Key Points
Real-time tracking of newly synthesized α-granule proteins delineates the recycling endosome as a key intermediate in α-granule biogenesis.
Disease-associated proteins VPS33B and VPS16B form a distinct complex, primarily functioning at recycling endosomes.
Abstract
Platelet α-granules play important roles in platelet function. They contain hundreds of proteins that are synthesized by the megakaryocyte or taken up by endocytosis. The trafficking pathways that mediate platelet α-granule biogenesis are incompletely understood, especially with regard to cargo synthesized by the megakaryocyte. Vacuolar-protein sorting 33B (VPS33B) and VPS16B are essential proteins for α-granule biogenesis, but they are largely uncharacterized. Here, we adapted a powerful method to directly map the pathway followed by newly synthesized cargo proteins to reach α-granules. Using this method, we revealed the recycling endosome as a key intermediate compartment in α-granule biogenesis. We then used CRISPR/Cas9 gene editing to knock out VPS33B in pluripotent stem cell–derived immortalized megakaryocyte cells (imMKCLs). Consistent with the observations in platelets from patients with VPS33B mutation, VPS33B-knockout (KO) imMKCLs have drastically reduced levels of α-granule proteins platelet factor 4, von Willebrand factor, and P-selectin. VPS33B and VPS16B form a distinct and small complex in imMKCLs with the same hydrodynamic radius as the recombinant VPS33B-VPS16B heterodimer purified from bacteria. Mechanistically, the VPS33B-VPS16B complex ensures the correct trafficking of α-granule proteins. VPS33B deficiency results in α-granule cargo degradation in lysosomes. VPS16B steady-state levels are significantly lower in VPS33B-KO imMKCLs, suggesting that VPS16B is destabilized in the absence of its partner. Exogenous expression of green fluorescent protein–VPS33B in VPS33B-KO imMKCLs reconstitutes the complex, which localizes to the recycling endosome, further defining this compartment as a key intermediate in α-granule biogenesis. These results advance our understanding of platelet α-granule biogenesis and open new avenues for the study of these organelles.
Introduction
Platelets are anucleate blood cells that serve key functions in hemostasis and have also been implicated in several other processes such as inflammation and angiogenesis.1-3 Normal platelet function depends on α-granules, secretory organelles that release their content upon platelet activation.1-6 α-Granules are unusual because they contain both proteins that are synthesized by the megakaryocyte (the platelet precursor) and proteins taken up by endocytosis.1 The intracellular trafficking pathway followed by endocytosed cargo was previously elucidated by applying a pulse chase approach with labeled proteins added to the culture media. Quickly after endocytosis, cargo populates early endosomes, then late endosomes/multivesicular bodies (MVBs), and finally arrive to α-granules.7,8 An analogous approach to establish the pathway followed by megakaryocyte-synthesized cargo has not yet been developed, thus leaving the biogenesis of α-granules incompletely understood.
The first proteins known to be essential for α-granule biogenesis are vacuolar-protein sorting 33B (VPS33B) and VPS16B (also known as VIPAR).9-12 Mutation of VPS33B and VPS16B cause arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome, which presents with α-granule deficiency and other manifestations.5,9 The VPS33B and VPS16B proteins are believed to form a complex, similar to paralog proteins VPS33A and VPS16A, which are also expressed in mammalian cells.11-13 Yeast cells contain only one form of VPS33 and VPS16, which associate with each other and are part of class C core vacuole/endosome tethering (CORVET) and homotypic fusion and vacuole protein-sorting (HOPS), 2 large hetero-hexameric complexes.14 Yeast CORVET and HOPS have been well studied; they mediate tethering and assist in the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE)–regulated fusion of vesicles with early and late endosomal compartments, respectively.14,15 Based on earlier reports, the subunit composition of mammalian CORVET and HOPS was less clear, and it was suggested that CORVET might contain VPS33B and VPS16B, and HOPS might contain VPS33A and VPS16A.14,16,17 However, more recent publications showed that both mammalian CORVET and HOPS contain VPS33A and VPS16A and are hetero-hexameric complexes similar to the yeast counterparts, and that VPS33B and VPS16B are not part of CORVET or HOPS.5,18-22 There is significant evidence suggesting VPS33A and VPS33B perform different functions, cannot substitute for each other, and are components of separate complexes.5,11,13,19 In fact, VPS33A mutation in mice causes platelet-dense granule deficiency without affecting α-granules.23,24 However, VPS33B and VPS16B have been less well characterized than VPS33A and VPS16A, and it is less clear if they are part of larger complexes akin to CORVET and HOPS. Recently published evidence in HEK293T cells indicates that VPS33B and VPS16B form a smaller complex than CORVET and HOPS.25 It is reasonable to predict that VPS33B and VPS16B function in intracellular vesicle trafficking, and there is information on their localization and connection with clathrin-dependent pathways on endosomes.10,12,17 Furthermore, VPS33B and VPS16B have been shown to function in cargo recycling in polarized epithelial cells, and VPS33B interacts physically with recycling endosome component CCDC22 in HEK293 cells.9,11,25,26 Nevertheless, we have a limited understanding of these crucial components of the α-granule biogenesis machinery. Key aspects to improve upon the current knowledge include: characterizing the VPS33B-VPS16B complex at a biochemical level, better defining the specific trafficking step(s) it mediates in megakaryocytes, and elucidating the fate of α-granule cargo in cells lacking its function.
