Key Points
The B-cell receptor signaling pathway is active in diffuse large B-cell lymphomas, with increased expression of MYC and BCL2 protein.
The overall response rate was 60% for relapsed/refractory non–germinal center double-expressor lymphoma patients treated with ibrutinib.
Introduction
Patients diagnosed with diffuse large B-cell lymphoma (DLBCL) may be cured with front-line immunochemotherapy or with high-dose chemotherapy, followed by autologous stem cell transplantation (ASCT), in the relapsed/refractory (R/R) setting. However, those diagnosed with certain high-risk molecular subtypes of DLBCL may experience poor outcomes following receipt of cytotoxic therapy-based treatments. One such subtype is double-expressor lymphoma (DEL), defined as DLBCL with expression of MYC ≥40% and BCL2 ≥50% by immunohistochemistry (IHC) staining.1 DEL represents ∼20% to 35% of newly diagnosed DLBCL, and DEL patients experience significantly shorter survival following treatment with front-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone),2-4 as well as second-line high-dose therapy with ASCT,5,6 compared to those without DEL.
Ibrutinib, a small molecule inhibitor of Bruton tyrosine kinase, a component of the B-cell receptor (BCR) signaling pathway, has shown efficacy in patients with R/R DLBCL, with an overall response rate of 20% to 30%. Higher response rates are experienced by patients with activated B cell (ABC) compared with germinal center B (GCB) cell of origin (COO) when defined by gene-expression profiling (GEP)7 but similar response rates in patients with non-GCB and GCB COO when defined by Hans algorithm.8
Analysis of DLBCL patient tumors demonstrating increased expression of MYC and BCL2 protein suggest upregulation of the BCR signaling pathway. In a cohort of untreated de novo DLBCL patient tissue samples, those with MYC expression >40% by IHC staining demonstrated increased expression of BCR-associated proteins pBLK and pSYK compared with samples with MYC expression ≤40%.9 In a separate cohort, MYC/BCL2 dual-expressing DEL (MYC expression >40% and BCL2 expression >50%) specimens were correlated with active BCR signaling (assessed by phosphorylation of LYN, SYK, and Bruton tyrosine kinase) and highly correlated with cytoplasmic localization of the AKT-dependent transcription factor FOXO1 compared with non-DEL specimens.10,11
Therefore, we hypothesized that DEL patients would be likely to respond to therapy targeting the BCR signaling pathway and investigated this hypothesis through retrospective analysis of a cohort of patients with R/R DEL treated with ibrutinib.
Methods
Cases reviewed were those of patients with R/R DLBCL or high-grade B-cell lymphoma (HGBL) treated with ibrutinib monotherapy at the University of Pennsylvania, The Ohio State University, or the Cleveland Clinic, with IHC stains for MYC and BCL2 from the tissue specimen obtained most recently prior to initiation of ibrutinib available for hematopathologist (HP) review. Patients receiving ibrutinib for R/R DLBCL/HGBL were identified through prescribing records. Exclusion criteria were HIV positivity, posttransplant lymphoproliferative disorder, prior chronic lymphocytic leukemia, and inadequate data. All available IHC stains for MYC and BCL2 were scored by an HP at the treating center (HP1), as well as by a second HP at one of the other centers (HP2), through review of digital slide images, but they were not centrally reviewed by a single HP. IHC staining for MYC and/or BCL2 was performed for this analysis if not already performed for clinical evaluation. In cases of disagreement, the score assigned in the clinical pathology report was used as a tiebreaker. Survival times were estimated via the Kaplan-Meier method and association of clinicopathologic and treatment characteristics with response were estimated via logistic regression, with statistical significance defined as a 2-tailed P < .05. Disease response, progression-free survival (PFS), duration of response, and overall survival (OS) were defined by the Revised Response Criteria for Malignant Lymphoma.12 Survival times were estimated via the Kaplan-Meier method, and association of clinicopathologic and treatment characteristics with response were estimated via logistic regression. Data were censored on 1 July 2018. All statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX). This protocol was approved by the Institutional Review Board of each participating center. Research was conducted by the tenets of the World Medical Association’s most recently revised Declaration of Helsinki.
