Key Points
The molecular events leading to primary and acquired resistance to ibrutinib in marginal zone lymphoma have not been studied.
We describe the first case of MZL with acquired resistance to ibrutinib in which mutations in both BTK (C481S) and PLCG2 are documented.
Introduction
Ibrutinib is approved by the US Food and Drug Administration for the treatment of patients with relapsed/refractory marginal zone lymphoma (MZL) who are progressing on at least 1 previous anti-CD20–based therapy. This approval was based on the results of a multicenter phase 2 study of ibrutinib in relapsed/refractory MZL (n = 60) that showed an overall response rate of 48% and a median progression-free survival of 14.2 months.1 Although mechanisms of acquired resistance to ibrutinib have been described in chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), Waldenstrom macroglobulinemia (WM), and diffuse large B-cell lymphoma (DLBCL),2-6 none have been reported for MZL. Because more patients with relapsed/refractory MZL are receiving treatment with ibrutinib, it is important to explore the possible mechanisms of ibrutinib resistance and understand the outcomes for patients who progress on ibrutinib. Here, we present a case of acquired ibrutinib resistance in a patient with relapsed/refractory MZL who was treated with ibrutinib.
Methods
A 71-year-old white man with a history of MZL was seen in consultation for progressively worsening abdominal pain, fatigue, and weakness that had lasted for 4 weeks. He was originally diagnosed with MZL involving the right hilar lymph nodes and spleen in May 2009 via endobronchial ultrasound-guided biopsy which demonstrated kappa-restricted monoclonal B cells that expressed CD20 and CD19 and were negative for CD5 and CD10. After an initial period of observation, he was treated in June 2010 with 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with a complete response and then after progression (June 2017, bone marrow biopsy–proven recurrence) with bendamustine and rituximab, which was discontinued after 2 cycles because of disease progression. He was started on ibrutinib in August 2017, and by October 2017, he had achieved a partial response on restaging scans. In February 2018, he presented with disease progression manifested by progressive splenomegaly and lymphadenopathy as well as increasing lymphocyte count with circulating lymphoma cells, the majority of which were large with prominent nucleoli.
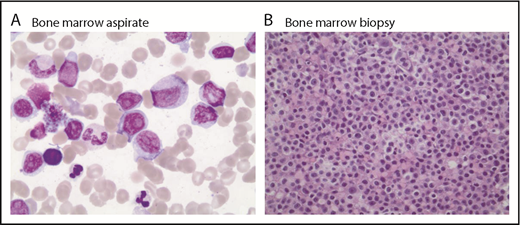
Because of concern for transformed lymphoma, a positron emission tomography scan and bone marrow biopsy were performed. The scan showed hypermetabolic lymphadenopathy throughout the neck, chest, and abdomen (standardized uptake value maximum, 11.7 in the mesenteric adenopathy), whereas bone marrow biopsy showed groups of medium to large lymphoid cells with moderate cytoplasm and prominent nucleoli occupying 10% to 15% of marrow cellularity with a Ki-67 of 30% to 40% consistent with large cell transformation of MZL (Figure 1). On cytogenetic analysis, there were additional abnormalities identified at progression that were not present before treatment with ibrutinib (supplemental Table 1).
Bone marrow aspirate and biopsy (postibrutinib). Large cells noted on bone marrow aspirate (A; magnification ×100, Wright Giemsa stain) and (B; magnification ×40, hematoxylin and eosin stain).
Bone marrow aspirate and biopsy (postibrutinib). Large cells noted on bone marrow aspirate (A; magnification ×100, Wright Giemsa stain) and (B; magnification ×40, hematoxylin and eosin stain).
Given the known association of ibrutinib resistance with BTK and phospholipase Cγ2 (PLCG2) mutations in CLL, a peripheral blood sample that contained transformed cells was sent to a clinical laboratory for ion torrent deep sequencing. A predominant BTK (C481S) and a minor PLCG2 (R665W) resistance mutation were detected in addition to TP53 mutation (Table 1). In contrast, a 15% to 25% involved bone marrow biopsy taken before ibrutinib was initiated showed only the same TP53 mutation but no BTK or PLCG2 mutations. The list of genes analyzed in the next-generation sequencing panel is provided in the supplemental Appendix (supplemental Table 2). Other than BTK (C481S) mutations and a minor PLCG2 (R665W) mutation, we did not identify any other drivers or passenger mutations at progression on peripheral blood sorted B cells.
