Key Points
The presence of butyrogenic bacteria after the onset of acute GVHD associates with subsequent steroid-refractory GVHD or chronic GVHD.
Butyrate inhibits human colonic stem cells from forming an intact epithelial monolayer.
Introduction
Butyrogenic bacteria are ubiquitously present in the healthy human gut microbiome.1 Butyrate is a major energy source for the gut epithelium,2 a potent histone deacetylase inhibitor3 and agonist for peroxisome proliferator-activated receptors.4 Studies have demonstrated an association between administration of butyrate and protection from acute graft-versus-host disease (GVHD)5 after allogeneic hematopoietic cell transplant (HCT).
Steroid-refractory acute GVHD causes substantial morbidity and is associated with about 50% mortality at 6 months.6 Effective second-line therapy for this condition has yet to be defined. Although several studies have established a link between gut bacteria and onset of acute GVHD,7,,-10 few studies have investigated the role of butyrogenic organisms in recovery from severe acute GVHD or the development of chronic GVHD of the gut. Exposure to antibiotics with anaerobic activity (butyrogens are obligate anaerobes) associated with higher risk for mortality from acute GVHD9 ; the presence of Blautia species associated with lower mortality.11
In the context of colitis resulting in the loss of crypt architecture, butyrate inhibits healing of the murine gut.12 Thus, there is evidence that butyrate could be beneficial or harmful in GVHD pathogenesis after onset of acute GVHD, depending on the status of the colonic mucosa. Here we assess whether the presence of butyrogenic organisms after the onset of acute GVHD colitis in a human HCT cohort predicts subsequent steroid-refractory or chronic GVHD of the gut.
Methods
In a previously described cohort of HCT recipients (n = 210)7 undergoing allogenic transplant, we collected weekly stool specimens extending from before transplant through day 100 posttransplant (institutional review board approved and with informed consent in accordance with the Declaration of Helsinki).
Stool specimens underwent DNA extraction and 16S rRNA gene amplicon sequencing, as previously described.7 Sequence variants were generated using DADA2,13 and then phylogenetically placed on a custom reference set.14,15 Butyrogens were identified based on prior surveys,16,17 as in Haak et al,18 and provided as a supplemental Data. The number of butyrogens and cumulative relative abundance of butyrogens was determined for each stool specimen. The SRA bioproject is PRJNA566174.
Standardized retrospective chart review was used to determine whether participants developed steroid-refractory GVHD (failed to respond to the equivalent of 2 mg/kg of methylprednisolone) or chronic GHVD of the gut, as defined by standardized clinical review during long-term follow-up.
Colonic epithelium from human colonic organoids (colonoids) were derived as described in Tsai et al19 and per the standard protocols of the Translational Tissue Modeling Laboratory of the University of Michigan adapted from Tsai et al.19 Four technical replicates were used for each condition. Trans-epithelial electrical resistance was measured daily.
Results and discussion
In a cohort of 210 HCT recipients, 27 recipients had severe acute GVHD of the gut (stage ≥2). Of those with severe acute GVHD of the gut, 4 went on to have chronic GVHD of the gut, and 11 had steroid-refractory acute GVHD (supplemental Table 1).
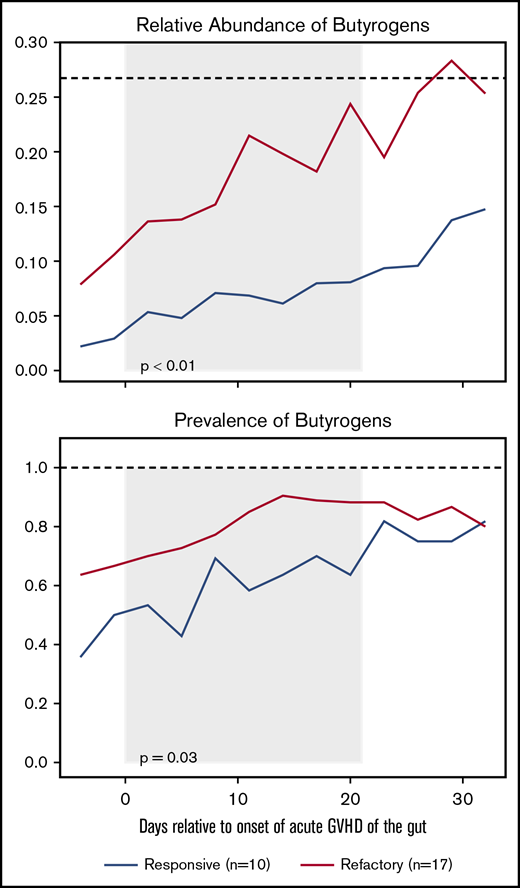
Increased relative abundance and having at least 1 butyrogenic bacterium present in the gut microbiome within 21 days after the onset of severe acute GVHD was associated with an increased risk of developing steroid-refractory GVHD (Figure 1; P < .05 mixed effects modeling). Chronic GVHD analysis was limited by the small number of recipients who developed chronic GVHD of the gut (n = 4); the presence of at least 1 butyrogen in the gut was also significantly associated with subsequent chronic GVHD of the gut (supplemental Figure 1; P < .05 by mixed effects modeling).
