follows: either thalidomide 100-200 mg/d, doxorubicin 9 mg/m2 (administered IV on days 1-4), and dexamethasone 20 mg/d (administered orally or IV on days 1-4 and 9-12); or thalidomide 100-200 mg/d, cyclophosphamide 500 mg/m2 (administered IV on days 1 and 8), and dexamethasone 20 mg/d (administered orally or IV on days 1-4 and 9-12).
Bortezomib-based therapy was carried out as follows: bortezomib 1.3 mg/m2 (BD; administered IV or subcutaneously on days 1, 4, 8, and 11); dexamethasone 20 mg/d (administered orally or IV on days 1, 2, 4, 5, 8, 9, 11, and 12); BCD, BD plus cyclophosphamide 500 mg/m2 (administered IV on days 1 and 8); or BD plus doxorubicin 9 mg/m2 (administered IV on days 1-4).
After at least 4 cycles of treatment with partial response or better response, patients underwent consolidation therapy, which was either autologous stem cell transplant or chemotherapy with the patient’s original regimen, according to their request. Subsequently, patients were treated with thalidomide (100-200 mg/day) for 1 year to maintain the response. When relapse or progression was observed, patients would receive a salvage regimen from the opposite group, as well as supportive treatment with zoledronic acid every 1 to 2 months and erythropoietin or granulocyte colony-stimulating factor. All patients were then treated with thalidomide (100-200 mg/d) for 1 year to maintain the response. The median duration of first-line therapy for NDMM was 11 months (interquartile range, 4.7-21.3 months).
The patient’s maximum response to therapy was categorized using international Uniform Response Criteria for MM: stable disease, partial response, very good partial response, complete response, stringent complete response, and progressive disease and clinical relapse.11 For measurable disease, M-protein detection by serum protein electrophoresis and immunofixation electrophoresis every 2 cycles of chemotherapy and bone marrow aspirate examination were both necessary. To evaluate the response of unmeasurable disease, we took effective measures, such as bone marrow aspiration and smears and minimal residual disease detection by flow cytometry. Bone disease and extramedullary disease were measured by total body low-dose CT or magnetic resonance imaging. On the basis of the results of these parameters, we can evaluate the response of most patients.
Cytogenetic analysis
All MM samples were purified using anti-CD138-coated magnetic beads (Miltenyi Biotech, Paris, France) before fluorescence in situ hybridization (FISH) analysis, as reported previously.12 Plasma cells were analyzed using DNA probes specific for del(13q14), del(17p), t(11;14), t(4;14), and t(14;16). Gains of 1q21 were assessed using a bacterial artificial chromosome (RP11-307C12) probe at 1q21. A total of 200 interphase nuclei were analyzed, and the cutoff values recommended by the European Myeloma Network were used: 20% for deletions and numerical aberrations and 10% for translocations in IgH locus, as well as other translocations.12
Statistical analysis
Eight groups (patients with measurable disease or unmeasurable disease including oligosecretory, nonsecretory, and nonproducer MM who received bortezomib or thalidomide-based therapy) were compared using the χ2 test for frequencies and Mann-Whitney U test for continuous variables. Survival curves were plotted using the Kaplan-Meier method, with differences assessed by the log-rank test. Multivariate analysis was estimated using COX models. Differences were considered statistically significant when P ≤ .05. OS was measured from the initiation of treatment to the date of death or last follow-up.13
Results
Characteristics of patients with MM with measurable disease and with oligosecretory, nonsecretory, nonproducer MM
The clinical characteristics of the 822 patients with MM at initial diagnosis are shown in Table 1. MM with unmeasurable disease accounted for 10.58% (87/822) of patients, including 7.42% (61/822) with oligosecretory, 2.31% (19/822) with nonsecretory, and 0.85% (7/822) with nonproducer MM. Patients with oligosecretory MM had more IgD and κ light chain subtype, with fewer IgG M protein. Patients with nonsecretory MM more commonly had low-risk International Staging System (ISS) MM. Oligosecretory and nonsecretory MM had higher levels of albumin compared with measurable disease; renal dysfunction was more common in oligosecretory than in nonsecretory, nonproducer, and measurable MM. Compared with measurable disease, oligosecretory, nonsecretory, and nonproducer MM had higher levels of lactate dehydrogenase (LDH), whereas oligosecretory MM had higher levels of hemoglobin. The plasma cells in bone marrow biopsy of nonproducer MM were predominantly plasmablasts. The sex, age, Durie-Salmon stage, median platelet count, serum calcium, number of plasma cells in bone marrow, and extramedullary disease were not statistically significantly different among the 8 groups.