One reason why there is a knowledge gap regarding α-granule biogenesis is that primary megakaryocytes are difficult cells to isolate, culture, and manipulate and have a very short life span.27 The recent development of pluripotent stem cell–derived immortalized megakaryocyte cells (imMKCLs) capable of generating functional platelets has provided a promising system to facilitate mechanistic studies at the cellular level.28-30 Despite this potential, imMKCLs have not been specifically used in α-granule biogenesis studies.
Here we adapted an approach that allows for a synchronized release of newly synthesized α-granule proteins from the endoplasmic reticulum (ER) and the tracking of their pathway to α-granules in real time. This analysis allowed mapping of the pathway and revealed the recycling endosome as a novel post–Golgi complex compartment involved in sorting of proteins to the α-granule. We applied CRISPR/Cas9 gene editing technology to generate VPS33B-knockout (KO) imMKCLs that recapitulate the α-granule deficiency observed in platelets from patients with ARC and show the lack of VPS33B results in the degradation of α-granule proteins in lysosomes. We also show that VPS33B and VPS16B form a distinct, smaller complex unlike CORVET and HOPS that localizes to recycling endosomes.
Methods
Cell culture, transfection, and generation of VPS33B-KO imMKCLs
imMKCLs were a gift from Koji Eto (Kyoto University). These cells were cultured and differentiated as described previously.28 The supplemental Methods present additional information and include a description of VPS33B-KO imMKCL generation.
DNA constructs
All DNA constructs were generated by using the In-Fusion HD Cloning Plus kit (Takara Bio). The retention using selective hooks (RUSH) constructs were created by modifying the original Str-KDEL_TNF-SBP-EGFP plasmid31 as follows: the TNF coding sequence was replaced with either human platelet-derived growth factor (PDGF) or platelet factor 4 (PF4) ORFs and EGFP with Cherry. The EmGFP-SiT-N-15 and GFP-Myo5b plasmids were a gift from Michael Davidson (Addgene plasmid #54255) and Richard Cheney (University of North Carolina), respectively.
Fluorescence microscopy, RUSH system, RNA analysis, and biochemical procedures
Fluorescence microscopy, RNA analysis, and biochemical procedures were performed as previously described.32-39 The supplemental Methods provide additional information.
Statistical analysis
Error bars indicate mean ± standard error of the mean. Statistical significance was determined via unpaired, 2-tailed Student t tests: *P ≤ .05; **P ≤ .01; ***P ≤ .001; and ****P ≤ .0001.
Results
A method to study the intracellular pathway taken by newly synthesized proteins to reach the α-granule
α-Granules are produced by the bone marrow megakaryocyte and contain numerous proteins synthesized by the cell and others that are incorporated by endocytosis. Staining of megakaryocytes for a specific cargo typically detects its steady-state localization, mostly the α-granule, but it is inadequate to reveal intermediate compartments. The path followed by the endocytosed proteins to reach the α-granule has been mapped out by using pulse chase experiments with labeled cargo added to the culture media.7,8 An equivalent method to study α-granule proteins produced by the megakaryocyte has not been available.
To develop an approach that synchronizes biosynthetic α-granule cargo traffic, we adapted a technique known as RUSH, originally designed to study integral membrane proteins destined for the plasma membrane in nonspecialized cells.31 The RUSH system consists of 2 components: (1) an ER-hook composed of the core streptavidin protein fused to the ER retention tetrapeptide signal KDEL; and (2) a cargo-reporter consisting of an α-granule protein (eg, PDGF, PF4) fused to the short streptavidin-binding peptide and a fluorescent protein (Cherry) (Figure 1A-B). When the 2 components of the RUSH system are expressed in the ER, the ER-hook is retained by binding to the endogenous and abundantly expressed KDEL receptor, thus capturing the cargo-reporter in the ER. Addition of biotin to the culture media triggers a synchronized release of the cargo-reporter from the ER that can now traffic to the α-granule while being visualized by fluorescence microscopy. The ER-hook and PDGF-reporter were cloned in a bicistronic vector to ensure all cells expressing PDGF-reporter would have it hooked in the ER in the absence of biotin. As a proof of concept, live MEG01 cells transfected with the RUSH bicistronic vector were imaged with a spinning disk confocal fluorescence microscope at 37°C (Figure 1C). Simultaneous expression of marker proteins for the ER (blue fluorescent protein 2–KDEL) and Golgi complex (trans-Golgi network protein–green fluorescent protein [GFP]), as well as labeling of α-granules with endocytosed and chased fibrinogen–Alexa Fluor 647, allowed monitoring of PDGF-reporter localization at all times. At 0 minutes, PDGF-reporter was retained in the ER as indicated by the colocalization with the ER marker. At 25 minutes after biotin addition, essentially all PDGF-reporter molecules had left the ER and localized at the Golgi complex. At 150 minutes, a substantial proportion of the PDGF-reporter had exited the Golgi complex and reached α-granules. The data thus show: (1) the system is not leaky, as the reporter is fully hooked and retained in the ER in the absence of biotin; (2) after release, the reporter synchronously exits the ER, traverses the Golgi complex, and correctly targets α-granules; (3) the ability exists to perform simultaneous multicolor imaging, allowing identification of reporter localization to specific compartments at all times; and (4) it takes hours for cargo to traffic to α-granules.
