Abstract
Since successful cloning of thrombopoietin (TPO) in 1994, significant advances have been made in the development of recombinant TPO receptor agonists. The US Food and Drug Administration (FDA) has approved 2 agents for use in patients with immune thrombocytopenia (ITP): eltrombopag and romiplostim. Romiplostim is a once-weekly subcutaneous injection that has been shown to increase the platelet count, lessen bleeding, and reduce concurrent medication use in adults with ITP. In December 2018, the US FDA approved romiplostim for use in pediatric patients ≥1 year of age with ITP of >6 months’ duration and insufficient response to corticosteroids, immunoglobulins, or splenectomy, based on similarly favorable clinical trial data. In addition, romiplostim is well tolerated, making it an attractive option for the treatment of children. Expansion of off-label romiplostim use is being reported in children for ITP <6 months, neonatal thrombocytopenia, hereditary thrombocytopenias, and chemotherapy- and bone marrow transplant–associated thrombocytopenia. We review here the development of romiplostim with a focus on pediatric use.
Introduction
Successful cloning of the thrombopoietin (TPO) receptor occurred 1994, with several laboratories simultaneously reporting their findings.1 Around that same time, the effects of TPO, a growth factor for megakaryocytes and platelet production, were elucidated. Knowledge several years later supported impaired thrombopoiesis and megakaryocyte apoptosis in patients with immune thrombocytopenia (ITP),2-5 thus making TPO receptor agonists (TPO-RAs) a viable option for treatment. Recombinant TPO increased the platelet count in patients with ITP; however, because of the development of antibodies that cross-reacted with endogenous TPO, further use of recombinant TPO was halted.1 Following initial testing in healthy volunteers and preclinical data, romiplostim was fast tracked by the US Food and Drug Administration (FDA); in 2008, it became the first US FDA–approved TPO-RA for use in adults with chronic ITP. In 2018, it was further approved for use in children ≥1 year of age with ITP of >6 months’ duration and insufficient response to corticosteroids, immunoglobulins, or splenectomy. Along a similar time course, in 2011, a second TPO-RA, eltrombopag, was approved for adults with chronic ITP who had insufficient response to corticosteroids, immunoglobulins, or splenectomy, and the label was expanded to children ≥1 year of age in 2016. Use of eltrombopag in the pediatric population is outlined in a 2018 review article6 ; this review will focus on romiplostim.
Preclinical data
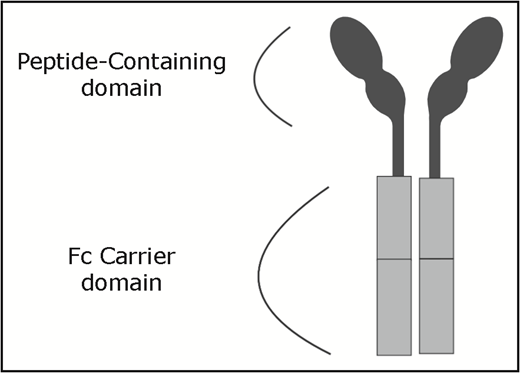
In 1997, romiplostim (formerly AMG531) was identified by searching recombinant peptide libraries to identify peptides that interacted with the TPO receptor but lacked sequence homology.7 The unstable nature of the peptide led to linking 2 of the peptides with 2 single-chain Fc domain subunits, resulting in a “peptibody”1 (Figure 1). The Fc carrier component binds with the FcRn salvage receptor, which extends the half-life by undergoing endothelial recirculation.8 The products are cleared via reticuloendothelial elimination and excreted in the urine.