Demographics and baseline clinical characteristics of newly diagnosed MM with measurable disease and with oligosecretory, nonsecretory, nonproducer myeloma
| Patients’ characteristics . | Oligosecretory myeloma (N = 61) . | Nonsecretory myeloma (N = 19) . | Nonproducer myeloma (N = 7) . | Measurable disease (N = 735) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bortezomib (N = 30) . | Thalidomide (N = 31) . | Bortezomib (N = 6) . | Thalidomide (N = 13) . | Bortezomib (N = 3) . | Thalidomide (N = 4) . | Bortezomib (N = 280) . | Thalidomide (N = 455) . | ||
| Sex, n/N (%) | .575 | ||||||||
| Female | 9/61 (14.75) | 15/61 (24.59) | 1/19 (5.26) | 4/19 (21.05) | 0/7 (0.00) | 1/7 (14.29) | 99/735 (13.47) | 172/735 (23.40) | |
| Male | 21/61 (34.43) | 16/61 (26.23) | 5/19 (26.32) | 9/19 (47.37) | 3/7 (42.86) | 3/7 (42.86) | 181/735 (24.63) | 283/735 (38.50) | |
| Median of age, y | 51 | 57 | 53 | 53 | 57 | 59 | 56 | 59 | .245 |
| Subtype of MM, n/N (%) | .000 | ||||||||
| IgG | 4/61 (6.56) | 5/61 (8.20) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 156/735 (21.22) | 251/735 (34.15) | |
| IgA | 8/61 (13.11) | 9/61 (14.75) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 65/735 (8.84) | 131/735 (17.82) | |
| IgD | 5/61 (8.20) | 7/61 (11.48) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 10/735 (1.36) | 7/735 (0.95) | |
| IgM | 2/61 (3.28) | 1/61 (1.64) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2/735 (0.27) | 0/735 (0.00) | |
| Light chain type, n/N (%) | |||||||||
| κ | 6/61 (9.84) | 5/61 (8.20) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 16/735 (2.18) | 21/735 (2.86) | |
| λ | 5/61 (8.20) | 4/61 (6.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 31/735 (4.22) | 45/735 (6.12) | |
| ISS stage, n/N (%) | .021 | ||||||||
| I | 8/61 (13.11) | 11/61 (18.03) | 2/19 (10.53) | 7/19 (36.84) | 0/7 (0.00) | 1/7 (14.29) | 58/735 (7.89) | 95/735 (12.93) | |
| II | 6/61 (9.84) | 11/61 (18.03) | 2/19 (10.53) | 3/19 (15.79) | 2/7 (28.57) | 2/7 (28.57) | 97/735 (13.20) | 162/735 (22.04) | |
| III | 16/61 (26.23) | 9/61 (14.75) | 2/19 (10.53) | 3/19 (15.79) | 1/7 (14.29) | 1/7 (14.29) | 125/735 (17.01) | 198/735 (26.94) | |
| Durie-Salmon stage, n/N (%) | .064 | ||||||||
| I | 2/61 (3.28) | 5/61 (8.20) | 0/19 (0.00) | 3/19 (15.79) | 0/7 (0.00) | 0/7 (0.00) | 6/735 (0.82) | 32/735 (4.35) | |
| II | 2/61 (3.28) | 4/61 (6.56) | 1/19 (5.26) | 1/19 (5.26) | 1/7 (14.29) | 0/7 (0.00) | 19/735 (2.59) | 51/735 (6.94) | |
| III | 26/61 (42.62) | 22/61 (36.07) | 4/19 (21.05) | 10/19 (52.63) | 3/7 (42.86) | 3/7 (42.86) | 255/735 (34.69) | 372/735 (50.61) | |
| Median albumin, g/L | 43.90 | 48.10 | 41.5 | 38.9 | 41.4 | 31.3 | 34.00 | 33.8 | .000 |
| Renal dysfunction, serum Cr ≥2.0 mg/dL, n/N (%) | 15/61 (24.59) | 6/61 (9.84) | 1/19 (5.26) | 2/19 (10.53) | 1/7 (14.29) | 0/7 (0.00) | 61/735 (8.30) | 73/735 (9.93) | .004 |
| Median hemoglobin, g/L | 100 | 97 | 105 | 80 | 87 | 92 | 91 | 86 | .002 |
| Median platelet count, ×109/L | 193 | 176 | 144 | 130 | 196 | 144 | 175 | 158 | .082 |
| Median LDH, U/L | 235 | 257 | 349 | 179 | 228 | 367 | 174 | 188 | .000 |
| Median calcium, mmol/L | 2.32 | 2.29 | 2.37 | 2.30 | 2.28 | 1.83 | 2.38 | 2.27 | .322 |
| Plasma cells in bone marrow, % | 25.5 | 27.5 | 38.5 | 27.0 | 47.5 | 29.5 | 33.5 | 37.0 | .067 |
| Extramedullary disease, % | 10 | 3 | 2 | 1 | 1 | 0 | 72 | 30 | .072 |
| Patients’ characteristics . | Oligosecretory myeloma (N = 61) . | Nonsecretory myeloma (N = 19) . | Nonproducer myeloma (N = 7) . | Measurable disease (N = 735) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bortezomib (N = 30) . | Thalidomide (N = 31) . | Bortezomib (N = 6) . | Thalidomide (N = 13) . | Bortezomib (N = 3) . | Thalidomide (N = 4) . | Bortezomib (N = 280) . | Thalidomide (N = 455) . | ||
| Sex, n/N (%) | .575 | ||||||||