RUSH: a system to monitor the transport of newly synthesized α-granule cargo in real time. (A-B) The RUSH system has 2 components: an ER-hook comprising the streptavidin protein fused to the ER retention signal KDEL, and a cargo-reporter composed of a cargo protein (PDGF) fused to streptavidin-binding peptide (SBP) and a fluorescent protein (Cherry). The cargo-reporter is retained in the ER by the ER-hook, which is bound to the KDEL-receptor. Biotin added to the culture media competes for binding to streptavidin, thus releasing the cargo-reporter from the ER-hook and allowing synchronized traffic to the α-granule. The ER-hook and cargo-reporter are cloned in a bicistronic plasmid to ensure all cells expressing the cargo-reporter have it hooked in the ER in the absence of biotin. (C) Confocal fluorescence microscopy analysis of live MEG01 cells cotransfected with the RUSH bicistronic plasmid and markers of different subcellular compartments: ER, blue fluorescent protein 2–KDEL (BFP2-KDEL); Golgi complex, trans-Golgi network protein–GFP (TGNP-GFP). The α-granules were labeled with endocytosed and chased fibrinogen–Alexa Fluor 647 (Fibrinogen-A647). Four-color images were acquired immediately before adding biotin (time = 0 minutes) and 25 and 150 minutes after the addition of biotin.
RUSH: a system to monitor the transport of newly synthesized α-granule cargo in real time. (A-B) The RUSH system has 2 components: an ER-hook comprising the streptavidin protein fused to the ER retention signal KDEL, and a cargo-reporter composed of a cargo protein (PDGF) fused to streptavidin-binding peptide (SBP) and a fluorescent protein (Cherry). The cargo-reporter is retained in the ER by the ER-hook, which is bound to the KDEL-receptor. Biotin added to the culture media competes for binding to streptavidin, thus releasing the cargo-reporter from the ER-hook and allowing synchronized traffic to the α-granule. The ER-hook and cargo-reporter are cloned in a bicistronic plasmid to ensure all cells expressing the cargo-reporter have it hooked in the ER in the absence of biotin. (C) Confocal fluorescence microscopy analysis of live MEG01 cells cotransfected with the RUSH bicistronic plasmid and markers of different subcellular compartments: ER, blue fluorescent protein 2–KDEL (BFP2-KDEL); Golgi complex, trans-Golgi network protein–GFP (TGNP-GFP). The α-granules were labeled with endocytosed and chased fibrinogen–Alexa Fluor 647 (Fibrinogen-A647). Four-color images were acquired immediately before adding biotin (time = 0 minutes) and 25 and 150 minutes after the addition of biotin.
To further validate the RUSH system for α-granule biogenesis studies, we tested it with another cargo, PF4, using imMKCLs. These cells can be differentiated in 5 days to mature megakaryocytes that produce functional platelets.28,29 Expression of the α-granule cargoes von Willebrand factor (VWF) and PF4 is enormously increased in differentiated vs undifferentiated cells, confirming that imMKCLs are a good model system to study α-granule biogenesis (supplemental Figure 1). We transfected imMKCLs on day 2 of differentiation with a RUSH system bicistronic plasmid containing the ER-hook and PF4-reporter. On day 4 of differentiation, we supplemented the culture media with fibrinogen–Alexa Fluor 488 and incubated the cells overnight. On day 5, we chased the fibrinogen for 3 hours to allow for the accumulation of fibrinogen in α-granules and the clearance of intermediate organelles. Confocal fluorescence microscopy images of the live cells were then acquired before and after the addition of biotin at various time points (Figure 2). As expected, PF4-reporter quickly cleared the ER, concentrated in the perinuclear area, where the Golgi complex is localized, and then started accumulating in fibrinogen-labeled α-granules (supplemental Figure 2). This analysis shows that the RUSH system is a robust approach to study α-granule biogenesis. Importantly, the fact that the cargo-reporter requires significant time to reach α-granules suggests a trafficking pathway that involves additional intermediate compartments after the Golgi complex.
Use of the RUSH system with imMKCLs and PF4 as the α-granule cargo. Differentiated imMKCLs were transfected with a RUSH bicistronic plasmid containing PF4-SBP-Cherry as a cargo-reporter, and their α-granules were labeled with endocytosed and chased fibrinogen–Alexa Fluor 488. Live cell confocal fluorescence microscopy images were acquired before and after the addition of biotin over a 5-hour period. (A) Quantification of the cargo-reporter percent colocalization with fibrinogen–Alexa Fluor 488 is represented with red bars (n = 5). The reciprocal colocalization of fibrinogen–Alexa Fluor 488 with the cargo-reporter is represented with green bars. (B) Images of the cargo-reporter and fibrinogen–Alexa Fluor 488 for one representative cell over a 5-hour period. Note that the cargo-reporter takes several hours to reach α-granules after leaving the ER.