Results and discussion
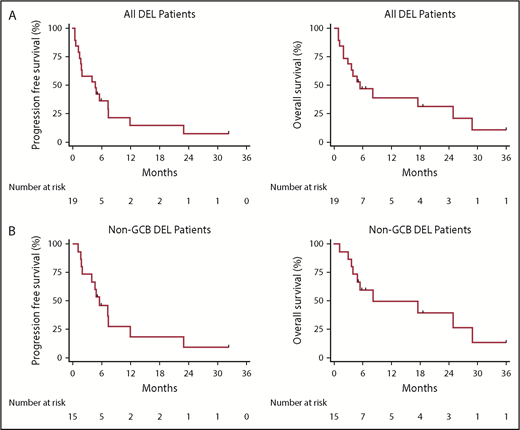
Twenty-five cases with IHC stains for MYC and BCL2 were reviewed, with 19 demonstrating MYC expression ≥40% and 23 demonstrating BCL2 expression 50% by IHC staining. All 19 cases with MYC ≥40% by IHC staining also demonstrated BCL2 ≥50% by IHC staining and, therefore, were classified as having DEL and were included in the outcomes analysis. Clinicopathologic characteristics at the time of ibrutinib initiation and treatment characteristics are listed in Table 1. The overall response rate (ORR) was 47%, with a complete response (CR) rate of 37%, median PFS of 4.7 months, median duration of response of 4.2 months, and median OS of 5.5 months. All responding patients were classified as having non-GCB COO. For non-GCB COO patients, the ORR and CR rate were 60% and 47%, respectively, and median PFS and OS were 5.5 months and 8.2 months, respectively. Kaplan-Meier plots depicting survival outcomes are shown in Figure 1. Other than COO, no baseline or treatment characteristic was significantly associated with response to ibrutinib. No patient known to have double-hit lymphoma responded to therapy. One responding patient discontinued ibrutinib due to cardiac toxicity. CR was achieved by 1 patient with and 1 patient without the L265P mutation present.
Clinicopathologic and treatment characteristics
| Characteristic . | All patients (N = 19) . |
|---|---|
| Age, median (range), y | 69 (33-98) |
| Sex, no. (%) | |
| Female | 6 (32) |
| Male | 13 (68) |
| International Prognostic Index score, no. (%) | |
| <3 | 6 (32) |
| ≥3 | 13 (68) |
| Histologic classification, no. (%) | |
| DLBCL | 14 (74) |
| HGBL | 5 (26) |
| Prior low-grade lymphoma, no. (%) | |
| No | 14 (74) |
| Yes | 5 (26) |
| Ki67, median (range), % | 80 (50-95) |
| COO by Hans algorithm, no. (%) | |
| GCB | 4 (21) |
| NGC | 15 (79) |
| Fluorescence in situ hybridization, no. (%) | |
| MYC rearrangement present | 7/14 (50) |
| BCL2 rearrangement present | 3/12 (25) |
| BCL6 rearrangement present | 1/7 (14) |
| Double-hit lymphoma | 3 |
| MYD88 L265P mutation present | 1/6 (17) |
| Front-line chemotherapy, no. (%) | |
| R-CHOP | 14 (74) |
| Intensive | 5 (26) |
| Second-line therapy, no. (%) | |
| Curative-intent cytotoxic therapy | 10 (53) |
| Other cytotoxic therapy | 2 (10) |
| Ibrutinib | 3 (16) |
| Other noncytotoxic therapy | 4 (21) |
| Prior ASCT, no. (%) | |
| No | 11 (58) |
| Yes | 8 (42) |
| Ibrutinib as line of therapy, no. (%) | |
| Second | 3 (16) |
| Third | 8 (42) |
| Fourth | 4 (21) |
| ≥Fifth | 4 (21) |
| Duration from start of prior therapy to start of ibrutinib, no. (%), mo | |
| 0-6 | 15 (79) |
| 6-12 | 3 (16) |
| >12 | 1 (5) |
| Characteristic . | All patients (N = 19) . |
|---|---|
| Age, median (range), y | 69 (33-98) |
| Sex, no. (%) | |
| Female | 6 (32) |
| Male | 13 (68) |
| International Prognostic Index score, no. (%) | |
| <3 | 6 (32) |
| ≥3 | 13 (68) |
| Histologic classification, no. (%) | |
| DLBCL | 14 (74) |
| HGBL | 5 (26) |
| Prior low-grade lymphoma, no. (%) | |
| No | 14 (74) |
| Yes | 5 (26) |
| Ki67, median (range), % | 80 (50-95) |
| COO by Hans algorithm, no. (%) | |
| GCB | 4 (21) |
| NGC | 15 (79) |
| Fluorescence in situ hybridization, no. (%) | |
| MYC rearrangement present | 7/14 (50) |
| BCL2 rearrangement present | 3/12 (25) |
| BCL6 rearrangement present | 1/7 (14) |
| Double-hit lymphoma | 3 |
| MYD88 L265P mutation present | 1/6 (17) |
| Front-line chemotherapy, no. (%) | |
| R-CHOP | 14 (74) |
| Intensive | 5 (26) |
| Second-line therapy, no. (%) | |
| Curative-intent cytotoxic therapy | 10 (53) |
| Other cytotoxic therapy | 2 (10) |
| Ibrutinib | 3 (16) |
| Other noncytotoxic therapy | 4 (21) |
| Prior ASCT, no. (%) | |
| No | 11 (58) |
| Yes | 8 (42) |
| Ibrutinib as line of therapy, no. (%) | |
| Second | 3 (16) |
| Third | 8 (42) |
| Fourth | 4 (21) |
| ≥Fifth | 4 (21) |
| Duration from start of prior therapy to start of ibrutinib, no. (%), mo | |
| 0-6 | 15 (79) |
| 6-12 | 3 (16) |
| >12 | 1 (5) |
Kaplan-Meier estimates of PFS and OS. PFS (left panels) and OS (right panels) for all DEL patients (A) and non-GCB DEL patients (B).