| Female | 9/61 (14.75) | 15/61 (24.59) | 1/19 (5.26) | 4/19 (21.05) | 0/7 (0.00) | 1/7 (14.29) | 99/735 (13.47) | 172/735 (23.40) | |
| Male | 21/61 (34.43) | 16/61 (26.23) | 5/19 (26.32) | 9/19 (47.37) | 3/7 (42.86) | 3/7 (42.86) | 181/735 (24.63) | 283/735 (38.50) | |
| Median of age, y | 51 | 57 | 53 | 53 | 57 | 59 | 56 | 59 | .245 |
| Subtype of MM, n/N (%) | .000 | ||||||||
| IgG | 4/61 (6.56) | 5/61 (8.20) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 156/735 (21.22) | 251/735 (34.15) | |
| IgA | 8/61 (13.11) | 9/61 (14.75) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 65/735 (8.84) | 131/735 (17.82) | |
| IgD | 5/61 (8.20) | 7/61 (11.48) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 10/735 (1.36) | 7/735 (0.95) | |
| IgM | 2/61 (3.28) | 1/61 (1.64) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2/735 (0.27) | 0/735 (0.00) | |
| Light chain type, n/N (%) | |||||||||
| κ | 6/61 (9.84) | 5/61 (8.20) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 16/735 (2.18) | 21/735 (2.86) | |
| λ | 5/61 (8.20) | 4/61 (6.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 31/735 (4.22) | 45/735 (6.12) | |
| ISS stage, n/N (%) | .021 | ||||||||
| I | 8/61 (13.11) | 11/61 (18.03) | 2/19 (10.53) | 7/19 (36.84) | 0/7 (0.00) | 1/7 (14.29) | 58/735 (7.89) | 95/735 (12.93) | |
| II | 6/61 (9.84) | 11/61 (18.03) | 2/19 (10.53) | 3/19 (15.79) | 2/7 (28.57) | 2/7 (28.57) | 97/735 (13.20) | 162/735 (22.04) | |
| III | 16/61 (26.23) | 9/61 (14.75) | 2/19 (10.53) | 3/19 (15.79) | 1/7 (14.29) | 1/7 (14.29) | 125/735 (17.01) | 198/735 (26.94) | |
| Durie-Salmon stage, n/N (%) | .064 | ||||||||
| I | 2/61 (3.28) | 5/61 (8.20) | 0/19 (0.00) | 3/19 (15.79) | 0/7 (0.00) | 0/7 (0.00) | 6/735 (0.82) | 32/735 (4.35) | |
| II | 2/61 (3.28) | 4/61 (6.56) | 1/19 (5.26) | 1/19 (5.26) | 1/7 (14.29) | 0/7 (0.00) | 19/735 (2.59) | 51/735 (6.94) | |
| III | 26/61 (42.62) | 22/61 (36.07) | 4/19 (21.05) | 10/19 (52.63) | 3/7 (42.86) | 3/7 (42.86) | 255/735 (34.69) | 372/735 (50.61) | |
| Median albumin, g/L | 43.90 | 48.10 | 41.5 | 38.9 | 41.4 | 31.3 | 34.00 | 33.8 | .000 |
| Renal dysfunction, serum Cr ≥2.0 mg/dL, n/N (%) | 15/61 (24.59) | 6/61 (9.84) | 1/19 (5.26) | 2/19 (10.53) | 1/7 (14.29) | 0/7 (0.00) | 61/735 (8.30) | 73/735 (9.93) | .004 |
| Median hemoglobin, g/L | 100 | 97 | 105 | 80 | 87 | 92 | 91 | 86 | .002 |
| Median platelet count, ×109/L | 193 | 176 | 144 | 130 | 196 | 144 | 175 | 158 | .082 |
| Median LDH, U/L | 235 | 257 | 349 | 179 | 228 | 367 | 174 | 188 | .000 |
| Median calcium, mmol/L | 2.32 | 2.29 | 2.37 | 2.30 | 2.28 | 1.83 | 2.38 | 2.27 | .322 |
| Plasma cells in bone marrow, % | 25.5 | 27.5 | 38.5 | 27.0 | 47.5 | 29.5 | 33.5 | 37.0 | .067 |
| Extramedullary disease, % | 10 | 3 | 2 | 1 | 1 | 0 | 72 | 30 | .072 |
Molecular cytogenetics of patients with MM with measurable disease and with oligosecretory, nonsecretory, and nonproducer MM
Cytogenetic analysis was performed in 636 of 822 patients with MM, including 563 with measurable disease, 53 oligosecretory, 14 nonsecretory, and 6 nonproducer MM. Metaphases karyotype analysis showed that there was no significant difference among the 8 groups. No analyzable metaphase was obtained in 47 patients including 30 with measurable disease, 11 oligosecretory, 4 nonsecretory, and 2 nonproducer MM. Interphase FISH analysis was performed in 621 of 822 patients with MM, and t(11,14) was observed frequently in oligosecretory, nonsecretory, and nonproducer MM, but there were no differences of other genetic aberrations among these 8 groups. We analyzed the immunophenotype by flow cytometry in 8 groups, and found a lower frequency of CD20-positive tumor cells in oligosecretory MM compared with measurable disease. In contrast, the frequencies of CD56, CD117, CD79a, CD33, and CD19 positivity were not statistically significantly different (P > .05; Table 2).