The relative abundance and prevalence (fraction of HCT recipients with at least 1 butyrogen present) of butyrogens after the onset of acute GVHD of the gut stratified by steroid responsive or refractory GVHD. All plots are rolling averages. P values from mixed-effects modeling from day 0 to +21 (with gray background) relative to the peak of acute GVHD symptoms. Black dashed line is the median value for healthy donors.
The relative abundance and prevalence (fraction of HCT recipients with at least 1 butyrogen present) of butyrogens after the onset of acute GVHD of the gut stratified by steroid responsive or refractory GVHD. All plots are rolling averages. P values from mixed-effects modeling from day 0 to +21 (with gray background) relative to the peak of acute GVHD symptoms. Black dashed line is the median value for healthy donors.
There was no association between the presence of a butyrogen at the point of neutrophil recovery and subsequent severe acute GVHD of the gut (supplemental Table 2; P = .3 per a Fisher’s exact test when comparing stage 0 or 1 acute gut GVHD with stage 2-4).
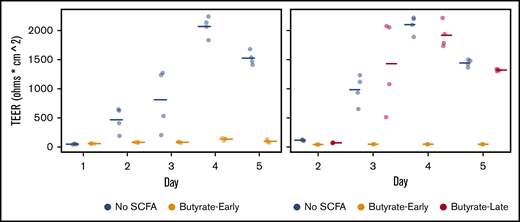
In the murine gut, butyrate suppresses the proliferation of colonic stem/progenitor cells12 ; in that model, the colonocyte metabolism of butyrate was determined to protect the crypts from exposure to butyrate, thereby protecting the stem cells at the base of the crypt from microbially produced butyrate in the lumen. To determine how butyrate affects human colonic stem cells, we employed an in vitro model of the human colon that uses colonoids, a primary human stem cell culture model. After dissociating and plating 3-dimensional colonoids on 2-dimensional transwells, the epithelium forms a monolayer with tight junctions associated with epithelial differentiation.20 The formation of a tight epithelial barrier over the course of several days in monolayer culture was impaired by the presence of physiologic concentrations of butyrate in the upper chamber of the transwell model (Figure 2) when exposure to butyrate occurred at the start of differentiation (P < .05 by a 2-tailed Student t test at day 4) in 2 biological replicates.
Transepithelial electrical resistance (TEER, in ohms * cm2) in differentiating colonic epithelium derived from primary human colonoids with varying timing of butyrate in the luminal compartment. With Hanks Buffered Salt Solution in the luminal compartment (No SCFA), a high level of TEER is achieved by differentiation day 4. With HBSS with 10 mM butyrate (Butyrate-Early) in the luminal compartment from day 1 onward, TEER does not develop. Butyrate (10 mM) from day 3 onward (Butyrate-Late) does not impair the development of TEER. Points are offset on the x-axis from the ordinal days for visual clarity.
Transepithelial electrical resistance (TEER, in ohms * cm2) in differentiating colonic epithelium derived from primary human colonoids with varying timing of butyrate in the luminal compartment. With Hanks Buffered Salt Solution in the luminal compartment (No SCFA), a high level of TEER is achieved by differentiation day 4. With HBSS with 10 mM butyrate (Butyrate-Early) in the luminal compartment from day 1 onward, TEER does not develop. Butyrate (10 mM) from day 3 onward (Butyrate-Late) does not impair the development of TEER. Points are offset on the x-axis from the ordinal days for visual clarity.