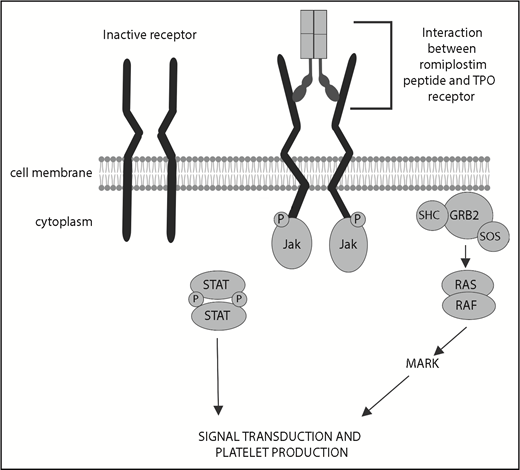
Romiplostim is able to stimulate megakaryocyte colony growth, as well as maturation. Early murine studies demonstrated that, compared with murine TPO, romiplostim resulted in similar megakaryocyte colony production, at higher concentrations (30 ng/mL compared with 1 ng/mL). Furthermore, the size, DNA content, acetylcholinesterase levels, and lobation of the megakaryocytes were identical in those induced with murine TPO or AMG531.9 Romiplostim also works in synergy with cytokines, such as erythropoietin and stem cell factor, to stimulate megakaryocyte growth and expansion. Lastly, although the effect of romiplostim is through interaction with the Mpl receptor, it did not inhibit the native role of TPO in megakaryocyte proliferation and could bind with Mpl, even in the presence of native TPO9 (Figure 2).
Pharmacodynamics and pharmacokinetics
Following exposure to exogenous recombinant TPO, platelet production peaks around 8 to 15 days and returns to baseline within ∼22 to 28 days.10 With regard to AMG531, early animal studies applied a starting dose of 10 μg/kg, and this was adapted in early pharmacodynamics studies in humans. Healthy subjects received a single IV or subcutaneous dose starting at 10 μg/kg, which was to be increased until a twofold increase in platelet count over baseline was achieved in ≥2 cohort subjects.10 Unexpectedly, there was a robust platelet count response (nearly sixfold) at 10 μg/kg; therefore, the starting dose was lowered to 0.1 μg/kg. The increased efficacy was conceptually related to increased affinity of romiplostim for the human Mpl receptor compared with the receptor in monkeys. In the IV and subcutaneous groups, the biologically active dose was determined to be 1 μg/kg.10 In this study and a study with dose escalation to 2 μg/kg,11 there was a dose-dependent increase in the platelet count. This increase is seen 1 to 3 days following a single IV dose or 4 to 9 days after a subcutaneous dose,10 with peak platelet counts occurring on roughly days 10 to 16.10,11 Platelet elevation lasted from 2 to 16 days, depending on the dose,11 even though serum concentrations fell below a detectable level, supporting the high potency of the drug to interact with the Mpl receptor.10,11
Romiplostim is eliminated primarily by the platelets and the reticuloendothelial system; therefore, clearance is dependent, in part, on platelet number. The pharmacokinetics showed that the drug had a biphasic distribution that was nonlinear with dose.10 Target-mediated drug disposition modeling established that weekly dosing would minimize fluctuations in the platelet count.8
The phase 1/2 pediatric clinical trial included assessment of pharmacokinetics.12 With weekly dosing, serum concentrations ranged from 16 to 51.1 pg/mL predose and from 17.7 to 274 pg/mL 2 days following a dose. In 55% of samples, the serum level was below the lower limit of detection postdosing. There was no difference across age cohorts and no relationship between serum concentration and dose.