Molecular cytogenetics and immunophenotype characteristics of newly diagnosed MM with measurable disease and with oligosecretory, nonsecretory, nonproducer myeloma
| Patients’ characteristics . | Oligosecretory myeloma (N = 61), n/N (%) . | Nonsecretory myeloma (N = 19), n/N (%) . | Nonproducer myeloma (N = 7), n/N (%) . | Measurable disease (N = 735), n/N (%) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bortezomib (N = 30) . | Thalidomide (N = 31) . | Bortezomib (N = 6) . | Thalidomide (N = 13) . | Bortezomib (N = 3) . | Thalidomide (N = 4) . | Bortezomib (N = 280) . | Thalidomide (N = 455) . | ||
| Cytogenetic abnormalities | .053 | ||||||||
| Diploidy | 26/53 (49.06) | 13/53 (24.53) | 2/14 (14.29) | 8/14 (57.14) | 2/6 (33.33) | 2/6 (33.33) | 205/563 (36.41) | 262/563 (46.54) | |
| Hyperdiploidy | 1/53 (1.89) | 1/53 (1.89) | 0/14 (0.00) | 0/14 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 8/563 (1.42) | 19/563 (3.37) | |
| Hypodiploidy | 1/53 (0.00) | 0/53 (0.00) | 0/14 (0.00) | 0/14 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 20/563 (3.55) | 19/563 (3.37) | |
| FISH | |||||||||
| del(13q) | 18/59 (30.51) | 9/5 9(15.25) | 2/15 (13.33) | 4/15 (26.67) | 0/5 (0.00) | 0/5 (0.00) | 111/537 (20.67) | 99/537 (18.44) | .733 |
| del(17p) | 3/57 (5.26) | 2/57 (3.51) | 1/15 (6.67) | 1/15 (6.67) | 0/6 (0.00) | 0/6 (0.00) | 19/541 (3.51) | 19/541 (3.51) | .585 |
| 1q21 gains | 19/53 (35.85) | 7/53 (13.21) | 2/15 (13.33) | 3/15 (0.2) | 0/5 (0.00) | 0/5 (0.00) | 94/514 (18.29) | 85/514 (16.54) | .691 |
| IgH translocations | |||||||||
| t(11;14) | 7/49 (14.29) | 4/49 (8.16) | 2/14 (14.29) | 2/14 (14.29) | 1/5 (20.00) | 1/5 (20.00) | 42/532 (7.89) | 32/532 (6.02) | .047 |
| t(4;14) | 5/43 (11.63) | 4/43 (9.30) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 47/529 (8.88) | 31/529 (5.86) | .711 |
| t(14;16) | 2/46 (4.35) | 0/46 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 9/513 (1.75) | 4/513 (0.78) | .449 |
| Immunophenotype | |||||||||
| CD56 positive | 16/57 (28.07) | 9/57 (15.79) | 1/18 (5.56) | 4/18 (22.22) | 0/7 (0.00) | 1/7 (14.29) | 209/679 (30.78) | 187/679 (27.54) | .128 |
| CD20 positive | 1/53 (1.89) | 0/53 (0.00) | 0/19 (0.00) | 3/19 (15.79) | 1/6 (16.67) | 0/6 (0.00) | 42/634 (6.62) | 51/634 (8.04) | .047 |
| CD117 positive | 6/54 (11.11) | 4/54 (7.41) | 0/18 (0.00) | 2/18 (11.11) | 1/7 (14.29) | 0/7 (0.00) | 105/629 (16.69) | 84/629 (13.35) | .785 |
| CD79a positive | 0/54 (0.00) | 0/54 (0.00) | 0/17 (0.00) | 0/17 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 3/607 (0.49) | 8/607 (1.32) | .603 |
| CD33 positive | 0/52 (0.00) | 0/52 (0.00) | 0/16 (0.00) | 0/16 (0.00) | 0/5 (0.00) | 0/5 (0.00) | 24/679 (3.53) | 19/679 (2.80) | .909 |
| CD19 positive | 1/57 (1.75) | 1/57 (1.75) | 0/18 (0.00) | 0/18 (0.00) | 0/7 (0.00) | 1/7 (14.29) | 10/529 (1.89) | 14/529 (2.65) | .057 |
| Patients’ characteristics . | Oligosecretory myeloma (N = 61), n/N (%) . | Nonsecretory myeloma (N = 19), n/N (%) . | Nonproducer myeloma (N = 7), n/N (%) . | Measurable disease (N = 735), n/N (%) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bortezomib (N = 30) . | Thalidomide (N = 31) . | Bortezomib (N = 6) . | Thalidomide (N = 13) . | Bortezomib (N = 3) . | Thalidomide (N = 4) . | Bortezomib (N = 280) . | Thalidomide (N = 455) . | ||
| Cytogenetic abnormalities | .053 | ||||||||
| Diploidy | 26/53 (49.06) | 13/53 (24.53) | 2/14 (14.29) | 8/14 (57.14) | 2/6 (33.33) | 2/6 (33.33) | 205/563 (36.41) | 262/563 (46.54) | |
| Hyperdiploidy | 1/53 (1.89) | 1/53 (1.89) | 0/14 (0.00) | 0/14 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 8/563 (1.42) | 19/563 (3.37) | |