Use of the RUSH system with imMKCLs and PF4 as the α-granule cargo. Differentiated imMKCLs were transfected with a RUSH bicistronic plasmid containing PF4-SBP-Cherry as a cargo-reporter, and their α-granules were labeled with endocytosed and chased fibrinogen–Alexa Fluor 488. Live cell confocal fluorescence microscopy images were acquired before and after the addition of biotin over a 5-hour period. (A) Quantification of the cargo-reporter percent colocalization with fibrinogen–Alexa Fluor 488 is represented with red bars (n = 5). The reciprocal colocalization of fibrinogen–Alexa Fluor 488 with the cargo-reporter is represented with green bars. (B) Images of the cargo-reporter and fibrinogen–Alexa Fluor 488 for one representative cell over a 5-hour period. Note that the cargo-reporter takes several hours to reach α-granules after leaving the ER.
Newly synthesized α-granule proteins traffic through recycling endosomes en route to α-granules
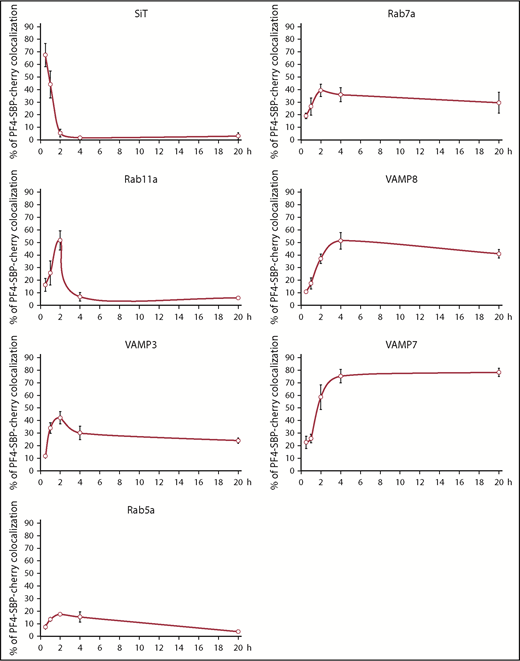
The RUSH system was then used in combination with various organelle markers to map intermediate post–Golgi compartments potentially navigated by newly synthesized cargo trafficking to α-granules. As described in the previous section, differentiated imMKCLs were transfected with the RUSH system bicistronic plasmid containing the ER-hook and PF4-reporter together with GFP-tagged markers of different organelles. Cells were fixed before and after the addition of biotin at different time points, numerous cells were imaged at each time point for each marker, and colocalization was quantified (Figure 3; supplemental Figure 3). As expected, PF4-reporter localization to the Golgi complex peaked at ∼30 minutes as determined by its colocalization with sialyltransferase. By 2 hours, PF4-reporter had completely cleared the Golgi complex. Remarkably, after passing through the Golgi complex, PF4-reporter was found in recycling endosomes labeled by Rab11a, peaking at ∼2 hours and totally clearing the organelle by ∼4 hours. Recycling endosomes also contain the SNARE protein VAMP3,40 which similarly exhibited significant colocalization with PF4-reporter and a maximum at ∼2 hours. In contrast, early endosomes did not seem to be significantly involved in the biosynthetic pathway to α-granules judging by the low colocalization level of PF4-reporter with Rab5a. However, we cannot rule out the possibility that PF4-reporter traffics too quickly through Rab5 compartments for efficient detection with this approach. In line with previous results that indicate α-granules have a late endocytic origin,7 PF4-reporter shows appreciable colocalization with the late endosome/MVB marker Rab7a before exhibiting maximal colocalization with markers of the more mature granules, VAMP7 and VAMP8, as well as fibrinogen (Figure 2; supplemental Figure 2).41-43 We used VAMP7 and VAMP8 as α-granule markers even though they have not been as well characterized in megakaryocytes compared with platelets because, in these studies, P-selectin–GFP did not express well enough to be used as a marker. Thus, the post–Golgi pathway followed by megakaryocyte-synthesized cargo is not direct but involves trafficking through recycling endosomes and late endosomes/MVBs before reaching the α-granules (Figure 7).
Mapping out the pathway taken by newly synthesized α-granule cargo. Differentiated imMKCLs were cotransfected with the RUSH bicistronic plasmid containing PF4-SBP-Cherry as a cargo-reporter and GFP-tagged markers of several compartments. Cells were fixed before and after the addition of biotin at various time points, and numerous cells were imaged for each marker at each time point (n ≥ 5 for each marker at each time point). The line graphs indicate the percent colocalization of the cargo-reporter with the GFP-tagged marker. SiT, sialyltransferase.