Dosing
A phase 1-2 open-label dose-escalation trial in ITP was conducted with the primary aim of evaluating the safety in adult patients; however, dosing was also assessed.13 Sixteen patients were assigned to 4 dosing cohorts: 30, 100, 300, or 500 μg. Each subject received 1 subcutaneous dose, with subsequent doses on day 15 or 22 based on the platelet count. Fourteen patients received 2 doses, with 2 patients (300 μg and 500 μg) receiving 1 dose only, secondary to development of a platelet count >1000 × 109/L. The initial patient in the 500-μg cohort had a platelet count of 1062 × 109/L, and subsequent patients were treated with 300 μg. The interval between injections ranged from 2 to 3 weeks for most patients. A platelet count double baseline, and between 50 and 450 × 109/L, was achieved in 25% of the 30-μg cohort, 100% of the 100-μg cohort, and 57% of the 300-μg cohort. When converted to weight-based dosing, the dosing range was between 1 and 10 μg/kg.13
Current dosing regimen begins with administration of 1 μg/kg and escalates by 1 μg/kg per week until a maximum dose of 10 μg/kg.14 The goal is to determine the lowest dose that will maintain a platelet count >50 × 109/L with subcutaneous weekly dosing. Thrombocytosis should be avoided, and the dose is adjusted when platelet count elevation is encountered (Table 1). Additional dose adjustments for weight should be considered every 12 weeks in pediatric patients. Romiplostim has not been investigated in children <1 year of age; however, in children ≥1 year of age, the serum concentrations appear to mirror those seen in adults, and no adjustments in dosing schema are necessary. If there is no response within 4 weeks of achieving the maximum weekly dose of 10 μg/kg, then additional treatment strategies should be considered,14 such as switching to an alternative TPO-RA, use of concomitant immunosuppression, or changing to therapy with a different mechanism of action. If a patient suddenly fails to maintain a response to romiplostim, neutralizing antibodies should be investigated by contacting the industry sponsor (Novartis).
Dosing nomogram for romiplostim
| Platelet count, × 109/L . | Action* . |
|---|---|
| <50 | Increase by 1 μg/kg |
| 50-200 | No change |
| >200 | If >200 × 109/L for 2 consecutive weeks, reduce dose by 1 μg/kg. |
| >400 | Do not give dose. Access weekly platelet count. Once platelet count is <200 × 109/L, resume at dose decreased by 1 μg/kg. |
| Platelet count, × 109/L . | Action* . |
|---|---|
| <50 | Increase by 1 μg/kg |
| 50-200 | No change |
| >200 | If >200 × 109/L for 2 consecutive weeks, reduce dose by 1 μg/kg. |
| >400 | Do not give dose. Access weekly platelet count. Once platelet count is <200 × 109/L, resume at dose decreased by 1 μg/kg. |
Begin at 1 μg/kg and increase to maximum dose of 10 μg/kg. If no response at 10 μg/kg for 4 weeks, discontinue romiplostim.
Dosing considerations
Romiplostim is supplied in single-use vials and must be reconstituted.14 For pediatric patients, it is important to note that romiplostim can be supplied in a single-use 250- or 500-μg/mL vial; therefore, for dose adjustment, determination of appropriate vial size may impact the amount of unused drug and cost. Alternative dosing strategies in adults have been reported using higher “on-demand” doses to maintain the platelet count or biweekly dosing with “rescue” dosing for a decline in platelet count.15,16 These approaches are currently not endorsed, and based on known pharmacokinetic and pharmacodynamic data8 and a small case series of biweekly dosing in which patients were unable to maintain a stable extended platelet count,17 weekly dosing remains ideal.
The ideal initial dose and titration nomogram are not known. Bleeding rates are highest during dose titration, and complications of thrombocytosis are rare; therefore, initiation at higher doses may minimize bleeding without increasing complications. In a real-world study of romiplostim (n = 356 adults), 31% received a starting dose >1 μ/kg without an increase in thrombotic rates.18 Data from the ITP Consortium of North America ICON2 trial revealed that, in practice (n = 51 children), the median starting dose was 2 μg/kg (range, 1-9 μg/kg).19 In the randomized phase 3 trial, the median most frequent dose during the final 8 weeks of the study was 5.5 μg/kg (interquartile range, 3-10 μg/kg).20 Therefore, for most children, several weeks of dose adjustment are required prior to achieving a therapeutic dose. Furthermore, a platelet count threshold other than 50 to 200 × 109/L is often tolerated in clinical practice. Additional real-world data may inform practical dosing nomograms.