| Hypodiploidy | 1/53 (0.00) | 0/53 (0.00) | 0/14 (0.00) | 0/14 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 20/563 (3.55) | 19/563 (3.37) | |
| FISH | |||||||||
| del(13q) | 18/59 (30.51) | 9/5 9(15.25) | 2/15 (13.33) | 4/15 (26.67) | 0/5 (0.00) | 0/5 (0.00) | 111/537 (20.67) | 99/537 (18.44) | .733 |
| del(17p) | 3/57 (5.26) | 2/57 (3.51) | 1/15 (6.67) | 1/15 (6.67) | 0/6 (0.00) | 0/6 (0.00) | 19/541 (3.51) | 19/541 (3.51) | .585 |
| 1q21 gains | 19/53 (35.85) | 7/53 (13.21) | 2/15 (13.33) | 3/15 (0.2) | 0/5 (0.00) | 0/5 (0.00) | 94/514 (18.29) | 85/514 (16.54) | .691 |
| IgH translocations | |||||||||
| t(11;14) | 7/49 (14.29) | 4/49 (8.16) | 2/14 (14.29) | 2/14 (14.29) | 1/5 (20.00) | 1/5 (20.00) | 42/532 (7.89) | 32/532 (6.02) | .047 |
| t(4;14) | 5/43 (11.63) | 4/43 (9.30) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 47/529 (8.88) | 31/529 (5.86) | .711 |
| t(14;16) | 2/46 (4.35) | 0/46 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 9/513 (1.75) | 4/513 (0.78) | .449 |
| Immunophenotype | |||||||||
| CD56 positive | 16/57 (28.07) | 9/57 (15.79) | 1/18 (5.56) | 4/18 (22.22) | 0/7 (0.00) | 1/7 (14.29) | 209/679 (30.78) | 187/679 (27.54) | .128 |
| CD20 positive | 1/53 (1.89) | 0/53 (0.00) | 0/19 (0.00) | 3/19 (15.79) | 1/6 (16.67) | 0/6 (0.00) | 42/634 (6.62) | 51/634 (8.04) | .047 |
| CD117 positive | 6/54 (11.11) | 4/54 (7.41) | 0/18 (0.00) | 2/18 (11.11) | 1/7 (14.29) | 0/7 (0.00) | 105/629 (16.69) | 84/629 (13.35) | .785 |
| CD79a positive | 0/54 (0.00) | 0/54 (0.00) | 0/17 (0.00) | 0/17 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 3/607 (0.49) | 8/607 (1.32) | .603 |
| CD33 positive | 0/52 (0.00) | 0/52 (0.00) | 0/16 (0.00) | 0/16 (0.00) | 0/5 (0.00) | 0/5 (0.00) | 24/679 (3.53) | 19/679 (2.80) | .909 |
| CD19 positive | 1/57 (1.75) | 1/57 (1.75) | 0/18 (0.00) | 0/18 (0.00) | 0/7 (0.00) | 1/7 (14.29) | 10/529 (1.89) | 14/529 (2.65) | .057 |
Effect of M-protein secretory status on PFS and OS of all patients with MM
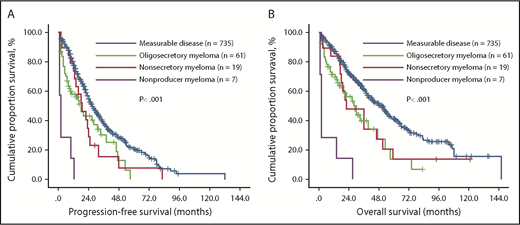
Patients with measurable disease, oligosecretory, nonsecretory, and nonproducer MM had median PFS of 27.0 (95% confidence interval [CI], 24.4-29.6), 18.0 (95% CI, 11.5-24.5), 19.0 (95% CI, 11.1-26.9), and 2.0 (95% CI, 0.8-3.2) months, respectively (P < .001; Figure 1A). We then performed a multivariate analysis including all the parameters identified to be associated with shorter PFS in all 822 patients with MM and identified 6 factors independently associated with shorter PFS in all patients with MM: ISS stage (HR, 1.318; 95% CI, 1.023-1.699; P = .033), 17p deletion (HR, 2.734; 95% CI:1.725-4.335]; P < .001), 1q21 gains (HR, 1.763; 95% CI, 1.285-2.419; P < .001), LDH concentration (HR, 1.001; 95% CI, 1.000-1.002; P = .017), thalidomide-based therapy strategy (HR, 0.695; 95% CI, 0.512-0.942; P = .019), and unmeasurable disease (HR, 2.190; 95% CI, 1.646-2.914; P < .001).
Effect of M-protein secretory status on PFS and OS of all patients with MM. Among 4 groups, the median PFS (A) and OS (B) of MM patients was significantly longer in patients with measurable disease than in those with oligosecretory, nonsecretory, and nonproducer MM. Within the unmeasurable group, patients with nonproducer myeloma showed the shortest PFS and OS.
Effect of M-protein secretory status on PFS and OS of all patients with MM. Among 4 groups, the median PFS (A) and OS (B) of MM patients was significantly longer in patients with measurable disease than in those with oligosecretory, nonsecretory, and nonproducer MM. Within the unmeasurable group, patients with nonproducer myeloma showed the shortest PFS and OS.