Mapping out the pathway taken by newly synthesized α-granule cargo. Differentiated imMKCLs were cotransfected with the RUSH bicistronic plasmid containing PF4-SBP-Cherry as a cargo-reporter and GFP-tagged markers of several compartments. Cells were fixed before and after the addition of biotin at various time points, and numerous cells were imaged for each marker at each time point (n ≥ 5 for each marker at each time point). The line graphs indicate the percent colocalization of the cargo-reporter with the GFP-tagged marker. SiT, sialyltransferase.
VPS33B and VPS16B define a distinct complex
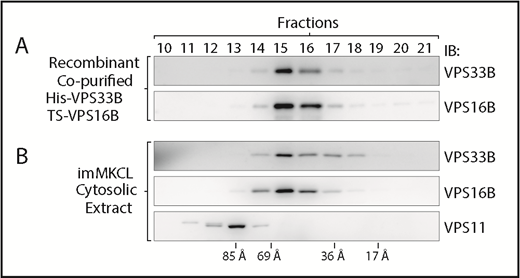
As mentioned in the Introduction, the VPS33B and VPS16B proteins have been previously shown to associate with each other,11,12 similar to their paralogs VPS33A and VPS16A.13 However, although the VPS33A-VPS16A unit is part of 2 larger complexes, CORVET and HOPS, the VPS33B-VPS16B complex is less well understood.5,12,13,18-22 We therefore proceeded to characterize the VPS33B-VPS16B complex. We cloned His-VPS33B and TwinStrep-VPS16B into a bicistronic vector and expressed them in Escherichiacoli. Sequential poly-histidine tag and Twin-Strep-tag purification (IBA Lifesciences) yielded pure VPS33B and VPS16B in equal amounts, suggesting a stable complex (supplemental Figure 4). Gel filtration chromatography and immunoblotting analysis of the recombinant VPS33B-VPS16B complex showed both proteins eluted in the same fractions, consistent with a stable complex (Figure 4A). We then obtained cytosolic extracts from imMKCLs and performed gel filtration chromatography under the same conditions. Endogenous VPS33B and VPS16B cofractionated and eluted in the exact same fractions as the purified recombinant complex, suggesting they associate with each other into a stable complex containing just those 2 proteins and do not form part of a larger complex. In addition, VPS11, a common component of both CORVET and HOPS, eluted in fractions corresponding to a bigger hydrodynamic radius consistent with the idea that the VPS33B-VPS16B complex is not part of CORVET, HOPS, or another complex of comparable architecture (Figure 4B). Similar gel filtration results were obtained with cytosolic extracts from MEG01 cells (supplemental Figure 5A). In addition, coimmunoprecipitation using an anti-VPS33B antibody was performed with each MEG01 gel filtration fraction containing VPS33B, VPS16B, or VPS11 (fractions 10-17). VPS16B but not VPS11 coimmunoprecipitated with VPS33B, consistent with a tight VPS33B-VPS16B complex that is not part of CORVET or HOPS (supplemental Figure 5B). These data are consistent with previous reports in which either coimmunoprecipitation or gel filtration analysis was performed.18,19,25 Therefore, VPS33B and VPS16B form a distinct stable complex in megakaryocytes, smaller than CORVET and HOPS, which likely does not include any additional protein. These results are consistent with a similar gel filtration analysis of HEK293T cell extracts showing that the VPS33B-VPS16B complex is smaller than CORVET and HOPS.25
VPS33B and VPS16B form a distinct and small complex. (A) Gel filtration chromatography analysis of recombinant copurified His-VPS33B/TS-VPS16B complex. Fractions were analyzed by immunoblotting with antibodies against VPS33B and VPS16B. (B) Gel filtration chromatography analysis of cytosolic extract obtained from imMKCLs. Fractions were analyzed by immunoblotting with antibodies against VPS33B, VPS16B, and VPS11. The column was calibrated with blue dextran and standard proteins of known hydrodynamic radius.
VPS33B and VPS16B form a distinct and small complex. (A) Gel filtration chromatography analysis of recombinant copurified His-VPS33B/TS-VPS16B complex. Fractions were analyzed by immunoblotting with antibodies against VPS33B and VPS16B. (B) Gel filtration chromatography analysis of cytosolic extract obtained from imMKCLs. Fractions were analyzed by immunoblotting with antibodies against VPS33B, VPS16B, and VPS11. The column was calibrated with blue dextran and standard proteins of known hydrodynamic radius.