Kaplan-Meier estimates of PFS and OS. PFS (left panels) and OS (right panels) for all DEL patients (A) and non-GCB DEL patients (B).
For all cases reviewed (n = 25), MYC IHC was scored by HP1 and HP2 and was available in the clinical pathology report for 100%, 84%, and 88%, respectively, and BCL2 IHC was scored by HP1 and HP2 and was available in the clinical pathology report for 100%, 84%, and 100%, respectively. Rates of concordance and κ coefficient for HP1 and HP2 were as follows: MYC, 85.7% (κ = 0.49), BCL2, 95.2% (κ = 0.64), and DEL, 84.0% (κ = 0.61). In 4 cases with disagreement between HP1 and HP2 with regard to DEL status, the clinical pathology report agreed with HP1.
An objective response to ibrutinib monotherapy was experienced by nearly half of the patients with R/R DEL in this series, suggesting that the BCR signaling pathway may be particularly active in this subtype of aggressive lymphoma. The ORR in patients with R/R DEL with non-GCB COO, as defined by Hans algorithm, included in this series (60%) is higher than that reported for patients with ABC COO, as defined by GEP (37%), in a phase 1/2 clinical trial of ibrutinib monotherapy in R/R DLBCL.7 Although it is difficult to draw conclusions about the impact of DEL status on the outcome across these 2 analyses, given that ours was retrospective and included only DEL patients and the other did not report MYC and BCL2 expression by IHC, the finding of differential expression of genes in ABC COO DLBCL based upon DEL status2 may explain this difference in response rates and could be investigated further in patients treated with ibrutinib. Additionally, analysis of outcomes based upon DEL status for those enrolled in the recently reported PHOENIX study, which randomized patients with untreated non-GCB DLBCL, as defined by IHC (68% of whom were classified as ABC COO by GEP), to receive R-CHOP, with or without ibrutinib,13 may be informative in the context of our findings.
The strengths of our study included multicenter HP review to increase accuracy in diagnosing cases of DEL, with interpathologist concordance and κ coefficient for MYC and BCL2 overexpression similar to previously published analyses using cutoffs of 40% and 50%, respectively,14,15 as well as reporting of extensive clinicopathologic and treatment characteristics of included cases. Weaknesses of our study include the retrospective nature and small sample size, the latter of which was a result of limiting the number of participating institutions to successfully coordinate multicenter HP review.
In conclusion, our data demonstrate activity of single-agent ibrutinib in R/R DEL patients. Our analysis supports the use of ibrutinib as a bridge to definitive cellular therapies, as well the development of prospective treatment protocols evaluating the efficacy of ibrutinib, along with correlative molecular studies, in this poor-prognosis patient population.
Authorship
Contribution: D.J.L. treated patients, designed research, collected data, analyzed data, performed statistical analyses, and wrote the manuscript; K.J.M. and B.T.H. treated patients, collected data, and edited the manuscript; M.E.H., A.K., D.B., L.G., A.M.W., S.L.O., E.D.H., and A.M.B. collected data and edited the manuscript; and S.D.N., J.S., and S.J.S. treated patients and edited the manuscript.
Conflict-of-interest disclosure: K.J.M. has received research funding and honoraria from Pharmacyclics/Janssen. B.T.H. has received honoraria from and is a member of the Board of Directors or advisory committee for Pharmacyclics. J.S. has acted as a consultant and received research funding from Pharmacyclics. A.B. has acted as a consultant for Janssen. The remaining authors declare no competing financial interests.
Correspondence: Daniel J. Landsburg, University of Pennsylvania, 3400 Civic Center Blvd, #12-182, Philadelphia, PA 19104; e-mail: daniel.landsburg@uphs.upenn.edu.