Among measurable disease, oligosecretory, nonsecretory, and nonproducer MM groups, the median OS was 51.0 (95% CI, 44.9-57.1), 30.0 (95% CI, 20.2-39.8), 22.0 (95% CI, 6.3-37.7), and 2.0 (95% CI, 1.2-2.8) months, respectively (P < .001; Figure 1B). We then performed a multivariate analysis including all the parameters that we identified to be associated with shorter OS in all 822 patients and found 6 factors to be independently associated with shorter OS: ISS stage (HR, 1.756; 95% CI, 1.280-2.409; P < .001), 17p deletion (HR, 2.101; 95% CI:1.268-3.482; P = .004), 1q21 gains (HR, 1.789; 95% CI, 1.246-2.568; P < .001), LDH concentration (HR, 1.001; 95% CI, 1.000-1.002; P = .023), thalidomide-based therapy strategy (HR, 0.695; 95% CI, 0.569-0.810; P = .002), and secretory status of monoclonal Ig (HR, 2.441; 95% CI, 1.809-3.294; P < .001).
Effect of thalidomide/bortezomib-based therapy on survival of different patients with M-protein secretory myeloma
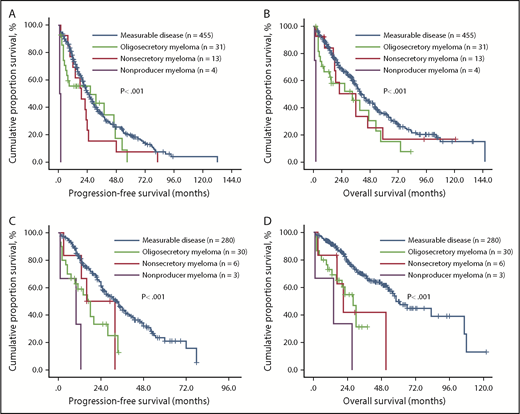
We compared the survival of these 4 groups receiving bortezomib- and thalidomide-based therapies. In patients with measurable disease, oligosecretory, nonsecretory, and nonproducer MM receiving thalidomide-based regimens, the median PFS was 25.0 (95% CI, 26.5-27.9), 26.5 (95% CI, 12.7-62.6), 19.0 (95% CI, 9.6-28.4), and 1.0 (95% CI, 0.3-1.9) months, respectively (P < .001; Figure 2A), and the OS of these 4 groups was 41.0 (95% CI, 36.0-46.0), 33.0 (95% CI, 6.6-59.4), 36.0 (95% CI, 17.9-54.1), and 2.0 (95% CI, 0.3-1.9) months, respectively (P < .001; Figure 2B). Importantly, in patients with measurable disease, oligosecretory, nonsecretory, and nonproducer MM receiving bortezomib-based regimens, the median PFS was 33.0 (95% CI, 27.7-38.3), 16.5 (95% CI, 9.9-23.1), 16.0 (95% CI, 4.6-27.4), and 10.0 (95% CI, 0.0-24.4) months, respectively (P < .001; Figure 2C), and the OS was 58.0 (95% CI, 48.8-67.2), 28.0 (95% CI, 20.3-35.7), 21.0 (95% CI, 11.6-30.4), and 14.0 (95% CI, 0.0-34.8) months, respectively (P < .001; Figure 2D).
Effect of M-protein secretory status on PFS and OS of patients with MM receiving thalidomide- or bortezomib-based therapy. The median PFS and OS of MM patients was significantly longer in patients with measurable disease than in those with oligosecretory, nonsecretory, and nonproducer MM regardless of thalidomide- (A-B) or bortezomib-based (C-D) therapy. Within the unmeasurable group, patients with nonproducer myeloma showed the shortest PFS and OS.
Effect of M-protein secretory status on PFS and OS of patients with MM receiving thalidomide- or bortezomib-based therapy. The median PFS and OS of MM patients was significantly longer in patients with measurable disease than in those with oligosecretory, nonsecretory, and nonproducer MM regardless of thalidomide- (A-B) or bortezomib-based (C-D) therapy. Within the unmeasurable group, patients with nonproducer myeloma showed the shortest PFS and OS.