Generation of VPS33B-KO imMKCLs
We generated 2 independent VPS33B-KO imMKCL clones by using the CRISPR/Cas9 genome editing technology.44 Immunoblotting analysis of total cell extracts showed the lack of VPS33B expression, and sequencing of genomic DNA confirmed the presence of insertion/deletions in the VPS33B-KO clones (supplemental Figures 6 and 7). The levels of VPS16B and α-granule cargo were analyzed by immunoblotting of total cell extracts of wild-type and VPS33B-KO imMKCLs in 3 independent experiments. The VPS16B levels were extremely reduced in imMKCLs lacking VPS33B, indicating that VPS16B is destabilized in the absence of its binding partner. As expected, 2 soluble α-granule cargoes (VWF and PF4) and a transmembrane α-granule protein (P-selectin) were considerably increased in differentiated vs undifferentiated wild-type imMKCLs. Importantly, differentiated VPS33B-KO cells displayed a significantly reduced level of all 3 α-granule proteins relative to the corresponding wild-type control. Accumulation of endocytosed fibrinogen–Alexa Fluor 488 quantified by using fluorescence microscopy was also reduced in differentiated VPS33B-KO cells relative to control cells (supplemental Figure 8). However, the defect in fibrinogen–Alexa Fluor 488 accumulation was less pronounced than that of megakaryocyte-synthesized α-granule proteins, perhaps reflecting different pathways taken by the cargoes. These results further validate the imMKCLs as a model to study α-granule biogenesis, are consistent with those obtained from platelets from ARC patients and with α-granule–deficient mouse models, and provide a new tool to investigate ARC at a cellular level.10,12,45
VPS33B deficiency results in degradation of α-granule proteins in lysosomes
The drastically reduced levels of α-granule cargo proteins in VPS33B-KO imMKCLs could be the result of mistrafficking and either degradation in lysosomes or secretion. To test for lysosome degradation, we incubated VPS33B-KO imMKCLs with the lysosome inhibitor bafilomycin A1 or vehicle, prepared total cell extracts, and assessed the amounts of PF4 and VWF by using immunoblotting (Figure 5). Quantification of 5 biological replicates normalized by the actin control showed a significant increase in PF4 and VWF overall levels in bafilomycin A1–treated cells, indicating lysosomal degradation of α-granule cargo in VPS33B-KO cells. Experiments performed in parallel with wild-type imMKCLs showed that bafilomycin A1 treatment caused a subtle decrease (rather than an increase) in PF4 and VWF. Conversely, by analyzing the culture media, we detected no mistrafficking to the plasma membrane or secretion of α-granule cargo in VPS33B-KO imMKCLs (data not shown).
VPS33B deficiency results in degradation of α-granule cargo in lysosomes. (A) Both wild-type (WT) and VPS33B-KO imMKCLs were differentiated after the standard 5-day protocol and treated with bafilomycin A1 (BafA1) or the vehicle dimethyl sulfoxide during the last 24 hours. Total cell extracts were prepared, and the overall VWF and PF4 protein levels were analyzed by immunoblotting (2 independent replicates are shown). (B) The bar graph shows the quantification of independent replicates normalized to the amount of actin that was used as a loading control (WT, n = 3; VPS33B-KO, n = 5). *P ≤ .05.
VPS33B deficiency results in degradation of α-granule cargo in lysosomes. (A) Both wild-type (WT) and VPS33B-KO imMKCLs were differentiated after the standard 5-day protocol and treated with bafilomycin A1 (BafA1) or the vehicle dimethyl sulfoxide during the last 24 hours. Total cell extracts were prepared, and the overall VWF and PF4 protein levels were analyzed by immunoblotting (2 independent replicates are shown). (B) The bar graph shows the quantification of independent replicates normalized to the amount of actin that was used as a loading control (WT, n = 3; VPS33B-KO, n = 5). *P ≤ .05.
The VPS33B-VPS16B complex primarily localizes to recycling endosomes
The VPS33B-VPS16B complex is expected to associate with membranes to function. The identity of the specific megakaryocyte compartment(s) in which the VPS33B–VPS16B complex functions has been investigated by immunofluorescence microscopy with anti-VPS33B antibodies in primary megakaryocytes10 and Dami cells expressing GFP-VPS16B.12 These studies suggested a broad distribution, including the trans-Golgi network/sorting endosomes (AP-1), late endosomes (Rab7), α-granules (VWF), and lysosomes (LAMP1). In light of our results with the RUSH system delineating the pathway followed by newly synthesized α-granule cargo (Figure 3), we attempted to define the primary subcellular localization of the VPS33B-VPS16B complex. One complication is that complex formation is key for its proper localization and function and, in our studies, this was not adequately achieved by exogenous expression of one tagged subunit in wild-type cells. Here, we took advantage of the VPS33B-KO imMKCLs and rescued them by transient transfection with a GFP-VPS33B plasmid. Immunoblotting analysis of these cells showed an increased level of endogenous VPS16B, indicating the formation of a GFP-VPS33B-VPS16B complex (Figure 6A). Both VPS33B-KO imMKCL clones showed this successful rescue in 2 independent experiments. We then used the rescued cells to visualize the GFP-VPS33B-VPS16B complex by live cell spinning disk confocal fluorescence microscopy. The GFP-VPS33B-VPS16 complex showed a clear association with Cherry-Rab11a–labeled compartments (Figure 6B). Tracking of the structures over time verified true colocalization of the 2 markers as opposed to random colocalization in 1 frame (an example is shown in Figure 6B, lower panels). More importantly, quantification showed that 85% ± 5% of the GFP-VPS33B-VPS16 puncta occurred on Cherry-Rab11a–labeled organelles (15 cells analyzed). Rab11a is a well-established marker of recycling endosomes in other cell types46 as well as in megakaryocytes.8 In other cell types, recycling endosomes are also defined by the molecular motor myosin 5B and function in the transport of endocytosed transferrin.47 The presence of GFP–myosin 5B and transferrin Alexa 647 taken up from the culture media on the Cherry-Rab11a–labeled organelles in imMKCLs further confirms these compartments as recycling endosomes (supplemental Figure 9). These data suggest that the VPS33B-VPS16B complex primarily localizes to recycling endosomes, which are functionally close to the trans-Golgi network and sorting endosomes. Localization of the VPS33B-VPS16B complex to recycling endosomes fits nicely with the RUSH data indicating that newly synthesized cargo traffics through this compartment before reaching the α-granule (Figure 7).