We propose that after the onset of severe acute GVHD of the gut, if butyrogens are present and active, the loss of epithelial architecture21 results in exposure of the colonic stem cells to microbially produced butyrate. This exposure may impair colonic mucosal recovery from acute GVHD, leading to increased risk for refractory and chronic GVHD. Thus, butyrogens may be helpful in protecting against the onset of acute GVHD,5 but once acute GVHD of the gut has occurred, butyrogens may impair recovery and lead to worsened outcomes.
This study infers butyrate levels in the gut by considering the presence, richness, and relative abundance of butyrogens in the stool (as in Haak et al18 ). However, a butyrogen being present does not mean it is active, nor does it give a 1:1 correspondence to the butyrate level. Measurement of butyrate levels in the stool requires carefully preserved specimens and is a focus of future prospective studies. Changes in intestinal transit time and treatments initiated for GVHD could skew our findings. This is a preliminary study into our focused question; studies in other transplant cohorts and further mechanistic studies are needed to confirm and extend our findings.
This proposed biphasic response to butyrate (helpful in prevention of acute GVHD; harmful to recovery once colitis has started) merits further exploration, but is broadly consistent with prior randomized controlled trials showing a benefit to gut decontamination for overall mortality22,23 ; in an era of less effective prevention strategies for acute GVHD, colitis may have been more common, tipping the balance to harm from a nominal microbiome in HCT recipients.
Third-party fecal microbiota transplant (FMT) has been attempted in HCT recipients.24,-26 We suspect the FMT could be responsible for a transient disruption in butyrate production by the gut microbiome long enough to allow for sufficient crypt reformation and for the beneficial effects of butyrate on intact colonic epithelium to predominate over the harms to colonic stem cells. There remains a lack of understanding of the optimal microbiome to transplant into HCT recipients via FMT (autologous or third party), and we are concerned about the assumption that microbiota that are beneficial in health will also be beneficial after HCT. Mixed results from the current FMT trials should not be interpreted as indicating that FMT post HCT is not effective but, rather, that careful attention must be made to both the colonization history and details of the specific gut microbiota being transplanted.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Maliha Berner, Kateryna Karpoff, Caroline McCarthy, and Angeline Wu from the Michigan Medicine Translational Tissue Modeling Laboratory for providing enteroid/colonoid 2-dimensional monolayer and 3-dimensional cultures. The Translational Tissue Modeling Laboratory is a University of Michigan-funded initiative (Center for Gastrointestinal Research, Office of the Dean, Comprehensive Cancer Center, Departments of Pathology, Pharmacology, and Internal Medicine) with support by the Endowment for Basic Sciences.
Funding for this study was provided by private donor funds, National Institutes of Health, National Institute of Allergy and Infectious Diseases AI-134808, and the American Society for Transplantation and Cellular Therapy New Investigator Award (J.L.G.). The National Institues of Health, National Cancer Institute cancer support grant CA15704 to the Fred Hutchinson Cancer Research Center supports the transplant outcome databases.
Authorship
Contribution: J.L.G. conceived the study and performed the in vitro modeling, primary data analysis, figure preparation, chart review, and writing of the manuscript; M.M.D. and T.L.F. prepared the amplicon libraries for sequencing after DNA extraction; T.L. oversees the study’s implementation, which includes identifying and consenting subjects and managing all samples collections throughout a subject’s participation in the study; Z.Z.Q. completed retrospective chart review; M.K.D. and S.S.S. were major contributors to model design, prepared colonoids and monolayers, and prepared and performed the in vitro modeling; M.C.W. conceived the statistical analysis plan; T.M.S. provided guidance on butyrogenesis; M.J.H. assisted with the colonoid model; M.M. contributed to analysis of the GVHD results; J.S. contributed to the design and interpretation of the in vitro model results and edited the manuscript; S.A.P. established the clinical cohort and supervises consent of participants; and D.N.F. contributed to the conception and editing of the manuscript, and gut bacterial community composition data were generated in his laboratory.
Conflict-of-interest disclosure: S.A.P. has received research support from Global Health Technologies, Inc. and participates in clinical trials with Chimerix. The remaining authors declare no competing financial interests.
Correspondence: Jonathan L Golob, University of Michigan, 1510E MSRB I, 1150 West Medical Center Dr, SPC 5666, Ann Arbor, MI 48109-5666; e-mail: jonathan@goloblab.org or golobj@med.umich.edu.