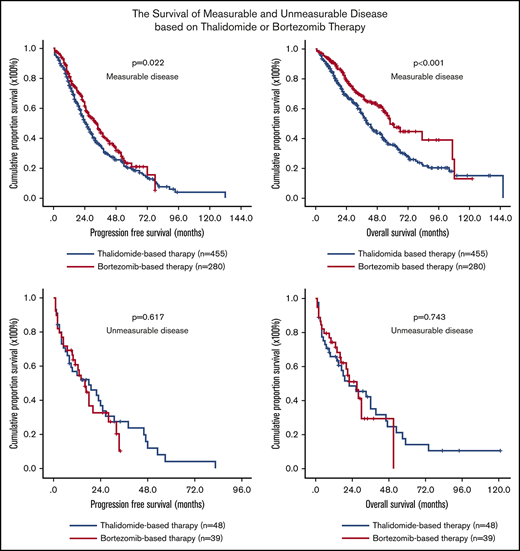
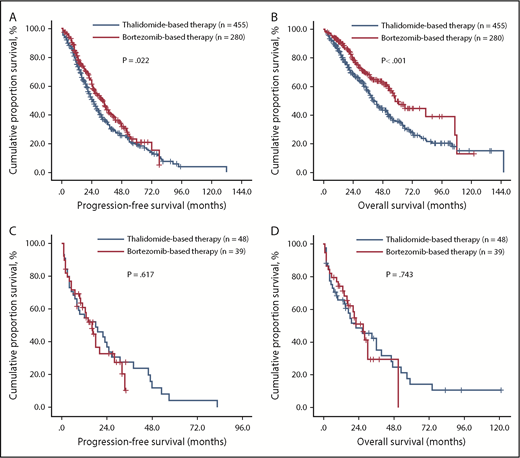
Finally, comparison of survival in measurable and unmeasurable disease between thalidomide-based and bortezomib-based therapies was performed. The median PFS of patients with measurable disease receiving thalidomide-based and bortezomib-based therapies was 25.0 (95% CI, 22.1-27.9) and 33.0 (95% CI, 27.7-38.3) months, respectively (P = .022; Figure 3A). The OS of these 2 groups was 41.0 (95% CI, 35.9-46.0) and 58.0 (95% CI, 48.8-67.2) months, respectively (P < .001; Figure 3B). For unmeasurable disease, the median PFS of patients receiving thalidomide-based and bortezomib-based therapies was 18.0 (95% CI, 4.49-31.51) and 16.0 (95% CI, 10.9-21.1) months, respectively (P = .617; Figure 3C). The OS of these 2 groups was 22.0 (95% CI, 5.5-38.5) and 27.0 (95% CI, 18.7-35.3 months, respectively (P = .743; Figure 3D).
Survival of newly diagnosed patients with MM with different M-protein secretory status after receiving thalidomide- or bortezomib-based therapy. PFS and OS were remarkably extended with chemotherapy incorporating the proteasome inhibitor bortezomib in patients with measurable disease (A-B), but not in patients with unmeasurable disease (C-D).
Survival of newly diagnosed patients with MM with different M-protein secretory status after receiving thalidomide- or bortezomib-based therapy. PFS and OS were remarkably extended with chemotherapy incorporating the proteasome inhibitor bortezomib in patients with measurable disease (A-B), but not in patients with unmeasurable disease (C-D).
Discussion
Bortezomib has been approved for the effective treatment of MM, at least in part through inducing a terminal unfolded protein response.8,14,15 To investigate the association between secretory status and response of bortezomib in MM in real-world China practice, we here analyzed the outcome of 822 patients with MM with different M-protein secretory status. We found that bortezomib significantly improved the PFS and OS of patients with MM with measurable disease compared with thalidomide-based treatment, whereas patients with unmeasurable disease had worse outcome regardless of whether they receive bortezomib- or thalidomide-based therapy. These results probably suggest that M-protein secretory status in patients with MM was associated with response to bortezomib therapy, and that patients with MM with unmeasurable disease benefited less from bortezomib treatment.
The frequency of unmeasurable disease of this study is consistent with previous reports.2,16 Compared with clinical baseline characteristics of patients with measurable disease, those with oligosecretory MM had higher frequency of renal dysfunction and were more commonly IgD MM. In our study, patients with oligosecretory MM had more renal dysfunction and those with unmeasurable disease had higher levels of LDH than those with measurable disease. Several prior studies have indicated that high LDH and renal dysfunction were associated with shorter survival in patients with symptomatic MM.17,18 Renal dysfunction (serum creatinine ≥2 mg/dL) was reported in approximately 20% of myeloma at diagnosis and a definite risk factor for inferior survival.19,20 The major causes of renal insufficiency are “myeloma kidney” from light chain or Ig deposition, with other causes including hypercalcemia, infections, use of nephrotoxic drugs, hypovolemia, and IgD MM.21,22 Although the precise causes of renal dysfunction in our patients with oligosecretory myeloma in our study were not defined, there was a high frequency of IgD MM, consistent with prior reports of shorter OS and higher frequency of renal dysfunction.22,23
We also analyzed the cytogenetic characteristics in patients with unmeasurable and measurable MM, and found that patients with unmeasurable disease were more commonly t(11;14). Other factors such as ploidy, del(17p), del(13p), 1q21 gains, t(4;14), and t(14;16) were not significantly different between patients with measurable and unmeasurable disease. t(11;14) is a common translocation in MM, including IgD and nonsecretory myeloma.24,25 Our previous study found that the outcome of patients with myeloma exhibiting t(11;14) was heterogeneous, and that patients within this subgroup with no CD20 expression showed significantly shorter survival, even after bortezomib-based treatment.26 Interestingly, some evidence confirmed that a very high incidence of t(11;14)(q13;q32) was detected in the nonsecretory myeloma, and a lower secreting capacity was associated with t(11;14).27,28
We analyzed the prognostic value of M-protein level in patients with MM and found that patients with oligosecretory, nonsecretory, and nonproducer MM had shorter PFS and OS than those patients with measurable disease. After bortezomib treatment, the survival of patients with measurable disease, but not those with unmeasurable disease, was significantly improved compared with thalidomide treatment. A multivariate analysis in all patients with 822 myeloma, including all the parameters identified to be associated with shorter OS in univariate analysis, showed that M-protein secretion status was independently associated with shorter PFS and OS.