Rescue of VPS33B-KO imMKCLs with GFP-VPS33B shows the VPS33B-VPS16B complex localizes to recycling endosomes. (A) The endogenous VPS16B protein level in 2 VPS33B-KO imMKCL clones transfected with GFP-VPS33B or untransfected control was analyzed by immunoblotting (2 independent replicates per clone). (B) Live cell confocal fluorescence microscopy analysis of VPS33B-KO imMKCL cells cotransfected with GFP-VPS33B and Cherry-Rab11a plasmids. The main panels correspond to the first frame of a movie and display each channel separately and the merge. The region indicated with a dotted rectangle is shown below with frames taken every 5 seconds and highlighting an example of GFP-VPS33B puncta localization to a Cherry-Rab11a compartment over time.
Rescue of VPS33B-KO imMKCLs with GFP-VPS33B shows the VPS33B-VPS16B complex localizes to recycling endosomes. (A) The endogenous VPS16B protein level in 2 VPS33B-KO imMKCL clones transfected with GFP-VPS33B or untransfected control was analyzed by immunoblotting (2 independent replicates per clone). (B) Live cell confocal fluorescence microscopy analysis of VPS33B-KO imMKCL cells cotransfected with GFP-VPS33B and Cherry-Rab11a plasmids. The main panels correspond to the first frame of a movie and display each channel separately and the merge. The region indicated with a dotted rectangle is shown below with frames taken every 5 seconds and highlighting an example of GFP-VPS33B puncta localization to a Cherry-Rab11a compartment over time.
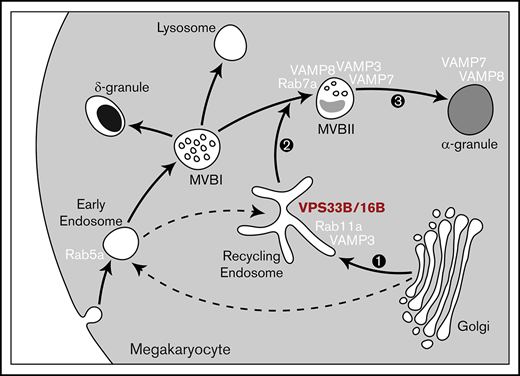
Model of the post–Golgi trafficking pathway followed by newly synthesized α-granule cargo. After leaving the Golgi complex, α-granule cargo traffics to recycling endosomes that contain Rab11a and VAMP3. The dotted arrows indicate the potential involvement of Rab5a compartments. The VPS33B-VPS16B complex also associates with recycling endosomes and is required for normal trafficking of cargo and α-granule biogenesis. Then, the α-granule proteins traffic to MVBs, possibly defining the transition between MVB type I and MVB type II, which contain higher levels of α-granule proteins and electron-dense lumen. The type II MVBs then mature to α-granules.
Model of the post–Golgi trafficking pathway followed by newly synthesized α-granule cargo. After leaving the Golgi complex, α-granule cargo traffics to recycling endosomes that contain Rab11a and VAMP3. The dotted arrows indicate the potential involvement of Rab5a compartments. The VPS33B-VPS16B complex also associates with recycling endosomes and is required for normal trafficking of cargo and α-granule biogenesis. Then, the α-granule proteins traffic to MVBs, possibly defining the transition between MVB type I and MVB type II, which contain higher levels of α-granule proteins and electron-dense lumen. The type II MVBs then mature to α-granules.
Discussion
The development of platelet α-granules is incompletely understood.1,5 The road taken by α-granule cargo of endocytic origin was elucidated by studies using a pulse chase method with labeled proteins.7,8 Endocytosed cargo progresses from early endosomes to late endosomes/MVBs and then to α-granules. An analogous approach to study proteins synthesized by the megakaryocyte was lacking. Here we adapted the RUSH system described for integral membrane proteins destined for the plasma membrane in nonspecialized cells31 to investigate the transport of newly synthesized soluble α-granule cargo proteins. First, the RUSH approach was validated by using 2 α-granule proteins, PDGF and PF4. Second, the transport pathway followed by PF4 was mapped out in detail (Figures 3 and 7). As a result, the recycling endosome was revealed as a key megakaryocyte compartment in α-granule biogenesis. The localization of key machinery (the VPS33B-VPS16B complex) to the recycling endosome further underscores the importance of this compartment for the biogenesis of α-granules. Interestingly, we observed the VPS33B-VPS16B complex on a subset of recycling endosomes, which might be specialized in the transport of α-granule cargo. The RUSH data support a model in which newly synthesized α-granule proteins then traffic from the recycling endosome to the late endosome/MVB, the known α-granule precursor.7
Two types of MVBs have been described in megakaryocytes: a less mature form (type I) containing little electron-dense material, and a more mature form (type II) containing electron-dense material that is more similar to the α-granule.7 The late endosome/MVB is also the precursor of platelet-dense granules and lysosomes (Figure 7).34,48 We suggest that such a common precursor is MVB type I and that the arrival of biosynthetic α-granule cargo from recycling endosomes defines the transition to MVB type II and then mature α-granule. This model is consistent with the electron microscopy characterization of megakaryocytes from VPS33B-deficient mice showing a reduction in both α-granules and mature type II MVBs.45 This model also fits nicely with the localization of a significant pool of the VPS33B-VPS16B complex to recycling endosomes.