Variant allele frequencies and nucleotide changes at baseline vs relapse
| Gene . | BMA, 15%-25% lymphoma June 2017 (before ibrutinib) . | Peripheral blood sorted B cells March 2018 (after ibrutinib) . |
|---|---|---|
| TP53 | ||
| Alteration | p.F113V | p.F113V |
| Nucleotide | c.337T>G | c.337T>G |
| VAF, % | 27.5 | 96.9 |
| BTK | ||
| Alteration | None | p.C481S |
| Nucleotide | c.1442G>C | |
| VAF, % | 76.5 | |
| PLCG2 | ||
| Alteration | None | p.R665W |
| Nucleotide | c.1993C>T | |
| VAF, % | 2.1 |
| Gene . | BMA, 15%-25% lymphoma June 2017 (before ibrutinib) . | Peripheral blood sorted B cells March 2018 (after ibrutinib) . |
|---|---|---|
| TP53 | ||
| Alteration | p.F113V | p.F113V |
| Nucleotide | c.337T>G | c.337T>G |
| VAF, % | 27.5 | 96.9 |
| BTK | ||
| Alteration | None | p.C481S |
| Nucleotide | c.1442G>C | |
| VAF, % | 76.5 | |
| PLCG2 | ||
| Alteration | None | p.R665W |
| Nucleotide | c.1993C>T | |
| VAF, % | 2.1 |
BMA, bone marrow aspirate; VAF, variant allele frequencies.
After ibrutinib therapy ended (March 2018), the patient was started on salvage therapy (rituximab, ifosfamide, carboplatin, and etoposide [RICE]), but he had a poor response to a single cycle of RICE. Given the progression of the disease and worsening performance status, the patient and the family opted for palliative care. The patient died shortly thereafter.
Results and discussion
MZL is the third most common form of non-Hodgkin lymphoma (NHL), accounting for 8% to 12% of all B-cell NHLs.7,8 Treatment of MZL has been adopted from other indolent NHLs with no standard treatment guidelines. Ibrutinib, a first-in-class BTK inhibitor, was studied in relapsed/refractory MZL in a phase 2 clinical trial and showed an overall response rate of 48% with complete response noted in 2 patients (3%). In the study, 5% of patients (n = 3) (based on independent assessment) had progressive disease (primary resistance), whereas 32% of study participants with an initial response discontinued the drug because of disease progression (acquired resistance).1 The underlying molecular events leading to the primary and acquired resistance to ibrutinib in MZL have not been studied.
Ibrutinib resistance has been studied in hematologic malignancies, including CLL, MCL, WM, and DLBCL. In CLL, the majority of cases of acquired resistance to ibrutinib are the result of the acquisition of mutations in BTK at the binding site (C481S mutations) or mutations in PLCG2, the kinase immediately downstream of BTK.2,3 BTK C481S decreases ibrutinib binding affinity for BTK.2,3 Notably, these mutations have not been demonstrated at high frequency in patients who undergo Richter’s transformation9 ; however, these reports were limited by a lack of transformed tissue in the majority of cases. BTK C481S mutations have been identified in MCL patients with late progression, but not in patients with early progression or primary resistance.4 BTK C481S has also been observed in WM, as well as mutations in CXCR4 that have been demonstrated to confer ibrutinib resistance.5,6 Recently, BTK C481S mutations have also been described in patients with DLBCL (n = 2) who progressed on ibrutinib therapy.10 Here, we describe the first case (to the best of our knowledge) of MZL with acquired resistance to ibrutinib in which mutations in both BTK (C481S) and PLCG2 are documented. Interestingly, this patient progressed with transformed lymphoma, and the mutations were demonstrated in the transformed cells, contrary to what has been reported in the CLL experience. With the recent approval by the US Food and Drug Administration of ibrutinib in MZL, identification of resistance mechanisms in this disease is of critical importance so that rational strategies for management can be developed.
Patients with CLL and MCL who progress on ibrutinib do poorly; MCL patients have a median overall survival of 2.5 to 8 months after treatment with ibrutinib has failed.11-13 The outcomes of patients with MZL who progress on ibrutinib has not been evaluated in a systematic fashion. In our case, the patient’s disease (after transformation) rapidly progressed despite aggressive salvage therapy. Further studies are needed to validate our findings and to perform functional characterization of mutations in patients with MZL who progress on ibrutinib. Given the relatively small population of patients with MZL compared with the population of patients who have CLL and MCL and are treated with ibrutinib, it will likely take time before comprehensive studies can be undertaken. In addition, it will be important to study the outcomes of patients with MZL who progress on ibrutinib in the context of current therapies and to develop dedicated studies of novel agents and combinations in this patient population.
The full-text version of this article contains a data supplement.
Authorship
Contribution: N.E. and J.A.W. conceived and designed the study; N.E., A.Y.S., and J.A.W. collected and assembled the data; N.E. and J.A.W. analyzed the data; N.E. prepared the first draft of the manuscript; and A.Y.S., D.J., B.A.C., S.A., K.M., and J.A.W. interpreted the data and provided critical comments on, helped revise, and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology, Department of Medicine, The Ohio State University, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210; e-mail: narendranath.epperla@osumc.edu.