To date, only limited in vitro data indicate that low levels of Ig in plasma cells confer resistance to bortezomib treatment.8,14 Some previous studies in vitro or on animal models indicated that a high level of Ig synthesis is more predictive for proteasome inhibitor-induced apoptosis. According to previous studies from many groups, high levels of protein synthesis were more predictive for proteasome inhibitor-induced apoptosis, and unmeasurable myeloma without sufficient Ig fails to activate UPR, which is the target of bortezomib in myeloma cells.15 These studies also suggested that proteasome inhibitors induced apoptosis in myeloma cells preferentially through high synthesis of Ig, which was associated with endoplasmic reticulum stress induced by the extensive unfolded proteins. In recent years, a few studies focused on the mechanism of resistance to bortezomib in myeloma cells and that UPR regulator Xbp1 is of great importance and is required for myeloma pathogenesis. Loss of Xbp1 was associated with decreased expression of Ig and immature plasma morphology, which resulted in induction of bortezomib resistance.29,30 However, sufficient Ig and UPR cannot fully explain the exceptional sensitivity of myeloma cells to bortezomib. For example, inhibition of the antiapoptotic transcription factor nuclear factor-KB apparently contributes to the antitumor effects of bortezomib. The role of the UPR would be ascertained with parallel correlative analyses of serial marrow samples.
Moreover, the prognosis of patients with unmeasurable MM treated with bortezomib-based treatments is not fully defined. When we compared the PFS and OS among patients with unmeasurable disease who received thalidomide- or bortezomib-based therapy, there were no significant differences. In other words, the PFS and OS of patients with unmeasurable disease were not prolonged in bortezomib-based compared with thalidomide-based treatment based on numbers of patients available for this analysis. In contrast, the survival in patients with measurable disease was significantly improved in bortezomib-based compared with thalidomide-based therapy. Although the patient outcome in MM has greatly improved with bortezomib treatment, our data indicate that those patients with unmeasurable disease benefitted no better with bortezomib compared with thalidomide-based treatment. A previous small study of bortezomib vs conventional chemotherapy as initial treatment in patients with unmeasurable MM similarly showed no significant differences.16
The most important finding of our study is the benefit of bortezomib treatment in patients in China with measurable disease. Previous reports have shown that high levels of protein synthesis are more predictive for proteasome inhibitor-induced apoptosis resulting from ER stress induced by UPR response, in contrast to oligosecretory or nonsecretory MM with only low levels of M-protein and activation of UPR response in tumor cells.8,14,29 These studies may provide the basis, at least in part, for the differences observed in our study comparing survival of patients with measurable vs unmeasurable MM after bortezomib-based therapies.
In conclusion, in unmeasurable myeloma groups, the difference of PFS and OS between thalidomide-based and bortezomib-based therapies was not significant. Our findings suggest that the secretory status of serum or urine M-protein may predict for differential survival in response to bortezomib therapy, and in contrast, that patients with unmeasurable myeloma may benefit less from bortezomib treatment. On the basis of our results, there remains an urgent need to develop new drugs and explore new combination therapies for the treatment of patients with unmeasurable (oligosecretory, nonsecretory, and nonproducer) MM. There are some shortcomings in this study. This is a retrospective analysis with a limited number of patients with unmeasurable disease. Although we found that patients with nonproducer myeloma had a poor prognosis, the number of patients in this group was small (only 7 cases), which resulted in weak statistical strength. The conclusion may have some selective bias and needs to be confirmed in larger, controlled studies in patients with MM. At the same time, a lack of serum free light chain information in patients with myeloma and unmeasurable disease cannot be reclassified according to the level of free light chain in serum. Moreover, some other factors such as socioeconomic factors and cognition of medical science had influenced the choice of treatment. The conclusion needs to be confirmed in other patient cohorts, preferably in a prospective trial.
Acknowledgments
This study was supported by the National Natural Science Foundation (81670202, 81630007, 81570181), Chinese Academy of Medical Science Innovation Fund for Medical Sciences (CIFMS 2017-I2M-1-015; 2017-I2M-1-005; 2016-I2M-3-013), and Shanxi Province Natural Science Foundation (201601D021149).
Authorship
Contribution: X.-Q.Q. and G.A. performed FISH, analyzed data, and drafted the paper; L.-T.L., Y.Q., and M.-R.Z. performed FISH; Z.-J.L., Y.X., X.-Y.F., S.-H.D., W.-W.S., W.-J.Y., Y.-R.Z., S.-H.Y., T.-Y.W., R.L., D.-H.Z., and Y.-Z.Z. treated the patients; L.-H.Y. and Y.-P.M. analyzed the data; and L.-G.Q. designed the research and gave the final approval of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lu-Gui Qiu, State Key Laboratory of Experimental Hematology, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Tianjin 300020, China; e-mail: qiulg@ihcams.ac.cn.
References
Author notes
X.-Q.Q. and G.A. contributed equally to this work.