The US FDA Medication Guide currently denotes administration by a health care provider; however, weekly self-administration was permitted in the initial open-label pediatric trials alongside monthly health care laboratory and administration visits. Post hoc analysis of adult clinical trials demonstrated no effect of self-administration on romiplostim dose, drug safety, or efficacy.21,22 In fact, self-administration of romiplostim resulted in a greater percentage of patients having platelet counts within range, longer maintenance of platelet counts, and lower rates of duration-adjusted adverse events.22 The European pharmacovigilance program allowed for self-administration in patients with a stable platelet count ≥50 × 109/L without a dose adjustment for ≥4 weeks. To facilitate proper drug administration, the manufacturer provided a home administration training pack to eligible patients. Following 4 weeks of home administration training pack training, the majority of patients were able to correctly reconstitute romiplostim (97.5%), deliver the proper dose (100%), and successfully administer the injection (100%).23 Data from the pediatric ICON2 trial found that ∼16% of children (n = 51) had received ≥1 dose of romiplostim at home.19
Clinical trials efficacy
Initial phase 1 and phase 2 clinical trials of romiplostim in adult patients with chronic ITP demonstrated platelet responses within the targeted platelet range, 50 to 450 × 109/L, and at least twice the baseline platelet count, to various dosing cohorts ranging from 1 to 10 μg/kg per week.24 Efficacy of romiplostim in splenectomized and nonsplenectomized adults was shown in 2 parallel phase 3 randomized trials, with 38% of splenectomized patients (n = 42) and 61% of nonsplenectomized subjects (n = 41) achieving a durable platelet response (platelet count >50 × 109/L for ≥6 of the last 8 weeks of therapy). Reduction or discontinuation of concurrent therapy (eg, corticosteroids) occurred in 87% of patients given romiplostim compared with 38% receiving placebo.25 Further long-term open-label adult studies (n = 142) with romiplostim treatment showed an overall platelet count response (>50 × 109/L) in 87% of all patients.26 Lastly, the phase 3 long-term trial, describing up to 5 years of therapy and nearly 300 adult patients, noted median platelet counts of 50 to 200 × 109/L maintained by all patients at a median 92% of study visits.27
The first randomized trial for romiplostim use in pediatrics was reported in 2011.12 In this phase 1/2 study, 22 children, with a diagnosis of ITP for ≥6 months and a platelet count <30 × 109/L, were randomized to receive romiplostim or placebo. Patients received romiplostim weekly, starting at 1 μg/kg and to a maximum of 10 μg/kg. Patients in the romiplostim group maintained a platelet response (≥50 × 109/L) for a median of 7 weeks (range, 0-11) compared with 0 weeks in the placebo group. The same was true for the efficacy end point of a platelet count ≥50 × 109/L and ≥20 × 109/L above baseline for 2 consecutive weeks (88% romiplostim vs 0% placebo, P = .0008).
Following completion of the phase 1/2 trial, patients could enroll in the open-label extension study.28 Patients received a median of 167 weeks of treatment (range, 12-242) at a median dose of 5.4 μg/kg (interquartile 1 [Q1], Q3: 4.3, 8.0 μg/kg). After the first 12 weeks, median platelet counts remained ≥50 × 109/L for a median 84.3% of the weeks that they received treatment (Q1, Q3: 69.9%, 90.8%).