Although there was an initial controversy regarding the possibility of VPS33B and VPS16B as part of CORVET and/or HOPS, more recent published evidence suggests biochemically separate complexes and functions.11,12,16-22,49 Accordingly, a recent review on platelet α-granule biogenesis concluded that VPS33B and VPS16B are not associated with CORVET/HOPS complexes.5 Results presented here clearly support the view of distinct complexes. In addition, several lines of evidence indicate that VPS33B and VPS16B associate into a stable complex that works as a unit. For example, lack of VPS33B causes a dramatic loss of VPS16B in imMKCLs. This result also resembles the converse situation of reduced VPS33B in platelets from a patient with ARC and a VPS16B mutation.12 Our results with imMKCLs and MEG01 cells are in agreement with a recent report showing that, in HEK293 cells, VPS33B and VPS16B are components of a complex smaller than CORVET and HOPS.25 Although we cannot rule out the transient association with additional proteins to form a larger complex, our comparison with recombinant VPS33B-VPS16B indicates that the complex does not stably associate with additional subunits. Such additional subunits confer CORVET and HOPS their ability to act as tethering complexes by mediating simultaneous interactions with 2 membrane-bound compartments via directly binding Rab proteins and/or the RILP complex.14,19 Therefore, the VPS33B-VPS16B complex may lack the intrinsic capacity to act as a tethering complex.
VPS33B is a member of the Sec1/Munc18 (SM) protein family that regulates SNAREs.9 Although SNARE proteins are critical for membrane fusion, their spontaneous assembly occurs with low efficiency, and SM proteins are needed to catalyze the reaction by acting as a template.15 SM proteins seem to recognize specific SNARE motifs and therefore contribute to the accuracy of vesicular transport. It is expected that VPS33B functions like other SM proteins, but the corresponding SNARE partners are not known. Regardless of the identity of the SNARE proteins involved, the function of VPS33B in ensuring transport fidelity is shown by our results indicating α-granule cargo degradation in lysosomes in VPS33B-deficient cells. Interestingly, a recent report found that NBEAL2 mutant mouse megakaryocytes, which have a deficiency in α-granules, fail to retain α-granule cargo and secrete it rather than degrade it.8 These contrasting results support the previously posited concept that the VPS33B-VPS16B complex and NBEAL2 likely function in different steps during α-granule biogenesis.5,12
Regarding SNARE proteins, VAMP3 is known to localize to recycling endosomes and to function in endosomal pathways.40,50 This scenario is consistent with the RUSH experiment showing maximal colocalization of cargo with VAMP3 at ∼2 hours. Interestingly, although at lower levels, cargo still shows appreciable colocalization with VAMP3 at 4 and 20 hours after release from the ER, when cargo is mostly at α-granules and colocalizes maximally with VAMP8, VAMP7, and fibrinogen. This finding implies that VAMP3 may have a broad distribution, possibly explaining a secondary function in granule fusion during platelet activation and redundancy between VAMPs.42,51,52
In conclusion, we developed innovative tools to study α-granule biogenesis, including the RUSH system for soluble α-granule cargo, generation of VPS33B-KO imMKCLs that recapitulate the α-granule deficiency observed in patients with ARC, and recombinant VPS33B-VPS16B complex. We further validated imMKCLs as a powerful system that allows a range of experimental approaches to study α-granule biogenesis at a cellular and molecular level. With these systems, we established the recycling endosome as a novel and key post–Golgi station in the pathway of newly synthesized cargo to α-granules, characterized VPS33B-VPS16B as a distinct and small complex localizing to recycling endosomes, and determined that α-granule proteins are degraded in lysosomes in VPS33B-deficient cells.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Koji Eto (Kyoto University) for the imMKCLs, Frank Perez and Graca Raposo (Institut Curie) for the original RUSH construct, and Richard Cheney (University of North Carolina) for the GFP-Myo5b plasmid.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants 1R01HL106186 and National Institutes of Health, National Institute of General Medical Sciences R01GM125619 (S.M.D.), as well as American Heart Association award 17GRNT33680196 (S.M.D.). The microscopes used in this work are supported in part by the CSU Microscope Imaging Network core infrastructure grant.
Authorship
Contribution: A.L.A. and S.M.D. designed the study, performed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Santiago M. Di Pietro, Department of Biochemistry and Molecular Biology, Colorado State University, 200 W Lake St, Fort Collins, CO 80523-1870; e-mail: santiago.dipietro@colostate.edu.