Subsequently, in a 24-week phase 3 randomized trial, children who had failed ≥1 additional therapy and continued to have a platelet count <30 × 109/L were eligible.20 A durable response, platelet count ≥50 × 109/L without rescue therapy in the previous 4 weeks for ≥6 of the final 8 study weeks, was exhibited by 52% of children in the treatment group compared with 10% in the placebo group (P = .002; odds ratio, 9.1; 95% confidence interval, 1.9-43.2). Romiplostim patients were also more likely to experience an overall platelet count response (≥4 platelet count responses between weeks 2 and 25), as well as any platelet response (platelet count ≥50 × 109/L). Further evidence of romiplostim efficacy was demonstrated by a reduction in the composite outcome of clinically significant bleeding (Common Terminology Criteria for Adverse Events grade ≥2), use of rescue medications, or both compared with placebo (P = .0001). More than 60 pediatric patients previously enrolled in a romiplostim ITP study continued on to a long-term extension study (median, 2.6 years; range, 0.1-7.0). Three fourths of the patients demonstrated a platelet count response ≥75% of the time.29
Reporting of patient-related outcomes is essential to understanding the complete impact of therapy on patients. In the adult phase 3 clinical trials, health-related quality of life (HRQoL) was evaluated via administration of the ITP patient assessment questionnaire at baseline and at weeks 4, 12, and 24. Pooled data, adjusted for splenectomy status, demonstrated improvement in 6 of the 10 scales.30 Data from the open-label 52-week study of ITP patients on romiplostim compared to those managed with medical standard of care revealed that treatment with romiplostim improved HRQoL from baseline.31
The pilot data from the pediatric phase 1/2 study showed that treatment with romiplostim significantly reduced parental burden (24 ± 17 vs −6 ± 8, P = .0008) based on the Kid’s ITP Tool (KIT). The study was unable to show changes in child-reported scores or parent proxy scores, likely because of the small number of enrolled children (n = 22).32 A more recent randomized trial applied the KIT as an exploratory outcome.33 The KIT was assessed at weeks 8, 16, and 25 in children receiving romiplostim (n = 42) or placebo (n = 20). Overall, this study showed greater changes in KIT scores from baseline to end of study in patients receiving romiplostim compared with placebo; the greatest effect was seen in the parent impact.
Safety
Adverse events
Adverse events for the initial adult phase 3 studies were primarily rated as mild to moderate in severity. Severe, life-threatening, or fatal bleeding events were reported in 12% of the placebo group and 7% of the romiplostim group (when platelet counts were <20 × 109/L); other mild and moderate bleeding events did not demonstrate a statistical difference among cohorts.
Nonbleeding events, such as dizziness, insomnia, myalgia, extremity pain, and abdominal pain, were increased in patients given romiplostim, with headache, fatigue, arthralgia, diarrhea, upper respiratory infections, back pain, nausea, cough, anxiety, and nasopharyngitis reported in >10% of patients in both treatment groups, yet the clinical significance in this small study population could not be fully assessed in these initial phase 3 studies.25 In the adult extension trials, headache (38%), nasopharyngitis (34%), upper respiratory tract infection (26%), and diarrhea (25%) were the most common nonbleeding adverse events. Thrombotic events were reported in 6.5% of patients, seen over the spectrum of platelet counts, with most events occurring when platelet counts were 200 to 400 × 109/L. It should be noted that nearly all patients had ≥1 preexisting cardiovascular risk factor.27
The first pediatric studies had no patients discontinuing therapy and no treatment-related serious adverse events. As in the adult studies, headache and epistaxis were the most commonly reported adverse events. Most bleeding adverse events occurred in the first 6 weeks of the treatment period, during the dose-escalation phase, and the platelet count was <30 × 109/L in >80% of these patients. The rate of bleeding events was 7.3 per 100 patient-weeks in the romiplostim group vs 11.9 per 100 patient-weeks in the placebo group.12
In the phase 3 pediatric trial, there were no grade 4 or 5 bleeding events, no cases of intracranial hemorrhage, and no age-related differences in bleeding, and most bleeding was grade 1 severity. The most frequently reported nonbleeding adverse events were headache, upper respiratory tract infections, vomiting, and oropharyngeal pain. Only the latter occurred more frequently in the romiplostim group, yet these were not serious events or deemed to be treatment related. There were no cases of thrombotic or thromboembolic events, with 1 patient experiencing thrombocytosis, a platelet count >1000 × 109/L.20
In the long-term extension trial, 54 serious adverse events occurred in 19 children, yet only 1 was reported to be treatment related, with grade 4 thrombocytopenia and grade 2 anemia associated with grade 3 epistaxis. The most commonly reported bleeding adverse events were contusion (51%), epistaxis (49%), and petechiae (31%), with 3 treatment-related bleeding events (injection site hemorrhage, injection site bruising, and epistaxis) and no case of intracranial hemorrhage. No thromboembolic or thrombotic adverse events were reported in these pediatric patients. Mirroring the adult data, headache (38%) and upper respiratory tract infection (32%) were the most commonly reported nonbleeding adverse events.29
Binding antibodies
As previously noted, the development of neutralizing autoantibodies to endogenous TPO in the initial recombinant TPO trials halted further development of these products. Reports of neutralizing antibodies to romiplostim, a second-generation TPO-RA, are predominantly described in case reports or case series. These antibodies have not been shown to cross-react with endogenous TPO.34 In the original pediatric studies, there were no reports of neutralizing antibodies to TPO or romiplostim.20,28 During the open-label extension trial, data at the 7-year time point demonstrated that antibody testing had been performed annually in 60 patients29 ; only 1 patient developed a neutralizing antibody, which was absent on subsequent testing 3 and 6 months following drug discontinuation. No patient in this trial developed anti-TPO–neutralizing antibodies.29
Bone marrow fibrosis
Of the 271 adult patients in a retrospective analysis, there were only 11 bone marrow examinations performed at the discretion of the provider. Although 10 samples demonstrated some degree of reticulin staining, only 5 had specimens pre- and posttherapy, and 4 of these identified an increase in reticulin grade. At follow-up, all patients had a decrease in reticulin grade, even 1 patient who continued on romiplostim therapy. Six of the 10 patients received doses >10 μg/kg. In the small prospective study, bone marrow stains were evaluable in 6 of 10 enrolled patients, and only 1 showed a 1-grade increase in reticulin staining, yet this remained within the normal range.35 In a long-term follow-up cohort, changes in bone marrow morphology in 131 evaluable biopsies from adult patients receiving romiplostim showed that 6.9% had increases that were ≥2 fibrosis grades, with 33% demonstrating reversibility after romiplostim discontinuation. As they relate to exposure, the numbers of patients with these changes in fibrosis grade were 3 of 41 at year 1, 1 of 28 at year 2, and 5 of 52 at year 3.36
The pediatric studies did not require bone marrow sampling and were performed only at the discretion of the treating provider. In the pediatric extension study, 5 bone marrow biopsies were conducted between weeks 34 and 87 after the start of treatment, and none showed bone marrow reticulin or fibrosis.28 In the long-term efficacy and safety study in the United States, bone marrow samples are not required,20 yet for those studies in the European Union, Switzerland, and Turkey, bone marrow assessment is being required at baseline and at 1 and 2 years and will likely contribute to a better understanding of the pediatric bone marrow response to romiplostim.37
Comparison with eltrombopag
Eltrombopag and romiplostim remain the only TPO-RAs approved by the US FDA for use in children. Data indicate that both agents are efficacious and safe in children compared with placebo; however, no randomized trial has directly compared the 2 TPO-RAs.
A systematic review showed that both drugs had higher rates of overall and durable responses compared with the control group in pediatric patients. Neither medication was associated with a reduction in overall bleeding events or severe bleeding events. Eltrombopag demonstrated reduced rates of rescue medication use compared with the control group; this effect was not seen with romiplostim.38 This may be reflective of the need to titrate romiplostim from a lower starting dose. An additional systematic review directly compared outcome and safety data for the 2 agents in children and found no significant differences.39
Both agents are associated with significant costs. A cost analysis of the 2 agents used in pediatric patients for 26 weeks showed that the cost per patient was $66 550 for eltrombopag, $101 056 for romiplostim, and $32 720 for an observational approach.40 It is important to note that this analysis used data that demonstrated a significantly higher rate of serious bleeding for romiplostim (5/37) than other trials, which contributed approximately $10 000 of additional romiplostim costs.
The decision about which agent to use is often driven by differences between the 2 treatments and patient preference. Eltrombopag is a once-daily oral medication, with certain dietary restrictions related to interaction with divalent cations.6 It is also structurally similar to agents involved in iron chelation, and limited data suggest that this may lead to iron deficiency in children receiving the drug.41 Additionally, eltrombopag is metabolized in the liver, and hepatotoxicity has occurred; therefore, hepatic function needs close monitoring. Lastly, until recently, a liquid preparation of eltrombopag was not available. Romiplostim, as outlined above, requires weekly subcutaneous injections and is not yet designated for home administration.
Indications and off-label use in pediatrics
Romiplostim is indicated for children ≥1 year of age who have had ITP for ≥6 months and an insufficient response to corticosteroids, immunoglobulins, or splenectomy. Use is to be reserved for patients whose platelet count represents an increased risk for bleeding; it is not to be used to “normalize” the platelet count.14 Despite narrow indications for ITP only, in clinical practice romiplostim is being used earlier and for reasons not specified on the label. One fourth (25.5%) of the patients who received romiplostim in the ICON2 analysis had newly diagnosed ITP, and 10% started it in an effort to resume activities or improve their HRQoL. It might be that the ideal timing of romiplostim use is earlier in the course of the disease when spontaneous remission may occur and avoiding immunosuppression would be ideal.
Off-label reports of romiplostim in pediatrics include use in neonatal alloimmune thrombocytopenia, congenital amegakaryocytic thrombocytopenia, chemotherapy-induced thrombocytopenia, and stem cell transplant–associated thrombocytopenia. Although eltrombopag has been used for the management of severe aplastic anemia, romiplostim does not appear to be as efficacious based on small studies, perhaps because of the difference in the location of interaction with the receptor.42,43
Neonatal alloimmune thrombocytopenia
Little is known about the role of TPO in fetal and neonatal megakaryocytopoiesis. Evidence suggests that TPO remains a regulator and can induce proliferation and differentiation of cord blood megakaryocyte progenitors.44 However, when neonatal and adult mice were exposed to a single dose of romiplostim, neonatal mice had a blunted platelet response, no increase in megakaryocyte size, and a lack of responsiveness of liver megakaryocytes.45 Lastly, eltrombopag has been shown to cross the blood–brain barrier and negatively impact neuronal development through effects as an iron chelator.46 Although romiplostim lacks the iron chelation effects of eltrombopag, the effects, if any, on neonatal neuronal development are unknown. Therefore, use of TPO-RAs in neonates is not indicated, and specific pharmacokinetics, pharmacodynamics, and safety trials would be needed.
There has been 1 case report on the use of romiplostim for the treatment of neonatal alloimmune thrombocytopenia.47 The infant received 4 doses of romiplostim (maximum dose of 3 μg/kg) starting at 34 days of life and was able to maintain stable platelet counts without any further treatment.
Hereditary thrombocytopenias
Current management for hereditary thrombocytopenias is usually with supportive care via platelet transfusions. However, this can result in platelet refractoriness and complicate bone marrow transplant, if needed. Splenectomy has been successful in Wiskott-Aldrich–associated thrombocytopenia, but at the expense of increased infection risk. Therefore, alternative options for management of severe thrombocytopenia in these conditions would be of high value.
Reports of romiplostim use are limited in this population. Two patients with myosin heavy chain-9–related disorders have received romiplostim therapy. Long-term use in 1 patient showed a decline in effectiveness; in the second patient, intermittent short-term use allowed for safe neurosurgery.48 A subgroup of patients with clinical congenital amegakaryocytic thrombocytopenia, specifically secondary to mutations in the TPHO gene, have received romiplostim. These mutations impair secretion and reduce the activity of native TPO. Five such patients have received romiplostim; all demonstrated a significant response in platelet count, as well as improvement in white cell counts and hemoglobin.49 There have been no reports of romiplostim use in Wiskott-Aldrich–associated thrombocytopenia; however, the use of eltrombopag in 8 patients showed a clinical or laboratory response in 5 patients.48
Chemotherapy-induced thrombocytopenia
A case series reported on the use of romiplostim in children with chemotherapy-induced thrombocytopenia (n = 5) secondary to treatment of solid malignancies. Given the importance of compressed therapy on outcomes, minimizing delays secondary to prolonged thrombocytopenia may provide an overall mortality benefit. In all 5 reported patients, romiplostim allowed for platelet recovery and the ability to resume chemotherapy after 2 or 3 doses.50
Stem cell transplant–associated thrombocytopenia
Secondary failure of platelet count recovery following stem cell transplant occurs in ∼20% of patients and can contribute significantly to morbidity and mortality. In a small case series (n = 7), children received romiplostim following stem cell transplant. Despite heterogeneous causes for thrombocytopenia, all but 1 patient had an excellent response to romiplostim therapy, with all requiring only 4 doses (3-7 μg/kg per dose) to increase the platelet count.51
Future directions
Future research could help to address some of the gaps in knowledge about romiplostim, including ideal dosing strategies, extended indications, optimum timing of initiation, and cost evaluation. Additionally, future understanding of the impact of romiplostim on the biology of the disease would inform use.
Current literature supports that changes occur that restore immune balance following exposure to TPO-RAs. The mechanism of this is not fully understood; however, it seems to resemble immune tolerance in which increased exposure to platelet autoantigens leads to reduction in autoantibodies. Data suggest that, following TPO-RA exposure, some individuals exhibit a reduction in levels of platelet antibodies,52 as well as improved regulatory T-cell number and function.53 A proportion of adult patients may be able to maintain a stable platelet count ≥50 × 109/L following the use of romiplostim. In a phase 2 trial extension trial, 24 adults (32% of all enrolled subjects) were able to continue to maintain a platelet count ≥50 × 109/L in the absence of additional ITP therapy for 24 consecutive weeks.54 It is important to note, however, that the majority of patients in this trial had a very short duration of ITP (median, 2.2 months; Q1, 0.9 months; Q3, 4.3 months), and pooled data from other clinical trials suggest that maintained platelet count rates may be lower in patients with chronic ITP.55 Although these trials did not include an observation group, spontaneous remission rates with observation alone are 9%.56
Additionally, treatment-free platelet response, defined as a platelet count >50 × 109/L for ≥6 months without receiving romiplostim or other medications to treat immune thrombocytopenia, was shown in nearly one fourth of the patients enrolled in the extension study. Younger age at diagnosis and at first romiplostim dose, as well as platelet response to ≥200 × 109/L within 4 weeks of therapy, were associated with treatment-free platelet response; because the natural history of ITP in childhood favors resolution, it is difficult to determine direct causality with the current data, yet this prompts further consideration and research.29 Retrospective real-world data from ICON2 showed that only 5% of children were in remission following TPO-RA exposure; however, this population represented a highly refractory group of patients.19 Further prospective data are needed in children to establish remission induction and biologic correlates.
Authorship
Contribution: C.E.N. and M.J.R. prepared and drafted the manuscript, made critical revisions, and approved the final version.
Conflict-of-interest disclosure: C.E.N. is a consultant for Sanofi-Genzyme. M.J.R. declares no competing financial interests.
Correspondence: Cindy E. Neunert, Department of Pediatrics, Columbia University Irving Medical Center, 3959 Broadway, CHN 10-03, New York, NY 10032; e-mail: cn2401@cumc.columbia.edu.